

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | -34.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92912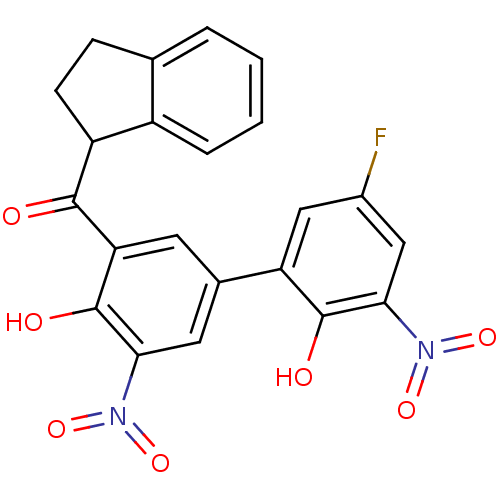 (Aryl 1-indanylketone, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 520 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92915 (Aryl 1-indanylketone, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92914 (Aryl 1-indanylketone, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92915 (Aryl 1-indanylketone, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.42E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase D (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.28E+3 | -27.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92914 (Aryl 1-indanylketone, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.60E+3 | -27.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92916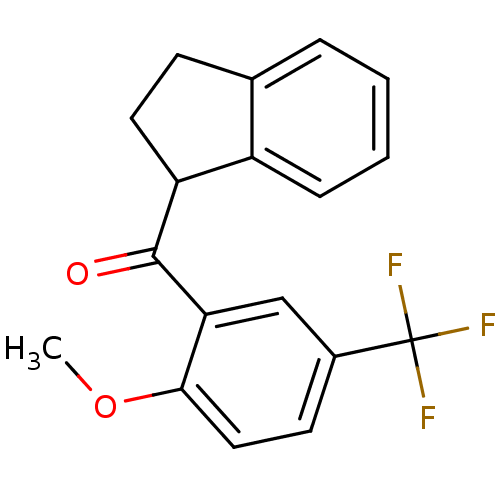 (Aryl 1-indanylketone, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92918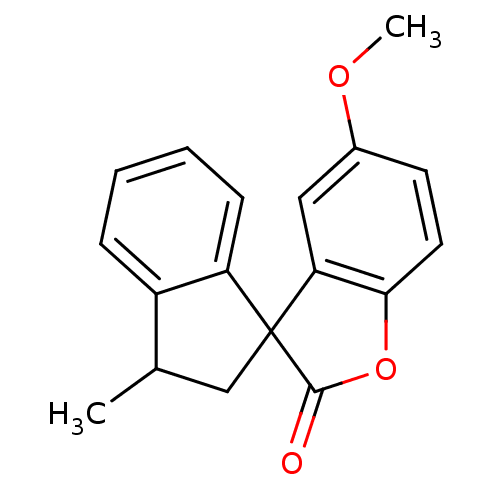 (Benzofuranone, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+4 | -24.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase-like 1 (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92920 (Benzofuranone, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30E+4 | -22.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92916 (Aryl 1-indanylketone, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92917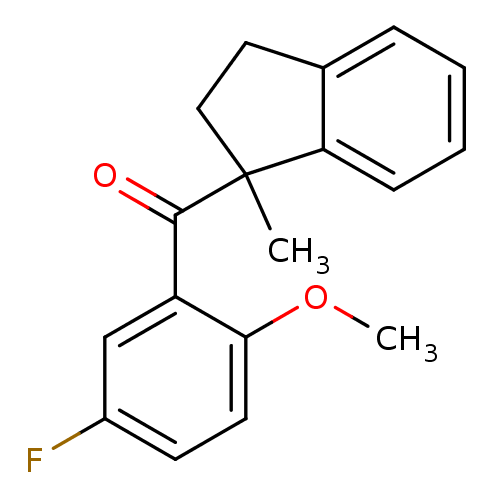 (Aryl 1-indanylketone, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92918 (Benzofuranone, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92919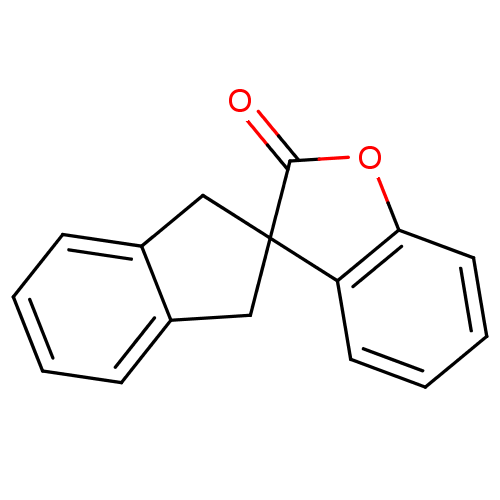 (Benzofuranone, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase C (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92919 (Benzofuranone, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase H (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase C (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase-like 1 (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92917 (Aryl 1-indanylketone, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase B (Homo sapiens (Human)) | BDBM92912 (Aryl 1-indanylketone, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM92920 (Benzofuranone, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase H (Homo sapiens (Human)) | BDBM92913 (Aryl 1-indanylketone, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research | Assay Description PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... | Biochemistry 48: 6268-77 (2009) Article DOI: 10.1021/bi9007287 BindingDB Entry DOI: 10.7270/Q2X34W26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426601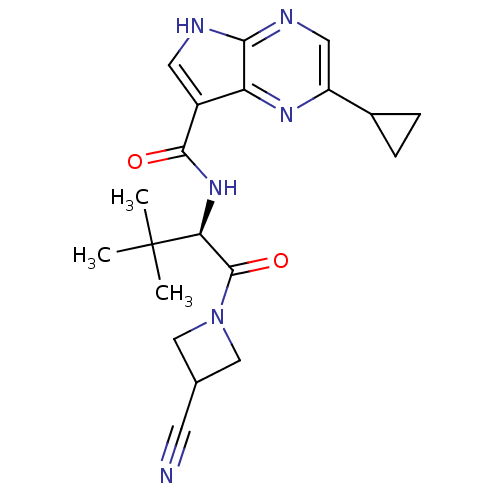 (CHEMBL2325895) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426599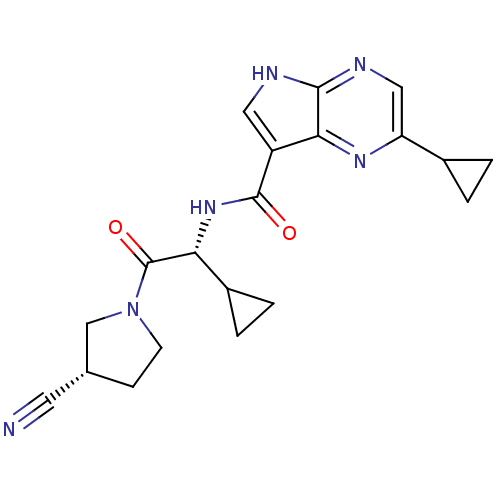 (CHEMBL2325898) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50426607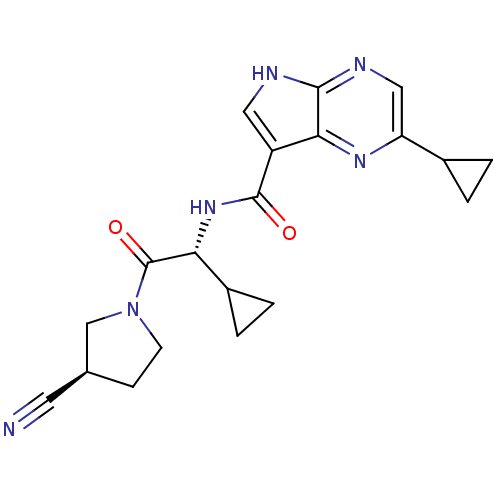 (CHEMBL2325897) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426599 (CHEMBL2325898) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50426599 (CHEMBL2325898) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426601 (CHEMBL2325895) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426606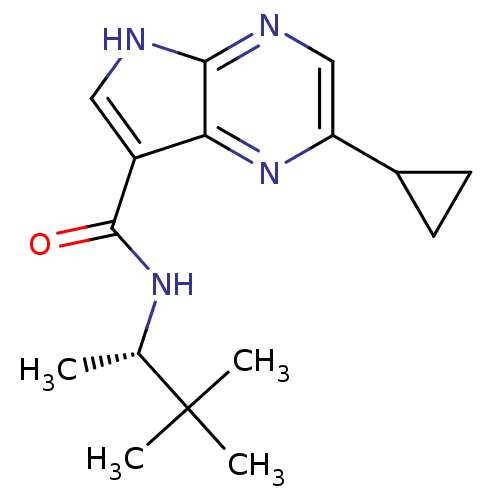 (CHEMBL2325903) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426604 (CHEMBL2325906) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50426601 (CHEMBL2325895) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK1 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426616 (CHEMBL2325899) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426602 (CHEMBL2325894) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426615 (CHEMBL2325900) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426603 (CHEMBL2325912) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426617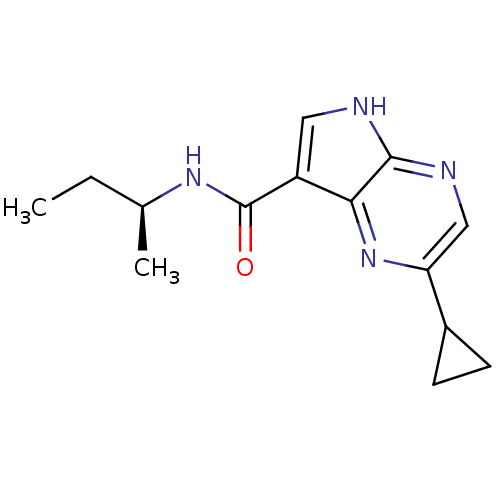 (CHEMBL2325902) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50498583 (CHEMBL3609639) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Boston Curated by ChEMBL | Assay Description Inhibition of gamma-secretase in human SH-SY5Y cells expressing beta-APP C-terminal fragment SPA4CT assessed as decrease of amyloid beta-42 level by ... | Bioorg Med Chem Lett 25: 3488-94 (2015) Article DOI: 10.1016/j.bmcl.2015.07.003 BindingDB Entry DOI: 10.7270/Q2MG7SHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426606 (CHEMBL2325903) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426604 (CHEMBL2325906) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426605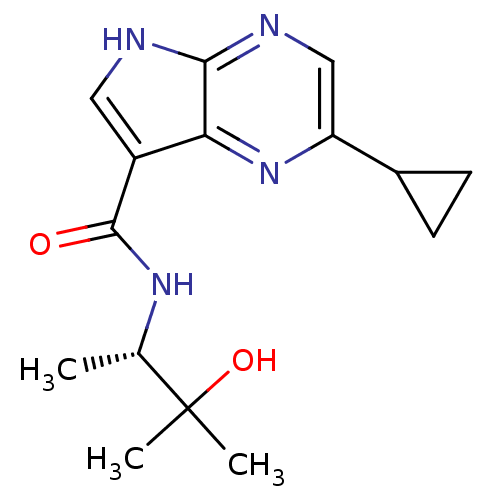 (CHEMBL2325904) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50426614 (CHEMBL2325905) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426615 (CHEMBL2325900) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50426603 (CHEMBL2325912) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Curated by ChEMBL | Assay Description Inhibition of JAK2 (unknown origin)-mediated phosphorylation of Biotin-KAIETDKEYYTVKD incubated for 10 mins prior to substrate addition measured afte... | J Med Chem 56: 345-56 (2013) Article DOI: 10.1021/jm301646k BindingDB Entry DOI: 10.7270/Q2Q241JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 221 total ) | Next | Last >> |