 Found 2202 hits with Last Name = 'tam' and Initial = 'k'
Found 2202 hits with Last Name = 'tam' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Escherichia coli) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026300

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-26-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-2)28-7-5-3-4-6-16(24)25/h9-11H,3-8H2,1-2H3,(H,24,25)(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026300

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-26-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-2)28-7-5-3-4-6-16(24)25/h9-11H,3-8H2,1-2H3,(H,24,25)(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026308
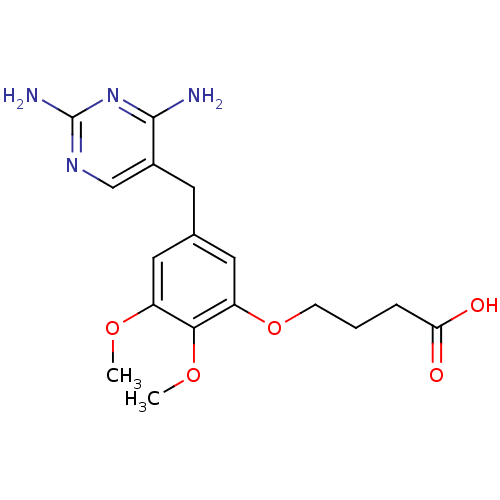
(4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C17H22N4O5/c1-24-12-7-10(6-11-9-20-17(19)21-16(11)18)8-13(15(12)25-2)26-5-3-4-14(22)23/h7-9H,3-6H2,1-2H3,(H,22,23)(H4,18,19,20,21) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026308

(4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C17H22N4O5/c1-24-12-7-10(6-11-9-20-17(19)21-16(11)18)8-13(15(12)25-2)26-5-3-4-14(22)23/h7-9H,3-6H2,1-2H3,(H,22,23)(H4,18,19,20,21) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026318
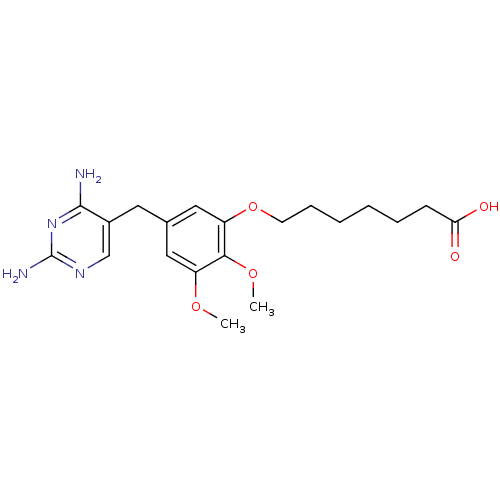
(7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C20H28N4O5/c1-27-15-10-13(9-14-12-23-20(22)24-19(14)21)11-16(18(15)28-2)29-8-6-4-3-5-7-17(25)26/h10-12H,3-9H2,1-2H3,(H,25,26)(H4,21,22,23,24) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026318

(7-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C20H28N4O5/c1-27-15-10-13(9-14-12-23-20(22)24-19(14)21)11-16(18(15)28-2)29-8-6-4-3-5-7-17(25)26/h10-12H,3-9H2,1-2H3,(H,25,26)(H4,21,22,23,24) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026314
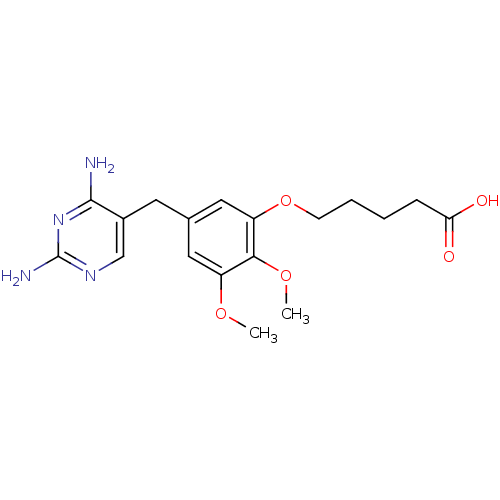
(5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C18H24N4O5/c1-25-13-8-11(7-12-10-21-18(20)22-17(12)19)9-14(16(13)26-2)27-6-4-3-5-15(23)24/h8-10H,3-7H2,1-2H3,(H,23,24)(H4,19,20,21,22) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026314

(5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C18H24N4O5/c1-25-13-8-11(7-12-10-21-18(20)22-17(12)19)9-14(16(13)26-2)27-6-4-3-5-15(23)24/h8-10H,3-7H2,1-2H3,(H,23,24)(H4,19,20,21,22) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324510
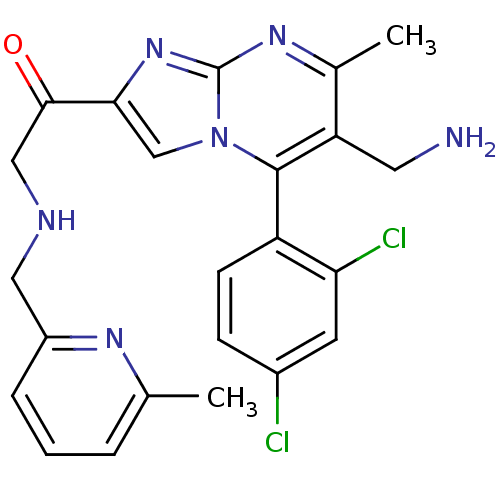
(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES Cc1cccc(CNCC(=O)c2cn3c(c(CN)c(C)nc3n2)-c2ccc(Cl)cc2Cl)n1 |(-4.59,-8.35,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,;-1.62,-5.59,;-.08,-5.54,;.65,-4.18,;2.19,-4.14,;2.92,-2.78,;2.11,-1.48,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;9.45,-1.81,;10.76,-1,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;-2.34,-6.93,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-13-4-3-5-16(28-13)10-27-11-21(32)20-12-31-22(17-7-6-15(24)8-19(17)25)18(9-26)14(2)29-23(31)30-20/h3-8,12,27H,9-11,26H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324512
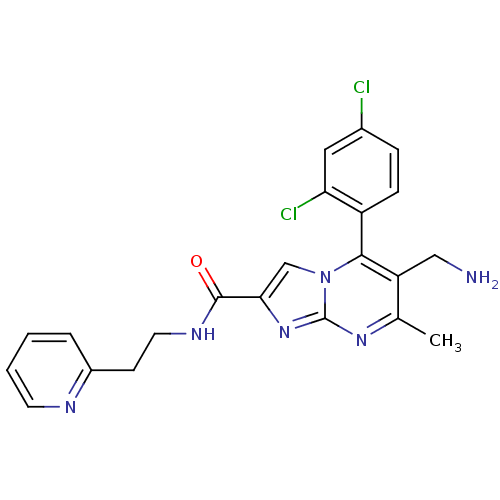
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1ccccn1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.93,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(11-25)20(16-6-5-14(23)10-18(16)24)30-12-19(29-22(30)28-13)21(31)27-9-7-15-4-2-3-8-26-15/h2-6,8,10,12H,7,9,11,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324523
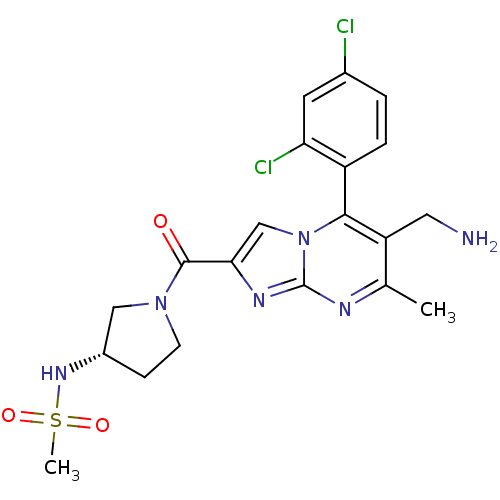
(CHEMBL1215018 | N-((3S)-1-(6-(aminomethyl)-5-(2,4-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CC[C@@H](C1)NS(C)(=O)=O |r,wD:25.30,(7.23,-5.85,;5.92,-6.66,;4.56,-5.95,;3.26,-6.75,;1.79,-6.32,;.92,-7.59,;1.87,-8.81,;3.31,-8.29,;4.66,-9.02,;5.97,-8.21,;7.33,-8.93,;8.64,-8.12,;4.63,-10.55,;3.28,-11.29,;3.25,-12.83,;4.57,-13.63,;4.54,-15.17,;5.92,-12.87,;5.95,-11.34,;7.29,-10.6,;-.62,-7.63,;-1.43,-6.33,;-1.34,-8.99,;-.88,-10.46,;-2.12,-11.36,;-3.37,-10.46,;-2.89,-8.99,;-4.91,-10.48,;-5.99,-9.37,;-7.4,-10.02,;-6.68,-7.99,;-4.84,-8.35,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-11-15(8-23)18(14-4-3-12(21)7-16(14)22)28-10-17(25-20(28)24-11)19(29)27-6-5-13(9-27)26-32(2,30)31/h3-4,7,10,13,26H,5-6,8-9,23H2,1-2H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50226128
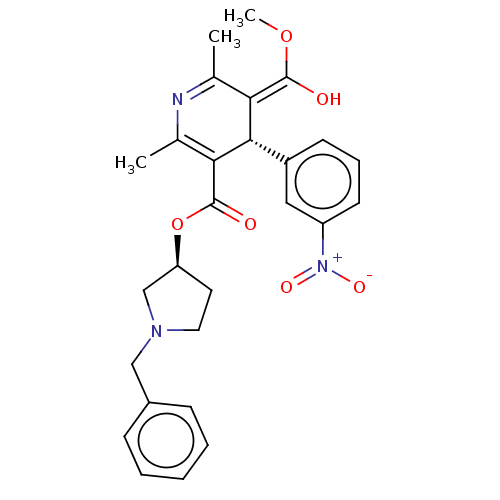
(CHEMBL2093893)Show SMILES Cl.[H][C@@]1(CCN(Cc2ccccc2)C1)OC(=O)C1=C(C)N=C(C)\C(=C(\O)OC)[C@]1([H])c1cccc(c1)[N+]([O-])=O |r,c:18,t:21| Show InChI InChI=1S/C27H29N3O6.ClH/c1-17-23(26(31)35-3)25(20-10-7-11-21(14-20)30(33)34)24(18(2)28-17)27(32)36-22-12-13-29(16-22)15-19-8-5-4-6-9-19;/h4-11,14,22,25,31H,12-13,15-16H2,1-3H3;1H/b26-23+;/t22-,25-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50396885
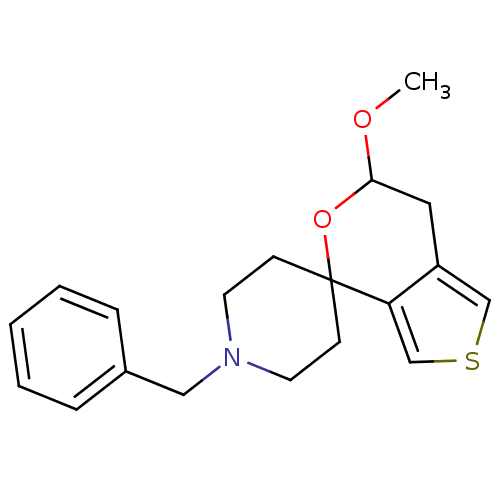
(CHEMBL2170669)Show InChI InChI=1S/C19H23NO2S/c1-21-18-11-16-13-23-14-17(16)19(22-18)7-9-20(10-8-19)12-15-5-3-2-4-6-15/h2-6,13-14,18H,7-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 120 mins by scintillation counting |
J Med Chem 55: 8047-65 (2012)
Article DOI: 10.1021/jm300894h
BindingDB Entry DOI: 10.7270/Q2G44RDB |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50396850
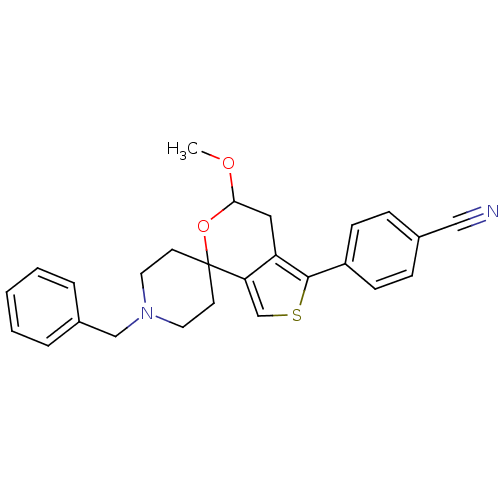
(CHEMBL2170496)Show SMILES COC1Cc2c(csc2-c2ccc(cc2)C#N)C2(CCN(Cc3ccccc3)CC2)O1 Show InChI InChI=1S/C26H26N2O2S/c1-29-24-15-22-23(18-31-25(22)21-9-7-19(16-27)8-10-21)26(30-24)11-13-28(14-12-26)17-20-5-3-2-4-6-20/h2-10,18,24H,11-15,17H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 120 mins by scintillation counting |
J Med Chem 55: 8047-65 (2012)
Article DOI: 10.1021/jm300894h
BindingDB Entry DOI: 10.7270/Q2G44RDB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324512

(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1ccccn1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.93,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(11-25)20(16-6-5-14(23)10-18(16)24)30-12-19(29-22(30)28-13)21(31)27-9-7-15-4-2-3-8-26-15/h2-6,8,10,12H,7,9,11,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324510

(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES Cc1cccc(CNCC(=O)c2cn3c(c(CN)c(C)nc3n2)-c2ccc(Cl)cc2Cl)n1 |(-4.59,-8.35,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,;-1.62,-5.59,;-.08,-5.54,;.65,-4.18,;2.19,-4.14,;2.92,-2.78,;2.11,-1.48,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;9.45,-1.81,;10.76,-1,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;-2.34,-6.93,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-13-4-3-5-16(28-13)10-27-11-21(32)20-12-31-22(17-7-6-15(24)8-19(17)25)18(9-26)14(2)29-23(31)30-20/h3-8,12,27H,9-11,26H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324525
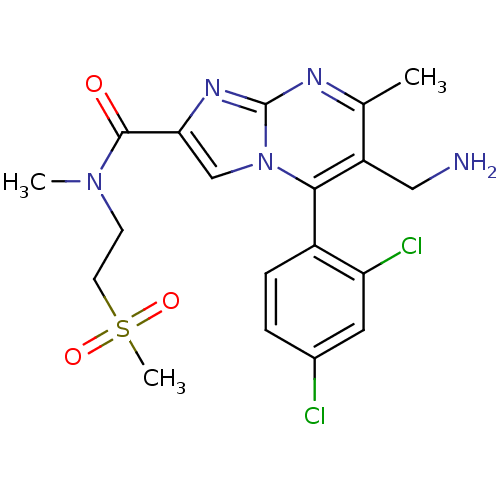
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...)Show SMILES CN(CCS(C)(=O)=O)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(2.96,-5.01,;2.15,-3.7,;.61,-3.75,;-.19,-2.43,;-1.73,-2.48,;-2.54,-1.17,;-3.23,-2.88,;-1.74,-4.02,;2.88,-2.34,;2.07,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.47,-2.91,;10.83,-3.64,;12.13,-2.83,;9.42,-1.37,;10.73,-.55,;8.06,-.65,;6.76,-1.46,;5.29,-1.03,;8.13,-5.25,;6.78,-5.99,;6.75,-7.53,;8.07,-8.33,;8.04,-9.87,;9.42,-7.58,;9.44,-6.05,;10.79,-5.3,)| Show InChI InChI=1S/C19H21Cl2N5O3S/c1-11-14(9-22)17(13-5-4-12(20)8-15(13)21)26-10-16(24-19(26)23-11)18(27)25(2)6-7-30(3,28)29/h4-5,8,10H,6-7,9,22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324511
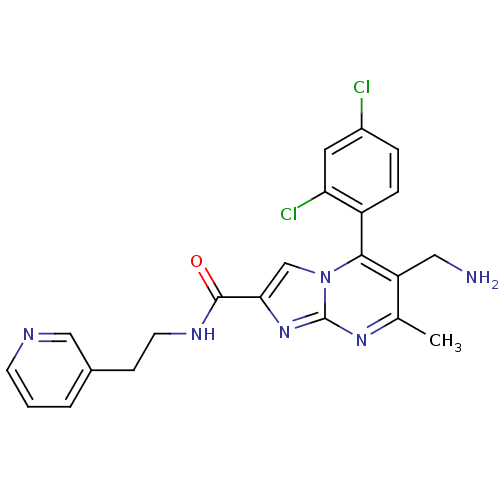
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1cccnc1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.94,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.32,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(10-25)20(16-5-4-15(23)9-18(16)24)30-12-19(29-22(30)28-13)21(31)27-8-6-14-3-2-7-26-11-14/h2-5,7,9,11-12H,6,8,10,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50396883
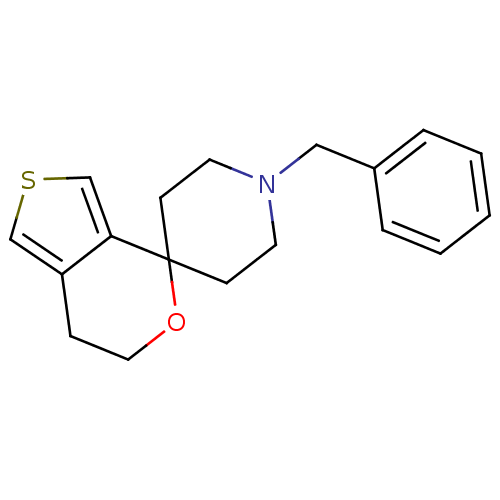
(CHEMBL2170667)Show InChI InChI=1S/C18H21NOS/c1-2-4-15(5-3-1)12-19-9-7-18(8-10-19)17-14-21-13-16(17)6-11-20-18/h1-5,13-14H,6-12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut f£r Pharmazeutische und Medizinische Chemie der Westf£lischen Wilhelms-Universit£t M£nster
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membranes after 120 mins by scintillation counting |
J Med Chem 55: 8047-65 (2012)
Article DOI: 10.1021/jm300894h
BindingDB Entry DOI: 10.7270/Q2G44RDB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026307
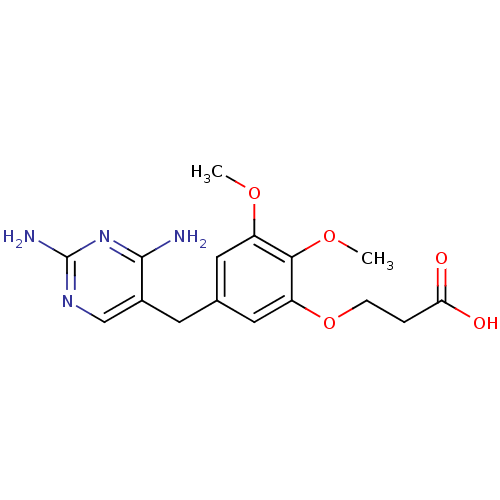
(3-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C16H20N4O5/c1-23-11-6-9(5-10-8-19-16(18)20-15(10)17)7-12(14(11)24-2)25-4-3-13(21)22/h6-8H,3-5H2,1-2H3,(H,21,22)(H4,17,18,19,20) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026307

(3-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C16H20N4O5/c1-23-11-6-9(5-10-8-19-16(18)20-15(10)17)7-12(14(11)24-2)25-4-3-13(21)22/h6-8H,3-5H2,1-2H3,(H,21,22)(H4,17,18,19,20) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324500
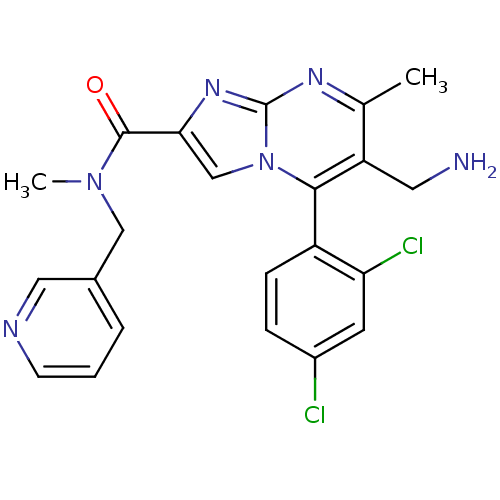
(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES CN(Cc1cccnc1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(4.9,-13.73,;4.13,-12.4,;2.59,-12.4,;1.82,-13.73,;.28,-13.72,;-.49,-15.05,;.28,-16.39,;1.83,-16.4,;2.59,-15.06,;4.91,-11.07,;4.14,-9.74,;6.44,-11.06,;7.34,-12.29,;8.8,-11.82,;10.13,-12.59,;11.47,-11.82,;12.8,-12.6,;14.14,-11.85,;11.46,-10.28,;12.8,-9.51,;10.13,-9.51,;8.8,-10.28,;7.34,-9.8,;10.13,-14.13,;8.79,-14.9,;8.8,-16.44,;10.13,-17.21,;10.14,-18.75,;11.45,-16.44,;11.45,-14.9,;12.8,-14.11,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(9-25)20(16-6-5-15(23)8-18(16)24)30-12-19(28-22(30)27-13)21(31)29(2)11-14-4-3-7-26-10-14/h3-8,10,12H,9,11,25H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324524
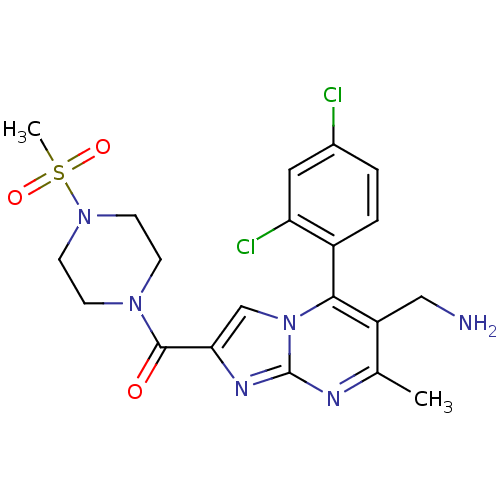
((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CCN(CC1)S(C)(=O)=O |(11.6,.54,;10.29,-.27,;8.93,.44,;7.63,-.37,;6.16,.07,;5.29,-1.2,;6.24,-2.42,;7.68,-1.9,;9.03,-2.63,;10.34,-1.82,;11.7,-2.54,;13.01,-1.73,;9,-4.16,;7.65,-4.9,;7.62,-6.44,;8.94,-7.24,;8.91,-8.78,;10.29,-6.48,;10.32,-4.95,;11.66,-4.21,;3.75,-1.24,;2.94,.06,;3.02,-2.6,;3.82,-3.91,;3.09,-5.26,;1.55,-5.3,;.75,-3.99,;1.48,-2.64,;.82,-6.65,;-.72,-6.7,;.4,-8.14,;2.15,-7.42,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-12-15(10-23)18(14-4-3-13(21)9-16(14)22)28-11-17(25-20(28)24-12)19(29)26-5-7-27(8-6-26)32(2,30)31/h3-4,9,11H,5-8,10,23H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026317
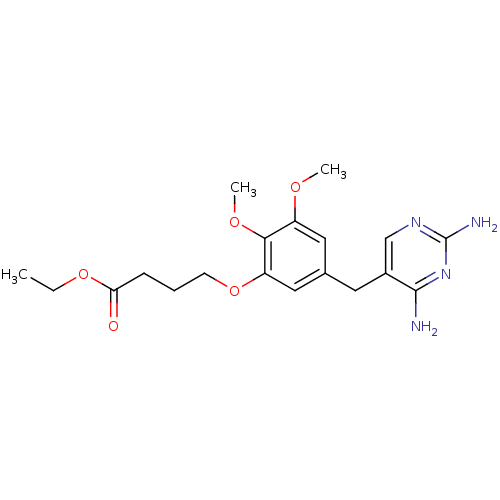
(4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-4-27-16(24)6-5-7-28-15-10-12(9-14(25-2)17(15)26-3)8-13-11-22-19(21)23-18(13)20/h9-11H,4-8H2,1-3H3,(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026317

(4-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-4-27-16(24)6-5-7-28-15-10-12(9-14(25-2)17(15)26-3)8-13-11-22-19(21)23-18(13)20/h9-11H,4-8H2,1-3H3,(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50226129
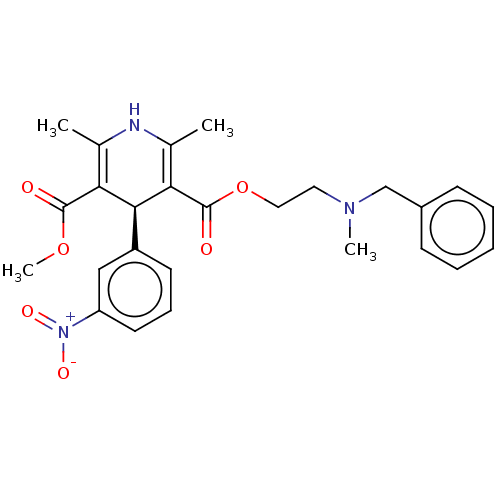
(CHEMBL1314450)Show SMILES COC(=O)C1=C(C)NC(C)=C([C@H]1c1cccc(c1)[N+]([O-])=O)C(=O)OCCN(C)Cc1ccccc1 |c:4,9| Show InChI InChI=1S/C26H29N3O6/c1-17-22(25(30)34-4)24(20-11-8-12-21(15-20)29(32)33)23(18(2)27-17)26(31)35-14-13-28(3)16-19-9-6-5-7-10-19/h5-12,15,24,27H,13-14,16H2,1-4H3/t24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324524

((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CCN(CC1)S(C)(=O)=O |(11.6,.54,;10.29,-.27,;8.93,.44,;7.63,-.37,;6.16,.07,;5.29,-1.2,;6.24,-2.42,;7.68,-1.9,;9.03,-2.63,;10.34,-1.82,;11.7,-2.54,;13.01,-1.73,;9,-4.16,;7.65,-4.9,;7.62,-6.44,;8.94,-7.24,;8.91,-8.78,;10.29,-6.48,;10.32,-4.95,;11.66,-4.21,;3.75,-1.24,;2.94,.06,;3.02,-2.6,;3.82,-3.91,;3.09,-5.26,;1.55,-5.3,;.75,-3.99,;1.48,-2.64,;.82,-6.65,;-.72,-6.7,;.4,-8.14,;2.15,-7.42,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-12-15(10-23)18(14-4-3-13(21)9-16(14)22)28-11-17(25-20(28)24-12)19(29)26-5-7-27(8-6-26)32(2,30)31/h3-4,9,11H,5-8,10,23H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324504
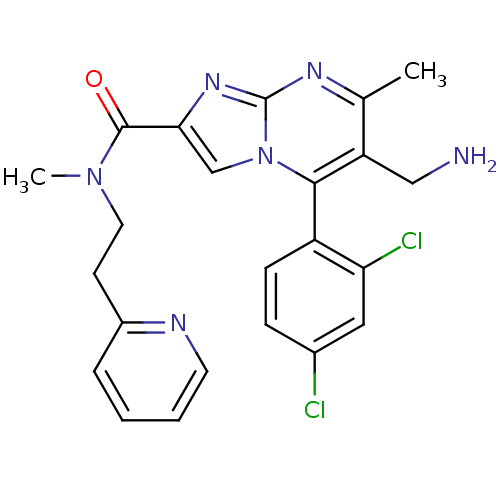
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...)Show SMILES CN(CCc1ccccn1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(7.39,-9.52,;6.58,-8.21,;5.04,-8.26,;4.23,-6.94,;2.69,-6.99,;1.89,-5.67,;.35,-5.72,;-.38,-7.08,;.44,-8.4,;1.97,-8.34,;7.3,-6.85,;6.49,-5.54,;8.85,-6.8,;9.79,-8.02,;11.23,-7.5,;12.59,-8.23,;13.89,-7.42,;15.25,-8.15,;16.56,-7.34,;13.85,-5.88,;15.15,-5.06,;12.48,-5.16,;11.19,-5.97,;9.71,-5.54,;12.55,-9.76,;11.2,-10.5,;11.17,-12.04,;12.49,-12.84,;12.58,-14.44,;13.85,-12.09,;13.87,-10.56,;15.21,-9.81,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-14-18(12-26)21(17-7-6-15(24)11-19(17)25)31-13-20(29-23(31)28-14)22(32)30(2)10-8-16-5-3-4-9-27-16/h3-7,9,11,13H,8,10,12,26H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
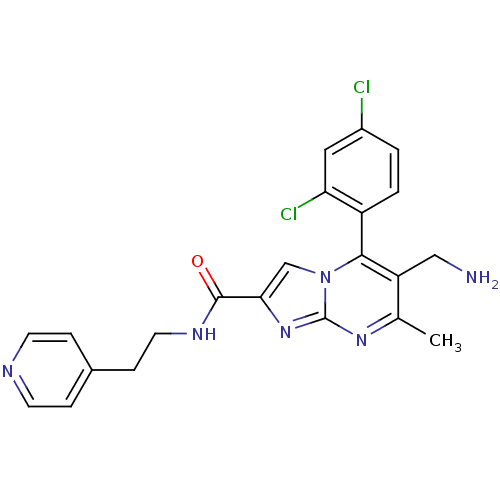
(Homo sapiens (Human)) | BDBM50324513
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1ccncc1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.42,-4.28,;-3.96,-4.32,;-4.69,-5.68,;-3.87,-6.99,;-2.34,-6.94,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(11-25)20(16-3-2-15(23)10-18(16)24)30-12-19(29-22(30)28-13)21(31)27-9-6-14-4-7-26-8-5-14/h2-5,7-8,10,12H,6,9,11,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324511

(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1cccnc1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.94,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.32,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(10-25)20(16-5-4-15(23)9-18(16)24)30-12-19(29-22(30)28-13)21(31)27-8-6-14-3-2-7-26-11-14/h2-5,7,9,11-12H,6,8,10,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324497
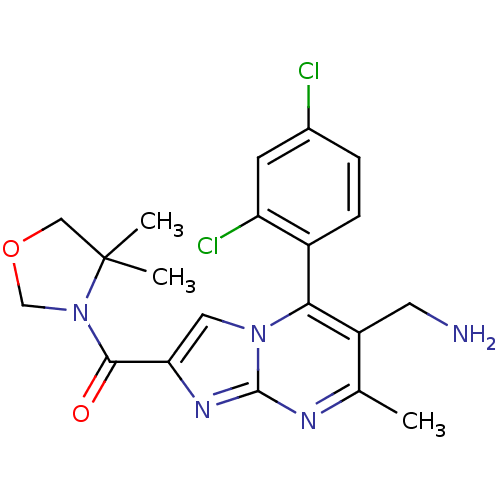
((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1COCC1(C)C |(10.72,-.55,;9.42,-1.37,;8.06,-.65,;6.76,-1.46,;5.28,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.46,-2.91,;10.82,-3.64,;12.13,-2.83,;8.13,-5.25,;6.78,-5.99,;6.74,-7.53,;8.07,-8.33,;8.03,-9.87,;9.42,-7.58,;9.44,-6.05,;10.79,-5.3,;2.88,-2.34,;2.07,-1.03,;2.15,-3.7,;2.66,-5.14,;1.44,-6.08,;.17,-5.2,;.61,-3.72,;-.99,-3.83,;-.16,-2.39,)| Show InChI InChI=1S/C20H21Cl2N5O2/c1-11-14(7-23)17(13-5-4-12(21)6-15(13)22)26-8-16(25-19(26)24-11)18(28)27-10-29-9-20(27,2)3/h4-6,8H,7,9-10,23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324500

(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES CN(Cc1cccnc1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(4.9,-13.73,;4.13,-12.4,;2.59,-12.4,;1.82,-13.73,;.28,-13.72,;-.49,-15.05,;.28,-16.39,;1.83,-16.4,;2.59,-15.06,;4.91,-11.07,;4.14,-9.74,;6.44,-11.06,;7.34,-12.29,;8.8,-11.82,;10.13,-12.59,;11.47,-11.82,;12.8,-12.6,;14.14,-11.85,;11.46,-10.28,;12.8,-9.51,;10.13,-9.51,;8.8,-10.28,;7.34,-9.8,;10.13,-14.13,;8.79,-14.9,;8.8,-16.44,;10.13,-17.21,;10.14,-18.75,;11.45,-16.44,;11.45,-14.9,;12.8,-14.11,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(9-25)20(16-6-5-15(23)8-18(16)24)30-12-19(28-22(30)27-13)21(31)29(2)11-14-4-3-7-26-10-14/h3-8,10,12H,9,11,25H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324508
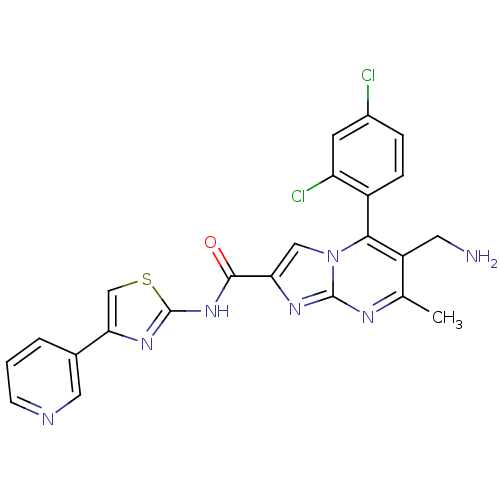
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)Nc1nc(cs1)-c1cccnc1 |(10.76,-1,;9.46,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.49,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.23,-5.46,;-1.7,-5.02,;-1.75,-3.48,;-.3,-2.97,;-2.84,-6.06,;-2.83,-7.6,;-4.15,-8.37,;-5.5,-7.61,;-5.51,-6.06,;-4.17,-5.29,)| Show InChI InChI=1S/C23H17Cl2N7OS/c1-12-16(8-26)20(15-5-4-14(24)7-17(15)25)32-10-18(29-22(32)28-12)21(33)31-23-30-19(11-34-23)13-3-2-6-27-9-13/h2-7,9-11H,8,26H2,1H3,(H,30,31,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324514
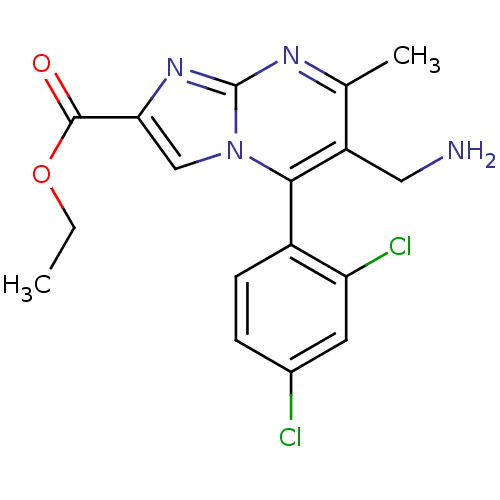
((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324514

((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324504

(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...)Show SMILES CN(CCc1ccccn1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(7.39,-9.52,;6.58,-8.21,;5.04,-8.26,;4.23,-6.94,;2.69,-6.99,;1.89,-5.67,;.35,-5.72,;-.38,-7.08,;.44,-8.4,;1.97,-8.34,;7.3,-6.85,;6.49,-5.54,;8.85,-6.8,;9.79,-8.02,;11.23,-7.5,;12.59,-8.23,;13.89,-7.42,;15.25,-8.15,;16.56,-7.34,;13.85,-5.88,;15.15,-5.06,;12.48,-5.16,;11.19,-5.97,;9.71,-5.54,;12.55,-9.76,;11.2,-10.5,;11.17,-12.04,;12.49,-12.84,;12.58,-14.44,;13.85,-12.09,;13.87,-10.56,;15.21,-9.81,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-14-18(12-26)21(17-7-6-15(24)11-19(17)25)31-13-20(29-23(31)28-14)22(32)30(2)10-8-16-5-3-4-9-27-16/h3-7,9,11,13H,8,10,12,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324494
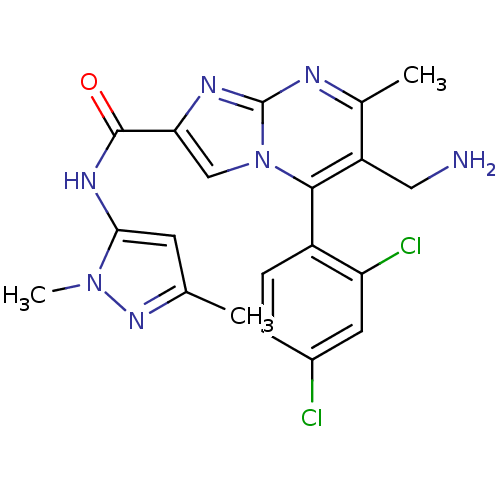
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N-(1,3-dime...)Show SMILES Cc1cc(NC(=O)c2cn3c(c(CN)c(C)nc3n2)-c2ccc(Cl)cc2Cl)n(C)n1 |(-2.95,-5.54,;-1.73,-4.59,;-.25,-5.02,;.61,-3.75,;2.15,-3.7,;2.88,-2.34,;2.07,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.47,-2.92,;10.83,-3.64,;12.14,-2.83,;9.42,-1.37,;10.73,-.55,;8.06,-.65,;6.76,-1.46,;5.29,-1.03,;8.13,-5.26,;6.78,-5.99,;6.75,-7.53,;8.07,-8.33,;8.04,-9.87,;9.42,-7.58,;9.45,-6.05,;10.79,-5.3,;-.33,-2.53,;.1,-1.06,;-1.78,-3.05,)| Show InChI InChI=1S/C20H19Cl2N7O/c1-10-6-17(28(3)27-10)26-19(30)16-9-29-18(13-5-4-12(21)7-15(13)22)14(8-23)11(2)24-20(29)25-16/h4-7,9H,8,23H2,1-3H3,(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026316
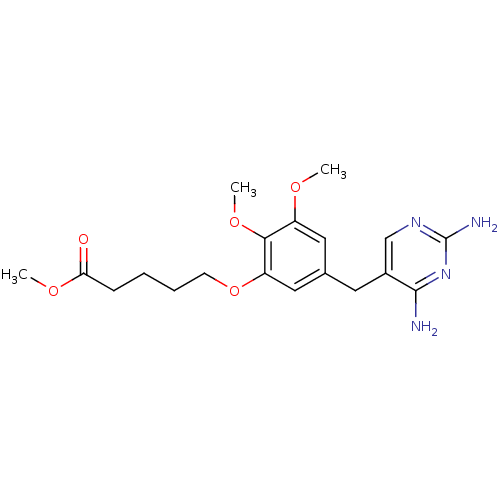
(5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-25-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-3)28-7-5-4-6-16(24)26-2/h9-11H,4-8H2,1-3H3,(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026316

(5-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C19H26N4O5/c1-25-14-9-12(8-13-11-22-19(21)23-18(13)20)10-15(17(14)27-3)28-7-5-4-6-16(24)26-2/h9-11H,4-8H2,1-3H3,(H4,20,21,22,23) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324524

((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CCN(CC1)S(C)(=O)=O |(11.6,.54,;10.29,-.27,;8.93,.44,;7.63,-.37,;6.16,.07,;5.29,-1.2,;6.24,-2.42,;7.68,-1.9,;9.03,-2.63,;10.34,-1.82,;11.7,-2.54,;13.01,-1.73,;9,-4.16,;7.65,-4.9,;7.62,-6.44,;8.94,-7.24,;8.91,-8.78,;10.29,-6.48,;10.32,-4.95,;11.66,-4.21,;3.75,-1.24,;2.94,.06,;3.02,-2.6,;3.82,-3.91,;3.09,-5.26,;1.55,-5.3,;.75,-3.99,;1.48,-2.64,;.82,-6.65,;-.72,-6.7,;.4,-8.14,;2.15,-7.42,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-12-15(10-23)18(14-4-3-13(21)9-16(14)22)28-11-17(25-20(28)24-12)19(29)26-5-7-27(8-6-26)32(2,30)31/h3-4,9,11H,5-8,10,23H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50101815

(CHEBI:7550 | Nicardipine)Show SMILES COC(=O)C1=C(C)NC(C)=C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OCCN(C)Cc1ccccc1 |c:4,9| Show InChI InChI=1S/C26H29N3O6/c1-17-22(25(30)34-4)24(20-11-8-12-21(15-20)29(32)33)23(18(2)27-17)26(31)35-14-13-28(3)16-19-9-6-5-7-10-19/h5-12,15,24,27H,13-14,16H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026306
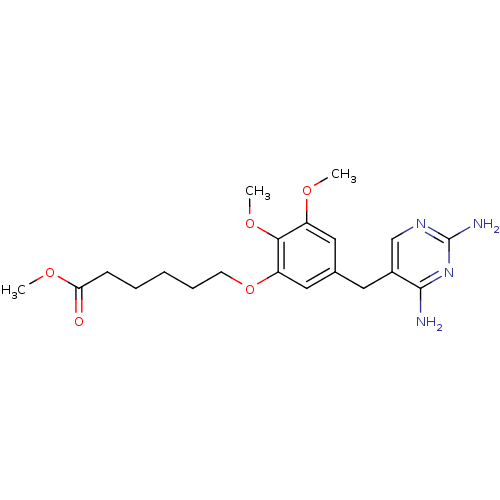
(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C20H28N4O5/c1-26-15-10-13(9-14-12-23-20(22)24-19(14)21)11-16(18(15)28-3)29-8-6-4-5-7-17(25)27-2/h10-12H,4-9H2,1-3H3,(H4,21,22,23,24) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for E. coli Dihydrofolate reductase |
J Med Chem 25: 1120-2 (1983)
BindingDB Entry DOI: 10.7270/Q28G8JRF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Escherichia coli) | BDBM50026306

(6-[5-(2,4-Diamino-pyrimidin-5-ylmethyl)-2,3-dimeth...)Show InChI InChI=1S/C20H28N4O5/c1-26-15-10-13(9-14-12-23-20(22)24-19(14)21)11-16(18(15)28-3)29-8-6-4-5-7-17(25)27-2/h10-12H,4-9H2,1-3H3,(H4,21,22,23,24) | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Dihydrofolate reductase Inhibitor of Escherichia coli |
J Med Chem 28: 303-11 (1985)
BindingDB Entry DOI: 10.7270/Q2J966Z0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324505
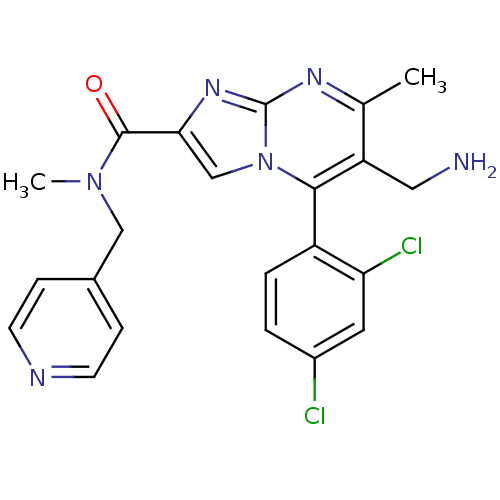
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...)Show SMILES CN(Cc1ccncc1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(4.39,-7.36,;3.62,-6.02,;2.08,-6.02,;1.31,-7.35,;2.08,-8.69,;1.31,-10.03,;-.23,-10.03,;-1,-8.68,;-.23,-7.35,;4.4,-4.69,;3.63,-3.36,;5.94,-4.68,;6.84,-5.92,;8.3,-5.44,;9.63,-6.21,;10.97,-5.44,;12.31,-6.23,;12.3,-7.77,;10.97,-3.9,;12.31,-3.13,;9.64,-3.13,;8.31,-3.9,;6.84,-3.42,;9.63,-7.75,;8.3,-8.53,;8.3,-10.07,;9.63,-10.84,;9.65,-12.38,;10.96,-10.07,;10.96,-8.52,;12.3,-7.74,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(10-25)20(16-4-3-15(23)9-18(16)24)30-12-19(28-22(30)27-13)21(31)29(2)11-14-5-7-26-8-6-14/h3-9,12H,10-11,25H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324494

(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N-(1,3-dime...)Show SMILES Cc1cc(NC(=O)c2cn3c(c(CN)c(C)nc3n2)-c2ccc(Cl)cc2Cl)n(C)n1 |(-2.95,-5.54,;-1.73,-4.59,;-.25,-5.02,;.61,-3.75,;2.15,-3.7,;2.88,-2.34,;2.07,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.47,-2.92,;10.83,-3.64,;12.14,-2.83,;9.42,-1.37,;10.73,-.55,;8.06,-.65,;6.76,-1.46,;5.29,-1.03,;8.13,-5.26,;6.78,-5.99,;6.75,-7.53,;8.07,-8.33,;8.04,-9.87,;9.42,-7.58,;9.45,-6.05,;10.79,-5.3,;-.33,-2.53,;.1,-1.06,;-1.78,-3.05,)| Show InChI InChI=1S/C20H19Cl2N7O/c1-10-6-17(28(3)27-10)26-19(30)16-9-29-18(13-5-4-12(21)7-15(13)22)14(8-23)11(2)24-20(29)25-16/h4-7,9H,8,23H2,1-3H3,(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324501
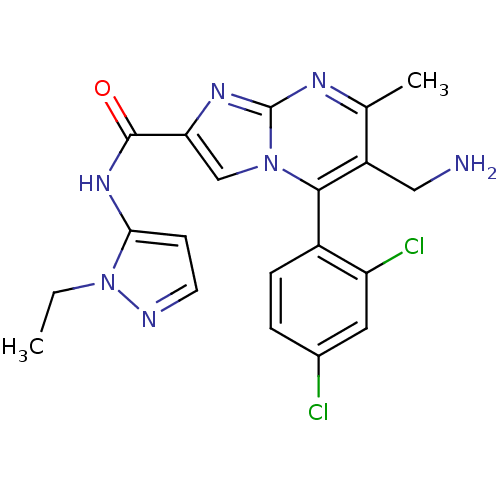
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N-(1-ethyl-...)Show SMILES CCn1nccc1NC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-3.58,-1.22,;-2.53,-2.33,;-2.95,-3.81,;-4.4,-4.33,;-4.36,-5.87,;-2.88,-6.31,;-2.01,-5.04,;-.47,-4.99,;.26,-3.63,;-.55,-2.32,;1.8,-3.58,;2.74,-4.81,;4.19,-4.28,;5.54,-5.01,;6.85,-4.21,;8.21,-4.93,;9.52,-4.12,;6.8,-2.66,;8.11,-1.84,;5.44,-1.94,;4.14,-2.75,;2.67,-2.32,;5.51,-6.55,;4.16,-7.29,;4.13,-8.83,;5.45,-9.63,;5.42,-11.17,;6.8,-8.87,;6.83,-7.34,;8.17,-6.59,)| Show InChI InChI=1S/C20H19Cl2N7O/c1-3-29-17(6-7-24-29)27-19(30)16-10-28-18(13-5-4-12(21)8-15(13)22)14(9-23)11(2)25-20(28)26-16/h4-8,10H,3,9,23H2,1-2H3,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324507
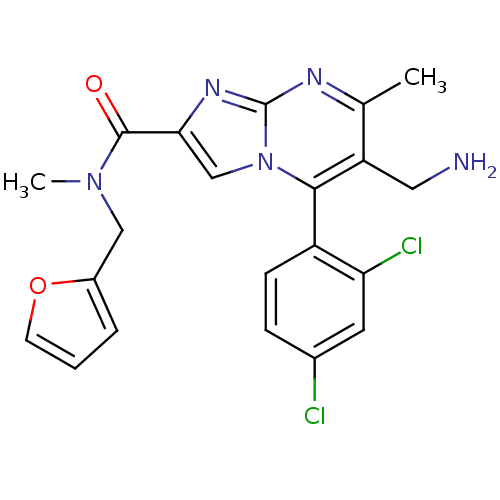
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N-(furan-2-...)Show SMILES CN(Cc1ccco1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(7.65,-13.28,;6.88,-11.95,;5.34,-11.95,;4.57,-13.28,;5.34,-14.61,;4.29,-15.77,;2.91,-15.13,;3.06,-13.6,;7.66,-10.61,;6.89,-9.28,;9.19,-10.6,;10.1,-11.84,;11.56,-11.37,;12.89,-12.13,;14.23,-11.36,;15.57,-12.15,;16.91,-11.39,;14.23,-9.82,;15.57,-9.05,;12.89,-9.06,;11.56,-9.83,;10.1,-9.36,;12.89,-13.68,;11.55,-14.45,;11.55,-16,;12.89,-16.76,;12.9,-18.3,;14.22,-16,;14.22,-14.45,;15.56,-13.67,)| Show InChI InChI=1S/C21H19Cl2N5O2/c1-12-16(9-24)19(15-6-5-13(22)8-17(15)23)28-11-18(26-21(28)25-12)20(29)27(2)10-14-4-3-7-30-14/h3-8,11H,9-10,24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324494

(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N-(1,3-dime...)Show SMILES Cc1cc(NC(=O)c2cn3c(c(CN)c(C)nc3n2)-c2ccc(Cl)cc2Cl)n(C)n1 |(-2.95,-5.54,;-1.73,-4.59,;-.25,-5.02,;.61,-3.75,;2.15,-3.7,;2.88,-2.34,;2.07,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.47,-2.92,;10.83,-3.64,;12.14,-2.83,;9.42,-1.37,;10.73,-.55,;8.06,-.65,;6.76,-1.46,;5.29,-1.03,;8.13,-5.26,;6.78,-5.99,;6.75,-7.53,;8.07,-8.33,;8.04,-9.87,;9.42,-7.58,;9.45,-6.05,;10.79,-5.3,;-.33,-2.53,;.1,-1.06,;-1.78,-3.05,)| Show InChI InChI=1S/C20H19Cl2N7O/c1-10-6-17(28(3)27-10)26-19(30)16-9-29-18(13-5-4-12(21)7-15(13)22)14(8-23)11(2)24-20(29)25-16/h4-7,9H,8,23H2,1-3H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324497

((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1COCC1(C)C |(10.72,-.55,;9.42,-1.37,;8.06,-.65,;6.76,-1.46,;5.28,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.46,-2.91,;10.82,-3.64,;12.13,-2.83,;8.13,-5.25,;6.78,-5.99,;6.74,-7.53,;8.07,-8.33,;8.03,-9.87,;9.42,-7.58,;9.44,-6.05,;10.79,-5.3,;2.88,-2.34,;2.07,-1.03,;2.15,-3.7,;2.66,-5.14,;1.44,-6.08,;.17,-5.2,;.61,-3.72,;-.99,-3.83,;-.16,-2.39,)| Show InChI InChI=1S/C20H21Cl2N5O2/c1-11-14(7-23)17(13-5-4-12(21)6-15(13)22)26-8-16(25-19(26)24-11)18(28)27-10-29-9-20(27,2)3/h4-6,8H,7,9-10,23H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data