 Found 1990 hits with Last Name = 'sze' and Initial = 'j'
Found 1990 hits with Last Name = 'sze' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
Bombesin receptor subtype-3
(Homo sapiens (Human)) | BDBM50275902

(CHEMBL525577 | D-Phe-Gln-Trp-Ala-Val-b-Ala-His-Phe...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34+,40-,42+,43+,44+,45+,46+,48+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to BRS-3 receptor (unknown origin) |
Bioorg Med Chem Lett 18: 5451-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.033
BindingDB Entry DOI: 10.7270/Q2H9952R |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289127
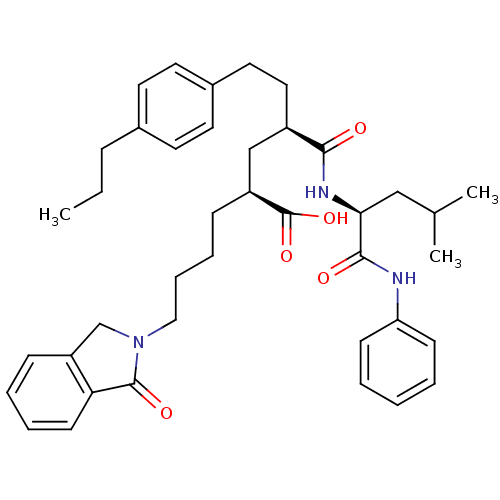
((2S,4R)-4-(3-Methyl-1-phenylcarbamoyl-butylcarbamo...)Show SMILES CCCc1ccc(CC[C@H](C[C@H](CCCCN2Cc3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C40H51N3O5/c1-4-12-29-18-20-30(21-19-29)22-23-31(37(44)42-36(25-28(2)3)38(45)41-34-15-6-5-7-16-34)26-32(40(47)48)13-10-11-24-43-27-33-14-8-9-17-35(33)39(43)46/h5-9,14-21,28,31-32,36H,4,10-13,22-27H2,1-3H3,(H,41,45)(H,42,44)(H,47,48)/t31-,32+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50354216
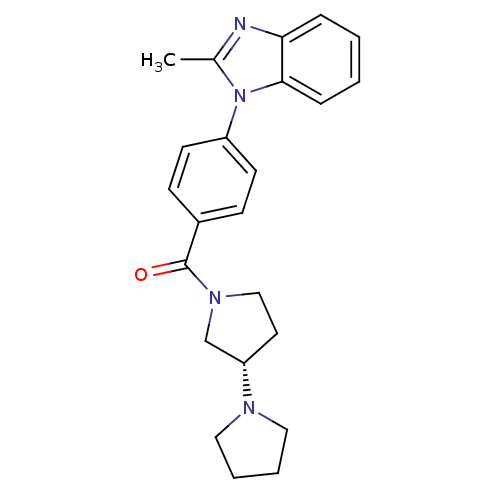
(CHEMBL1836004)Show SMILES Cc1nc2ccccc2n1-c1ccc(cc1)C(=O)N1CC[C@@H](C1)N1CCCC1 |r| Show InChI InChI=1S/C23H26N4O/c1-17-24-21-6-2-3-7-22(21)27(17)19-10-8-18(9-11-19)23(28)26-15-12-20(16-26)25-13-4-5-14-25/h2-3,6-11,20H,4-5,12-16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)alpha-methylhistamine from human histamine H3 receptor expressed in human HEK293T cells |
Bioorg Med Chem Lett 21: 5957-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.061
BindingDB Entry DOI: 10.7270/Q2QN6753 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50146441

(3-Methyl-5-pyridin-2-yl-8-(thiophen-2-ylsulfanyl)-...)Show InChI InChI=1S/C20H17NOS2/c1-13-11-15-14(16-5-2-3-9-21-16)7-8-18(20(15)17(22)12-13)24-19-6-4-10-23-19/h2-10,13H,11-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
Bioorg Med Chem Lett 14: 2871-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.054
BindingDB Entry DOI: 10.7270/Q2KW5GK5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364921
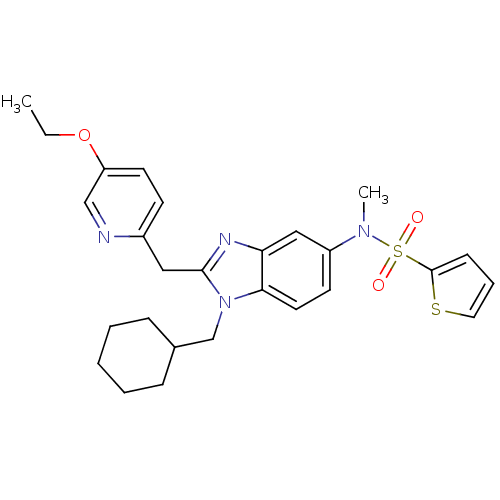
(CHEMBL1950329)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CC2CCCCC2)N(C)S(=O)(=O)c2cccs2)nc1 Show InChI InChI=1S/C27H32N4O3S2/c1-3-34-23-13-11-21(28-18-23)16-26-29-24-17-22(30(2)36(32,33)27-10-7-15-35-27)12-14-25(24)31(26)19-20-8-5-4-6-9-20/h7,10-15,17-18,20H,3-6,8-9,16,19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274186
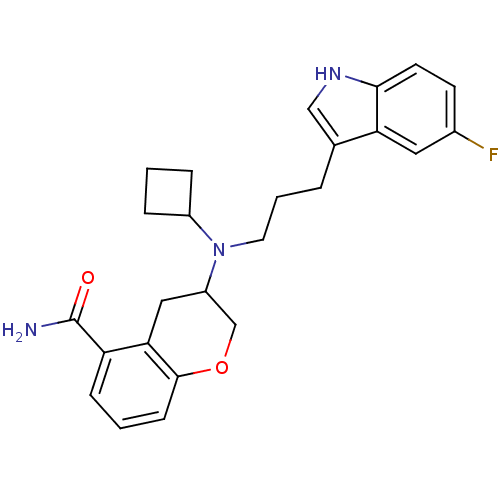
((+)-3-{Cyclobutyl[3-(5-fluoro-1H-indol-3-yl)propyl...)Show SMILES NC(=O)c1cccc2OCC(Cc12)N(CCCc1c[nH]c2ccc(F)cc12)C1CCC1 Show InChI InChI=1S/C25H28FN3O2/c26-17-9-10-23-21(12-17)16(14-28-23)4-3-11-29(18-5-1-6-18)19-13-22-20(25(27)30)7-2-8-24(22)31-15-19/h2,7-10,12,14,18-19,28H,1,3-6,11,13,15H2,(H2,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274782
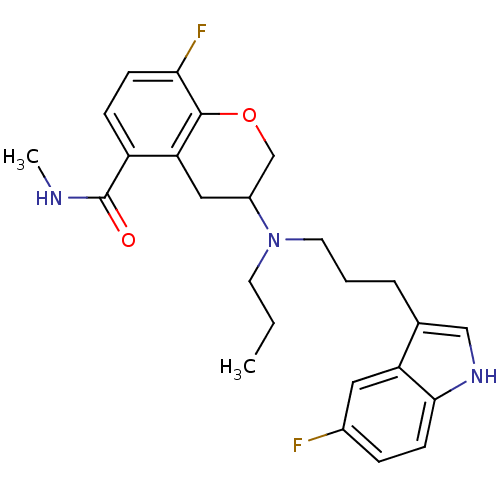
(8-Fluoro-3-{[3-(5-fluoro-1H-indol-3-yl)propyl](pro...)Show SMILES CCCN(CCCc1c[nH]c2ccc(F)cc12)C1COc2c(F)ccc(C(=O)NC)c2C1 Show InChI InChI=1S/C25H29F2N3O2/c1-3-10-30(11-4-5-16-14-29-23-9-6-17(26)12-20(16)23)18-13-21-19(25(31)28-2)7-8-22(27)24(21)32-15-18/h6-9,12,14,18,29H,3-5,10-11,13,15H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274784
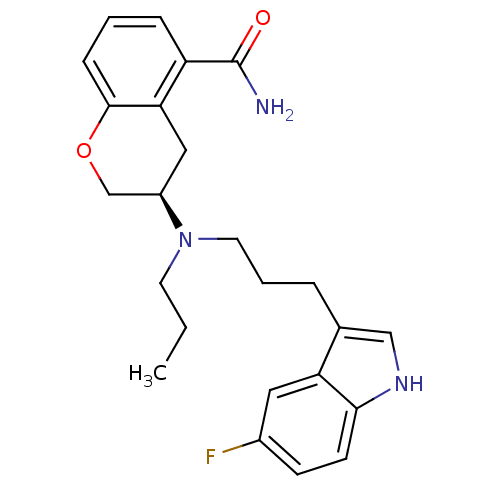
(3-{[3-(5-Fluoro-1H-indol-3-yl)propyl](propyl)amino...)Show SMILES CCCN(CCCc1c[nH]c2ccc(F)cc12)[C@H]1COc2cccc(C(N)=O)c2C1 |r| Show InChI InChI=1S/C24H28FN3O2/c1-2-10-28(11-4-5-16-14-27-22-9-8-17(25)12-20(16)22)18-13-21-19(24(26)29)6-3-7-23(21)30-15-18/h3,6-9,12,14,18,27H,2,4-5,10-11,13,15H2,1H3,(H2,26,29)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50275902

(CHEMBL525577 | D-Phe-Gln-Trp-Ala-Val-b-Ala-His-Phe...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C57H76N14O10/c1-6-7-21-42(49(60)73)66-55(79)44(26-36-18-12-9-13-19-36)69-56(80)46(28-38-30-61-31-63-38)68-50(74)33(4)65-57(81)48(32(2)3)71-51(75)34(5)64-54(78)45(27-37-29-62-41-22-15-14-20-39(37)41)70-53(77)43(23-24-47(59)72)67-52(76)40(58)25-35-16-10-8-11-17-35/h8-20,22,29-34,40,42-46,48,62H,6-7,21,23-28,58H2,1-5H3,(H2,59,72)(H2,60,73)(H,61,63)(H,64,78)(H,65,81)(H,66,79)(H,67,76)(H,68,74)(H,69,80)(H,70,77)(H,71,75)/t33-,34+,40-,42+,43+,44+,45+,46+,48+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to gastrin releasing peptide receptor (unknown origin) |
Bioorg Med Chem Lett 18: 5451-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.033
BindingDB Entry DOI: 10.7270/Q2H9952R |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50146443
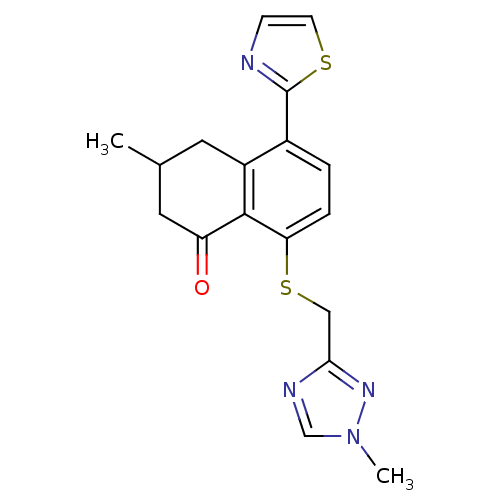
(3-Methyl-8-(1-methyl-1H-[1,2,4]triazol-3-ylmethyls...)Show SMILES CC1CC(=O)c2c(SCc3ncn(C)n3)ccc(-c3nccs3)c2C1 Show InChI InChI=1S/C18H18N4OS2/c1-11-7-13-12(18-19-5-6-24-18)3-4-15(17(13)14(23)8-11)25-9-16-20-10-22(2)21-16/h3-6,10-11H,7-9H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
Bioorg Med Chem Lett 14: 2871-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.054
BindingDB Entry DOI: 10.7270/Q2KW5GK5 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50146433
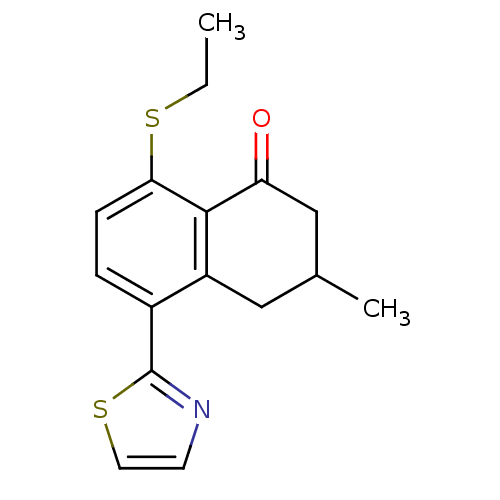
(8-Ethylsulfanyl-3-methyl-5-thiazol-2-yl-3,4-dihydr...)Show InChI InChI=1S/C16H17NOS2/c1-3-19-14-5-4-11(16-17-6-7-20-16)12-8-10(2)9-13(18)15(12)14/h4-7,10H,3,8-9H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
Bioorg Med Chem Lett 14: 2871-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.054
BindingDB Entry DOI: 10.7270/Q2KW5GK5 |
More data for this
Ligand-Target Pair | |
Beta-secretase 2
(Homo sapiens (Human)) | BDBM50392835

(CHEMBL2151181)Show SMILES CC#Cc1cncc(c1)-c1cc(Cl)c(s1)[C@]1(C)CC(=O)N(C)C(N)=N1 |r,c:25| Show InChI InChI=1S/C18H17ClN4OS/c1-4-5-11-6-12(10-21-9-11)14-7-13(19)16(25-14)18(2)8-15(24)23(3)17(20)22-18/h6-7,9-10H,8H2,1-3H3,(H2,20,22)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE2 by FRET assay |
ACS Med Chem Lett 3: 897-902 (2012)
Article DOI: 10.1021/ml3001165
BindingDB Entry DOI: 10.7270/Q23779SN |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085044

((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(5-methyl-2-...)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)-c1ccccc1 Show InChI InChI=1S/C34H30N2O5/c1-23-29(36-33(41-23)26-12-6-3-7-13-26)20-21-40-27-18-16-24(17-19-27)22-31(34(38)39)35-30-15-9-8-14-28(30)32(37)25-10-4-2-5-11-25/h2-19,31,35H,20-22H2,1H3,(H,38,39)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274487
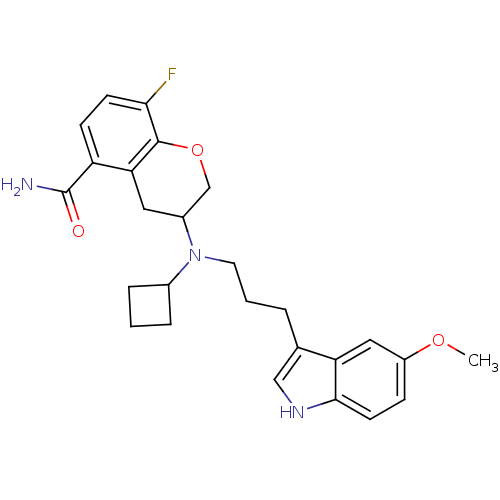
(3-{Cyclobutyl[3-(5-methoxy-1H-indol-3-yl)propyl]am...)Show SMILES COc1ccc2[nH]cc(CCCN(C3CCC3)C3COc4c(F)ccc(C(N)=O)c4C3)c2c1 Show InChI InChI=1S/C26H30FN3O3/c1-32-19-7-10-24-21(13-19)16(14-29-24)4-3-11-30(17-5-2-6-17)18-12-22-20(26(28)31)8-9-23(27)25(22)33-15-18/h7-10,13-14,17-18,29H,2-6,11-12,15H2,1H3,(H2,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50191615
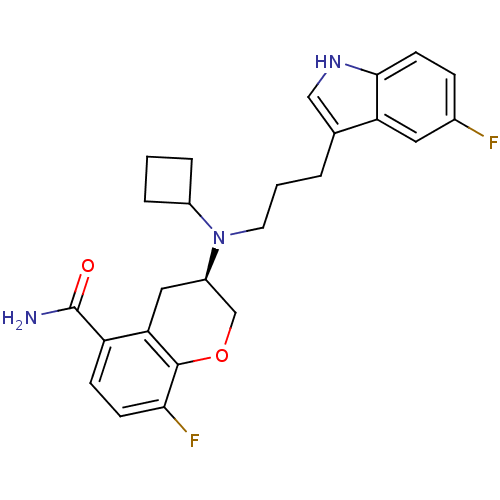
((3R)-(-)-3-{Cyclobutyl[3-(5-fluoro-1H-indol-3-yl)p...)Show SMILES NC(=O)c1ccc(F)c2OC[C@@H](Cc12)N(CCCc1c[nH]c2ccc(F)cc12)C1CCC1 |r| Show InChI InChI=1S/C25H27F2N3O2/c26-16-6-9-23-20(11-16)15(13-29-23)3-2-10-30(17-4-1-5-17)18-12-21-19(25(28)31)7-8-22(27)24(21)32-14-18/h6-9,11,13,17-18,29H,1-5,10,12,14H2,(H2,28,31)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by PDSP Ki Database
| |
J Med Chem 40: 2706-25 (1997)
Article DOI: 10.1021/jm970265x
BindingDB Entry DOI: 10.7270/Q27M06F9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274224
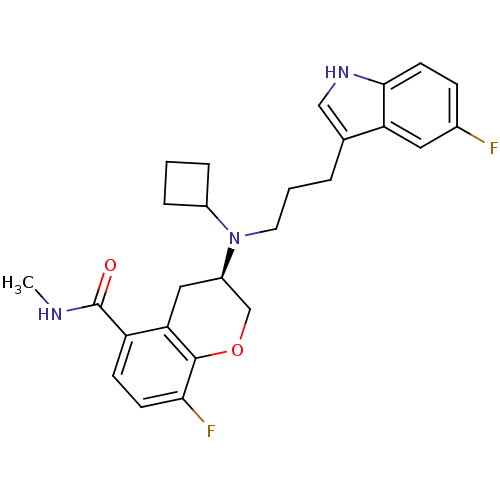
((3R)-(-)-3-{Cyclobutyl[3-(5-fluoro-1H-indol-3-yl)p...)Show SMILES CNC(=O)c1ccc(F)c2OC[C@@H](Cc12)N(CCCc1c[nH]c2ccc(F)cc12)C1CCC1 |r| Show InChI InChI=1S/C26H29F2N3O2/c1-29-26(32)20-8-9-23(28)25-22(20)13-19(15-33-25)31(18-5-2-6-18)11-3-4-16-14-30-24-10-7-17(27)12-21(16)24/h7-10,12,14,18-19,30H,2-6,11,13,15H2,1H3,(H,29,32)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50164512
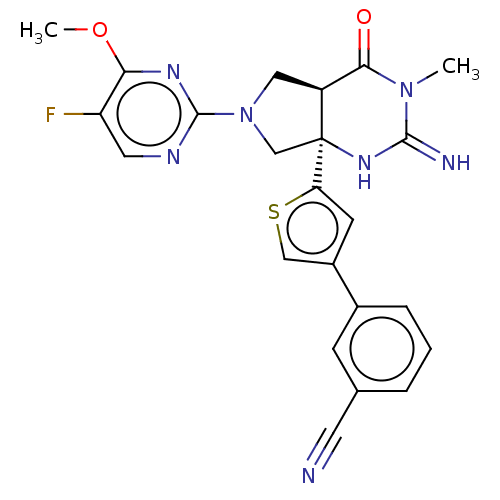
(CHEMBL3800286)Show SMILES [H][C@@]12CN(C[C@@]1(NC(=N)N(C)C2=O)c1cc(cs1)-c1cccc(c1)C#N)c1ncc(F)c(OC)n1 |r| Show InChI InChI=1S/C23H20FN7O2S/c1-30-20(32)16-10-31(22-27-9-17(24)19(28-22)33-2)12-23(16,29-21(30)26)18-7-15(11-34-18)14-5-3-4-13(6-14)8-25/h3-7,9,11,16H,10,12H2,1-2H3,(H2,26,29)/t16-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BACE1 preincubated for 30 mins followed QSY7EISEVNLDAEFC-Eu-amide substrate addition measured after 90 mins by FRET a... |
J Med Chem 59: 3231-48 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01995
BindingDB Entry DOI: 10.7270/Q2CR5W8V |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50191615

((3R)-(-)-3-{Cyclobutyl[3-(5-fluoro-1H-indol-3-yl)p...)Show SMILES NC(=O)c1ccc(F)c2OC[C@@H](Cc12)N(CCCc1c[nH]c2ccc(F)cc12)C1CCC1 |r| Show InChI InChI=1S/C25H27F2N3O2/c26-16-6-9-23-20(11-16)15(13-29-23)3-2-10-30(17-4-1-5-17)18-12-21-19(25(28)31)7-8-22(27)24(21)32-14-18/h6-9,11,13,17-18,29H,1-5,10,12,14H2,(H2,28,31)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from serotonin transporter in Sprague-Dawley rat frontal cortical membrane |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50191619
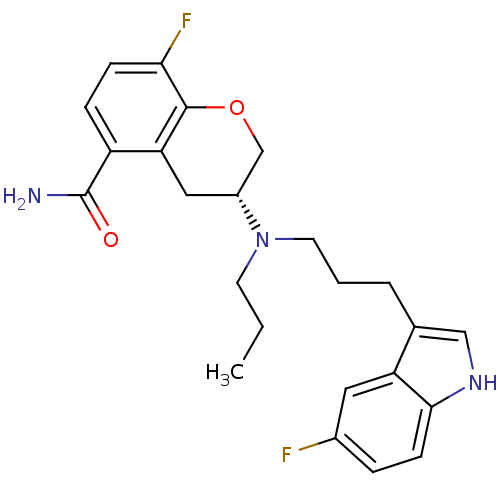
((3R)-(-)-8-Fluoro-3-{[3-(5-fluoro-1H-indol-3-yl)pr...)Show SMILES CCCN(CCCc1c[nH]c2ccc(F)cc12)[C@H]1COc2c(F)ccc(C(N)=O)c2C1 |r| Show InChI InChI=1S/C24H27F2N3O2/c1-2-9-29(10-3-4-15-13-28-22-8-5-16(25)11-19(15)22)17-12-20-18(24(27)30)6-7-21(26)23(20)31-14-17/h5-8,11,13,17,28H,2-4,9-10,12,14H2,1H3,(H2,27,30)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50418564
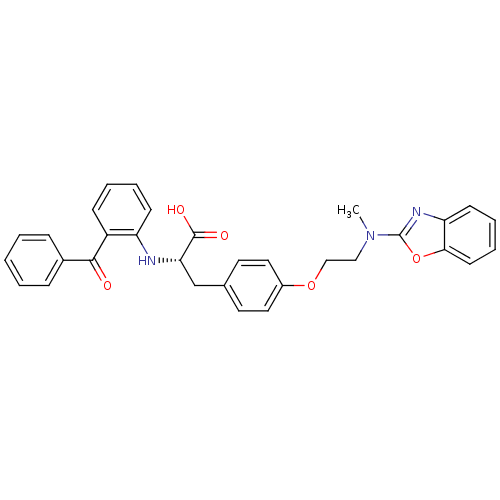
(CHEMBL423026)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1nc2ccccc2o1 Show InChI InChI=1S/C32H29N3O5/c1-35(32-34-27-13-7-8-14-29(27)40-32)19-20-39-24-17-15-22(16-18-24)21-28(31(37)38)33-26-12-6-5-11-25(26)30(36)23-9-3-2-4-10-23/h2-18,28,33H,19-21H2,1H3,(H,37,38)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
In vitro binding to peroxisome proliferator activated receptor gamma (PPAR gamma) using [3H]-BRL 49653 as radioligand in scintillation proximity assa... |
J Med Chem 41: 5020-36 (1999)
Checked by Author
Article DOI: 10.1021/jm9804127
BindingDB Entry DOI: 10.7270/Q20K2B28 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50354214
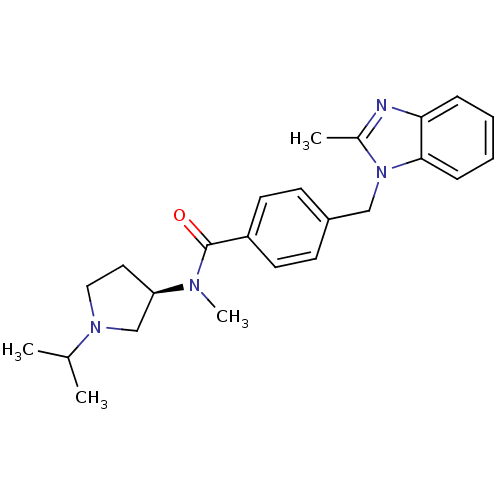
(CHEMBL1835992)Show SMILES CC(C)N1CC[C@H](C1)N(C)C(=O)c1ccc(Cn2c(C)nc3ccccc23)cc1 |r| Show InChI InChI=1S/C24H30N4O/c1-17(2)27-14-13-21(16-27)26(4)24(29)20-11-9-19(10-12-20)15-28-18(3)25-22-7-5-6-8-23(22)28/h5-12,17,21H,13-16H2,1-4H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)alpha-methylhistamine from human histamine H3 receptor expressed in human HEK293T cells |
Bioorg Med Chem Lett 21: 5957-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.061
BindingDB Entry DOI: 10.7270/Q2QN6753 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50392835

(CHEMBL2151181)Show SMILES CC#Cc1cncc(c1)-c1cc(Cl)c(s1)[C@]1(C)CC(=O)N(C)C(N)=N1 |r,c:25| Show InChI InChI=1S/C18H17ClN4OS/c1-4-5-11-6-12(10-21-9-11)14-7-13(19)16(25-14)18(2)8-15(24)23(3)17(20)22-18/h6-7,9-10H,8H2,1-3H3,(H2,20,22)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 by FRET assay |
ACS Med Chem Lett 3: 897-902 (2012)
Article DOI: 10.1021/ml3001165
BindingDB Entry DOI: 10.7270/Q23779SN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274528
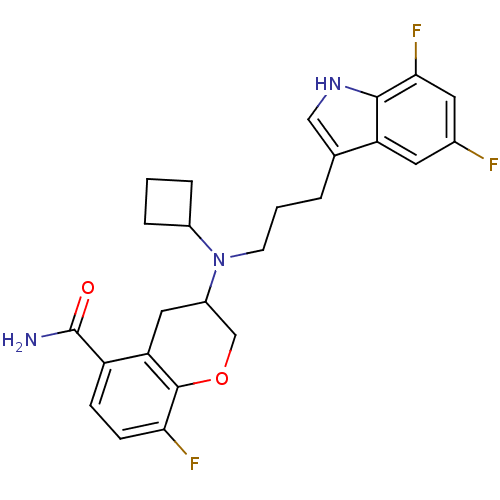
((+)-3-{Cyclobutyl[3-(5,7-difluoro-1H-indol-3-yl)pr...)Show SMILES NC(=O)c1ccc(F)c2OCC(Cc12)N(CCCc1c[nH]c2c(F)cc(F)cc12)C1CCC1 Show InChI InChI=1S/C25H26F3N3O2/c26-15-9-19-14(12-30-23(19)22(28)10-15)3-2-8-31(16-4-1-5-16)17-11-20-18(25(29)32)6-7-21(27)24(20)33-13-17/h6-7,9-10,12,16-17,30H,1-5,8,11,13H2,(H2,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274528

((+)-3-{Cyclobutyl[3-(5,7-difluoro-1H-indol-3-yl)pr...)Show SMILES NC(=O)c1ccc(F)c2OCC(Cc12)N(CCCc1c[nH]c2c(F)cc(F)cc12)C1CCC1 Show InChI InChI=1S/C25H26F3N3O2/c26-15-9-19-14(12-30-23(19)22(28)10-15)3-2-8-31(16-4-1-5-16)17-11-20-18(25(29)32)6-7-21(27)24(20)33-13-17/h6-7,9-10,12,16-17,30H,1-5,8,11,13H2,(H2,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274186

((+)-3-{Cyclobutyl[3-(5-fluoro-1H-indol-3-yl)propyl...)Show SMILES NC(=O)c1cccc2OCC(Cc12)N(CCCc1c[nH]c2ccc(F)cc12)C1CCC1 Show InChI InChI=1S/C25H28FN3O2/c26-17-9-10-23-21(12-17)16(14-28-23)4-3-11-29(18-5-1-6-18)19-13-22-20(25(27)30)7-2-8-24(22)31-15-19/h2,7-10,12,14,18-19,28H,1,3-6,11,13,15H2,(H2,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274186

((+)-3-{Cyclobutyl[3-(5-fluoro-1H-indol-3-yl)propyl...)Show SMILES NC(=O)c1cccc2OCC(Cc12)N(CCCc1c[nH]c2ccc(F)cc12)C1CCC1 Show InChI InChI=1S/C25H28FN3O2/c26-17-9-10-23-21(12-17)16(14-28-23)4-3-11-29(18-5-1-6-18)19-13-22-20(25(27)30)7-2-8-24(22)31-15-19/h2,7-10,12,14,18-19,28H,1,3-6,11,13,15H2,(H2,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50354205

(CHEMBL1835999)Show SMILES CN([C@@H]1CCN(C1)C1CCC1)C(=O)c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C19H24N4O/c1-21(18-10-13-22(14-18)16-4-2-5-16)19(24)15-6-8-17(9-7-15)23-12-3-11-20-23/h3,6-9,11-12,16,18H,2,4-5,10,13-14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)alpha-methylhistamine from human histamine H3 receptor expressed in human HEK293T cells |
Bioorg Med Chem Lett 21: 5957-60 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.061
BindingDB Entry DOI: 10.7270/Q2QN6753 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274414
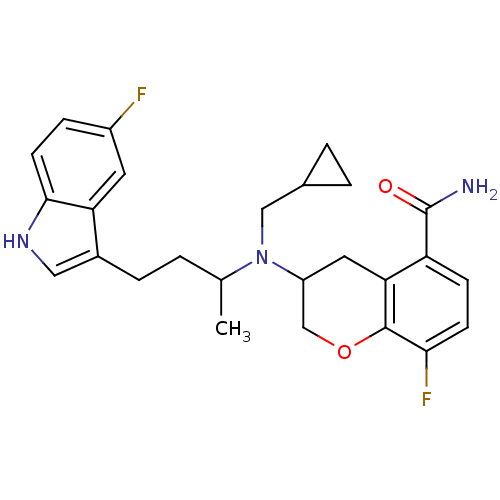
((+)-3-{(Cyclopropylmethyl)[3-(5-fluoro-1H-indol-3-...)Show SMILES CC(CCc1c[nH]c2ccc(F)cc12)N(CC1CC1)C1COc2c(F)ccc(C(N)=O)c2C1 Show InChI InChI=1S/C26H29F2N3O2/c1-15(2-5-17-12-30-24-9-6-18(27)10-21(17)24)31(13-16-3-4-16)19-11-22-20(26(29)32)7-8-23(28)25(22)33-14-19/h6-10,12,15-16,19,30H,2-5,11,13-14H2,1H3,(H2,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274414

((+)-3-{(Cyclopropylmethyl)[3-(5-fluoro-1H-indol-3-...)Show SMILES CC(CCc1c[nH]c2ccc(F)cc12)N(CC1CC1)C1COc2c(F)ccc(C(N)=O)c2C1 Show InChI InChI=1S/C26H29F2N3O2/c1-15(2-5-17-12-30-24-9-6-18(27)10-21(17)24)31(13-16-3-4-16)19-11-22-20(26(29)32)7-8-23(28)25(22)33-14-19/h6-10,12,15-16,19,30H,2-5,11,13-14H2,1H3,(H2,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289128
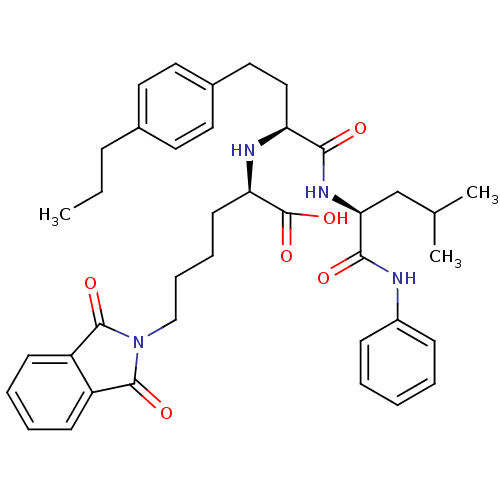
((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](CCCCN2C(=O)c3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C39H48N4O6/c1-4-12-27-18-20-28(21-19-27)22-23-32(35(44)42-34(25-26(2)3)36(45)40-29-13-6-5-7-14-29)41-33(39(48)49)17-10-11-24-43-37(46)30-15-8-9-16-31(30)38(43)47/h5-9,13-16,18-21,26,32-34,41H,4,10-12,17,22-25H2,1-3H3,(H,40,45)(H,42,44)(H,48,49)/t32-,33+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50146438

(8-Ethylsulfanyl-3-methyl-5-pyridin-2-yl-3,4-dihydr...)Show InChI InChI=1S/C18H19NOS/c1-3-21-17-8-7-13(15-6-4-5-9-19-15)14-10-12(2)11-16(20)18(14)17/h4-9,12H,3,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
Bioorg Med Chem Lett 14: 2871-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.054
BindingDB Entry DOI: 10.7270/Q2KW5GK5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274413

((+)-3-[[3-(5-Cyano-1H-indol-3-yl)propyl](cyclobuty...)Show SMILES NC(=O)c1ccc(F)c2OCC(Cc12)N(CCCc1c[nH]c2ccc(cc12)C#N)C1CCC1 Show InChI InChI=1S/C26H27FN4O2/c27-23-8-7-20(26(29)32)22-12-19(15-33-25(22)23)31(18-4-1-5-18)10-2-3-17-14-30-24-9-6-16(13-28)11-21(17)24/h6-9,11,14,18-19,30H,1-5,10,12,15H2,(H2,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50274413

((+)-3-[[3-(5-Cyano-1H-indol-3-yl)propyl](cyclobuty...)Show SMILES NC(=O)c1ccc(F)c2OCC(Cc12)N(CCCc1c[nH]c2ccc(cc12)C#N)C1CCC1 Show InChI InChI=1S/C26H27FN4O2/c27-23-8-7-20(26(29)32)22-12-19(15-33-25(22)23)31(18-4-1-5-18)10-2-3-17-14-30-24-9-6-16(13-28)11-21(17)24/h6-9,11,14,18-19,30H,1-5,10,12,15H2,(H2,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50166849
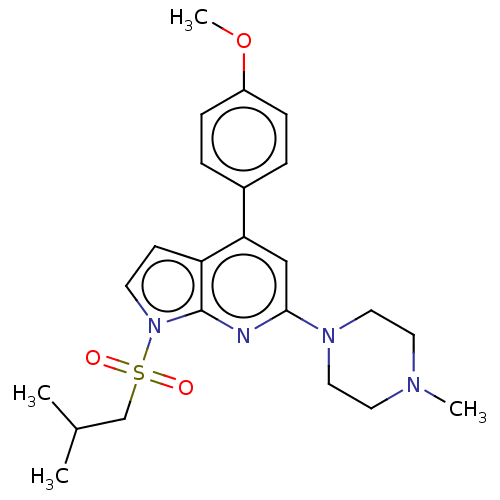
(CHEMBL3797651)Show SMILES COc1ccc(cc1)-c1cc(nc2n(ccc12)S(=O)(=O)CC(C)C)N1CCN(C)CC1 Show InChI InChI=1S/C23H30N4O3S/c1-17(2)16-31(28,29)27-10-9-20-21(18-5-7-19(30-4)8-6-18)15-22(24-23(20)27)26-13-11-25(3)12-14-26/h5-10,15,17H,11-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Selvita S.A.
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor in HEK293 cell membrane incubated for 1 hr by scintillation counting method |
Bioorg Med Chem Lett 26: 2610-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.024
BindingDB Entry DOI: 10.7270/Q2VM4F48 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 3884-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.128
BindingDB Entry DOI: 10.7270/Q28P61JV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057073
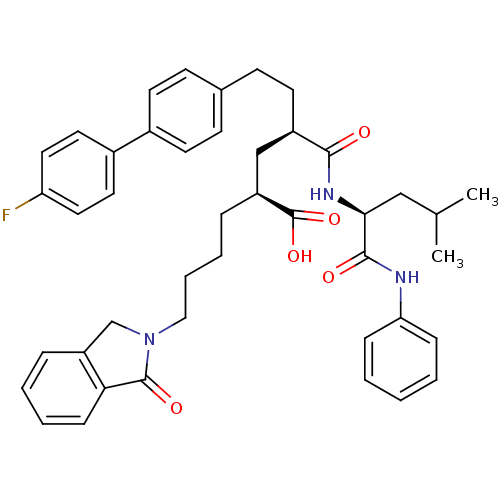
((2S,4R)-6-(4'-Fluoro-biphenyl-4-yl)-4-((S)-3-methy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccc(cc1)-c1ccc(F)cc1)C[C@H](CCCCN1Cc2ccccc2C1=O)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C43H48FN3O5/c1-29(2)26-39(41(49)45-37-12-4-3-5-13-37)46-40(48)33(20-17-30-15-18-31(19-16-30)32-21-23-36(44)24-22-32)27-34(43(51)52)10-8-9-25-47-28-35-11-6-7-14-38(35)42(47)50/h3-7,11-16,18-19,21-24,29,33-34,39H,8-10,17,20,25-28H2,1-2H3,(H,45,49)(H,46,48)(H,51,52)/t33-,34+,39+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057050
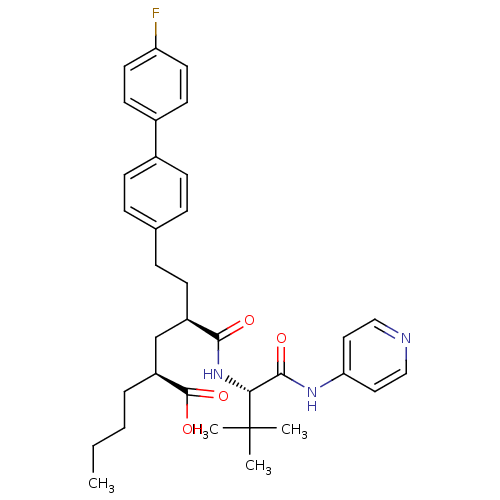
((2S,4R)-2-Butyl-4-[(S)-2,2-dimethyl-1-(pyridin-4-y...)Show SMILES CCCC[C@@H](C[C@@H](CCc1ccc(cc1)-c1ccc(F)cc1)C(=O)N[C@H](C(=O)Nc1ccncc1)C(C)(C)C)C(O)=O Show InChI InChI=1S/C34H42FN3O4/c1-5-6-7-27(33(41)42)22-26(13-10-23-8-11-24(12-9-23)25-14-16-28(35)17-15-25)31(39)38-30(34(2,3)4)32(40)37-29-18-20-36-21-19-29/h8-9,11-12,14-21,26-27,30H,5-7,10,13,22H2,1-4H3,(H,38,39)(H,41,42)(H,36,37,40)/t26-,27+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50146440

(8-Ethylsulfanyl-3-methyl-5-pyrazin-2-yl-3,4-dihydr...)Show InChI InChI=1S/C17H18N2OS/c1-3-21-16-5-4-12(14-10-18-6-7-19-14)13-8-11(2)9-15(20)17(13)16/h4-7,10-11H,3,8-9H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
Bioorg Med Chem Lett 14: 2871-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.054
BindingDB Entry DOI: 10.7270/Q2KW5GK5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50191627
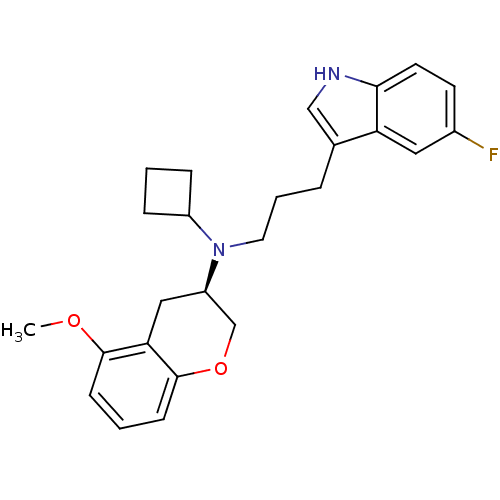
((3R)-(-)-N-Cyclobutyl-N-[3-(5-fluoro-1H-indol-3-yl...)Show SMILES COc1cccc2OC[C@@H](Cc12)N(CCCc1c[nH]c2ccc(F)cc12)C1CCC1 |r| Show InChI InChI=1S/C25H29FN2O2/c1-29-24-8-3-9-25-22(24)14-20(16-30-25)28(19-6-2-7-19)12-4-5-17-15-27-23-11-10-18(26)13-21(17)23/h3,8-11,13,15,19-20,27H,2,4-7,12,14,16H2,1H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human cloned 5HT1A receptor expressed in CHO cells |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50065229
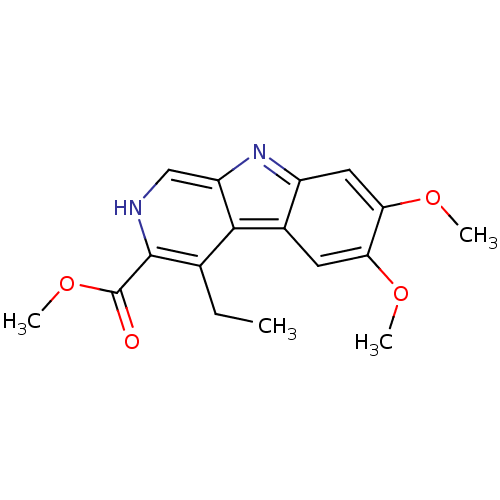
(4-Ethyl-6,7-dimethoxy-9H-beta-carboline-3-carboxyl...)Show InChI InChI=1S/C17H18N2O4/c1-5-9-15-10-6-13(21-2)14(22-3)7-11(10)19-12(15)8-18-16(9)17(20)23-4/h6-8,18H,5H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
Bioorg Med Chem Lett 14: 2871-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.054
BindingDB Entry DOI: 10.7270/Q2KW5GK5 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50146441

(3-Methyl-5-pyridin-2-yl-8-(thiophen-2-ylsulfanyl)-...)Show InChI InChI=1S/C20H17NOS2/c1-13-11-15-14(16-5-2-3-9-21-16)7-8-18(20(15)17(22)12-13)24-19-6-4-10-23-19/h2-10,13H,11-12H2,1H3 | UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to recombinant human gamma-aminobutyric-acid A receptor alpha-1-beta-3-gamma-2 |
Bioorg Med Chem Lett 14: 2871-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.054
BindingDB Entry DOI: 10.7270/Q2KW5GK5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50274782

(8-Fluoro-3-{[3-(5-fluoro-1H-indol-3-yl)propyl](pro...)Show SMILES CCCN(CCCc1c[nH]c2ccc(F)cc12)C1COc2c(F)ccc(C(=O)NC)c2C1 Show InChI InChI=1S/C25H29F2N3O2/c1-3-10-30(11-4-5-16-14-29-23-9-6-17(26)12-20(16)23)18-13-21-19(25(31)28-2)7-8-22(27)24(21)32-15-18/h6-9,12,14,18,29H,3-5,10-11,13,15H2,1-2H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from serotonin transporter in Sprague-Dawley rat frontal cortical membrane |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50274413

((+)-3-[[3-(5-Cyano-1H-indol-3-yl)propyl](cyclobuty...)Show SMILES NC(=O)c1ccc(F)c2OCC(Cc12)N(CCCc1c[nH]c2ccc(cc12)C#N)C1CCC1 Show InChI InChI=1S/C26H27FN4O2/c27-23-8-7-20(26(29)32)22-12-19(15-33-25(22)23)31(18-4-1-5-18)10-2-3-17-14-30-24-9-6-16(13-28)11-21(17)24/h6-9,11,14,18-19,30H,1-5,10,12,15H2,(H2,29,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from serotonin transporter in Sprague-Dawley rat frontal cortical membrane |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50274413

((+)-3-[[3-(5-Cyano-1H-indol-3-yl)propyl](cyclobuty...)Show SMILES NC(=O)c1ccc(F)c2OCC(Cc12)N(CCCc1c[nH]c2ccc(cc12)C#N)C1CCC1 Show InChI InChI=1S/C26H27FN4O2/c27-23-8-7-20(26(29)32)22-12-19(15-33-25(22)23)31(18-4-1-5-18)10-2-3-17-14-30-24-9-6-16(13-28)11-21(17)24/h6-9,11,14,18-19,30H,1-5,10,12,15H2,(H2,29,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from serotonin transporter in Sprague-Dawley rat frontal cortical membrane |
J Med Chem 51: 6980-7004 (2008)
Article DOI: 10.1021/jm8007097
BindingDB Entry DOI: 10.7270/Q2TT4RWM |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50146436
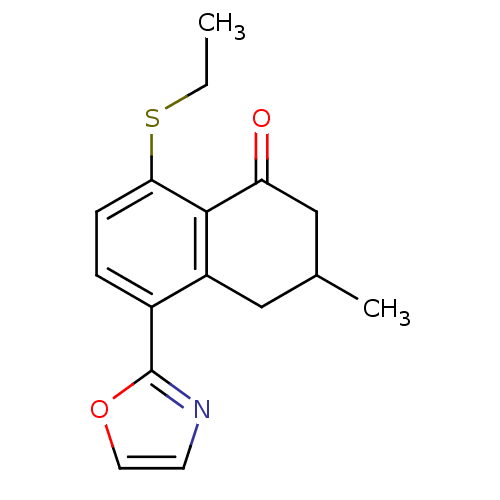
(8-Ethylsulfanyl-3-methyl-5-oxazol-2-yl-3,4-dihydro...)Show InChI InChI=1S/C16H17NO2S/c1-3-20-14-5-4-11(16-17-6-7-19-16)12-8-10(2)9-13(18)15(12)14/h4-7,10H,3,8-9H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
Bioorg Med Chem Lett 14: 2871-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.054
BindingDB Entry DOI: 10.7270/Q2KW5GK5 |
More data for this
Ligand-Target Pair | |
GABA-A receptor; alpha-5/beta-3/gamma-2
(Homo sapiens (Human)) | BDBM50146442
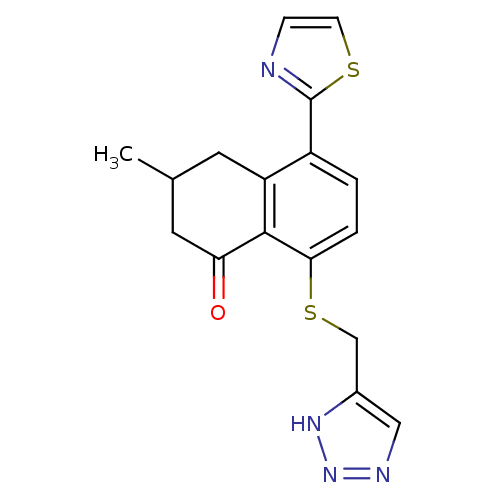
(3-Methyl-5-thiazol-2-yl-8-(3H-[1,2,3]triazol-4-ylm...)Show SMILES CC1CC(=O)c2c(SCc3cnn[nH]3)ccc(-c3nccs3)c2C1 Show InChI InChI=1S/C17H16N4OS2/c1-10-6-13-12(17-18-4-5-23-17)2-3-15(16(13)14(22)7-10)24-9-11-8-19-21-20-11/h2-5,8,10H,6-7,9H2,1H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-Ro- 15-1788 binding to human GABA-A receptor alpha-5-beta-3-gamma-2 subunits |
Bioorg Med Chem Lett 14: 2871-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.054
BindingDB Entry DOI: 10.7270/Q2KW5GK5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50289128

((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](CCCCN2C(=O)c3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C39H48N4O6/c1-4-12-27-18-20-28(21-19-27)22-23-32(35(44)42-34(25-26(2)3)36(45)40-29-13-6-5-7-14-29)41-33(39(48)49)17-10-11-24-43-37(46)30-15-8-9-16-31(30)38(43)47/h5-9,13-16,18-21,26,32-34,41H,4,10-12,17,22-25H2,1-3H3,(H,40,45)(H,42,44)(H,48,49)/t32-,33+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2(MMP-2) |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data