

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313984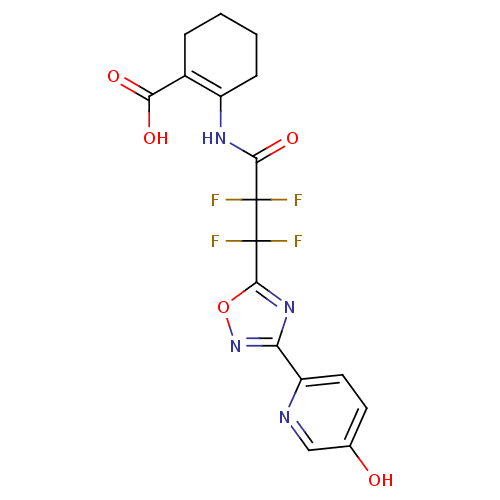 (2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313977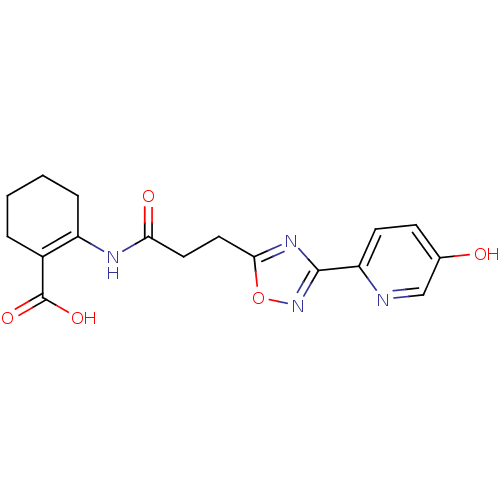 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313976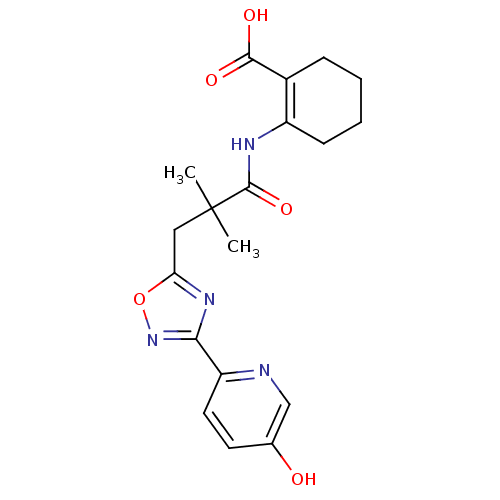 (2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23533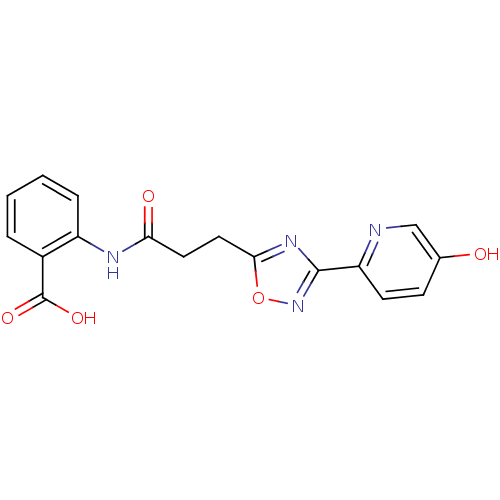 (2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313979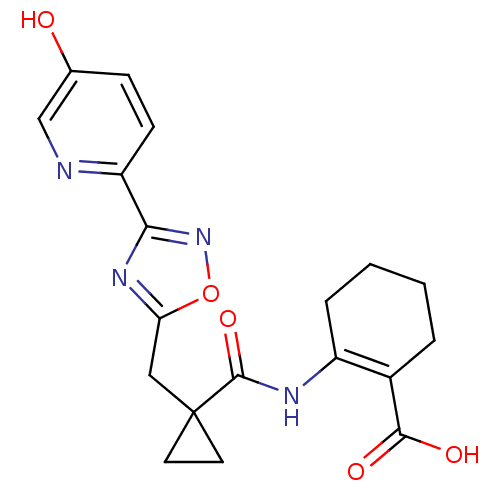 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313981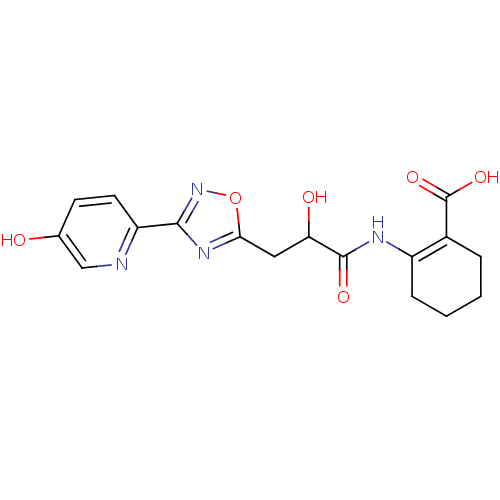 (CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313982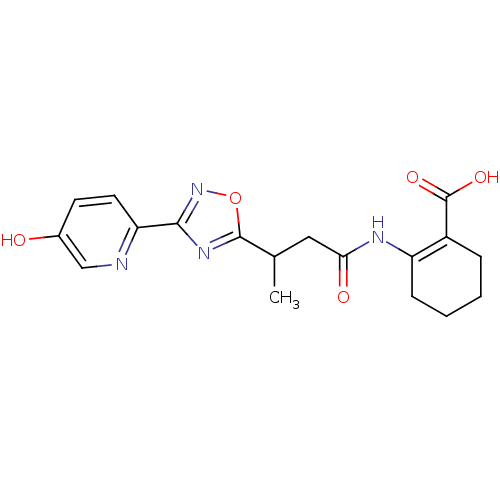 (CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells | J Med Chem 51: 5101-8 (2008) Article DOI: 10.1021/jm800258p BindingDB Entry DOI: 10.7270/Q2CF9PWV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23515 (CHEMBL573 | Niacin | Nicotinic Acid | [5, 6-3H]-ni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM23533 (2-{3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313983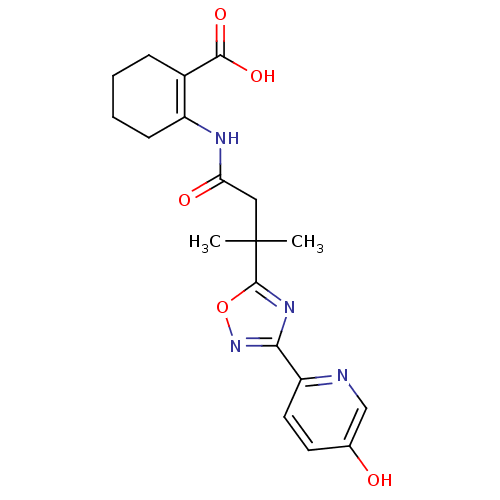 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313977 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313978 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313980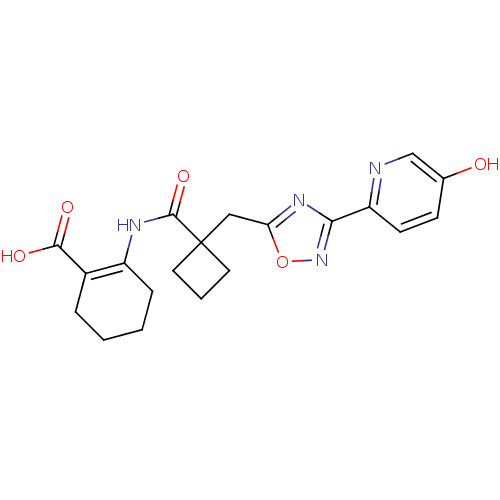 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50273099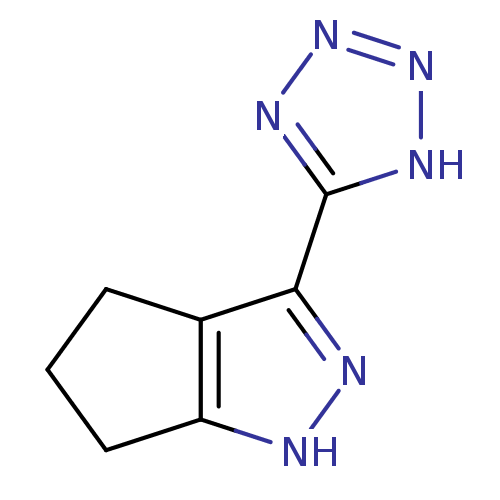 (3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydrocyclopenta[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]nicotinic acid from human GPR109a receptor expressed in CHO cells | J Med Chem 51: 5101-8 (2008) Article DOI: 10.1021/jm800258p BindingDB Entry DOI: 10.7270/Q2CF9PWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313976 (2-({3-[3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 595 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313981 (CHEMBL1088213 | rac-2-(2-hydroxy-3-(3-(5-hydroxypy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313984 (2-(2,2,3,3-tetrafluoro-3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313980 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313982 (CHEMBL1084393 | rac-2-(3-(3-(5-hydroxypyridin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313979 (2-(1-((3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hydroxycarboxylic acid receptor 2 (Homo sapiens (Human)) | BDBM50313983 (2-(3-(3-(5-hydroxypyridin-2-yl)-1,2,4-oxadiazol-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]NA from cloned human GPR109A receptor expressed in CHO-K1 cells by spectrophotometry in presence of 4% human serum albumin | J Med Chem 53: 2666-70 (2010) Article DOI: 10.1021/jm100022r BindingDB Entry DOI: 10.7270/Q2NS0V2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9113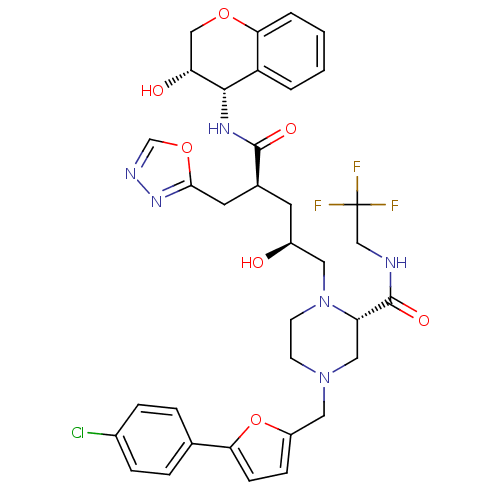 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9112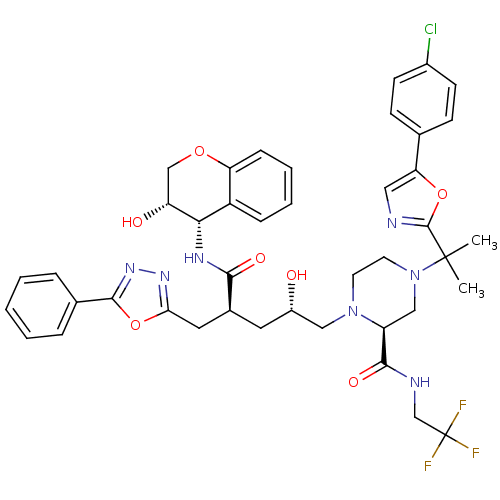 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9111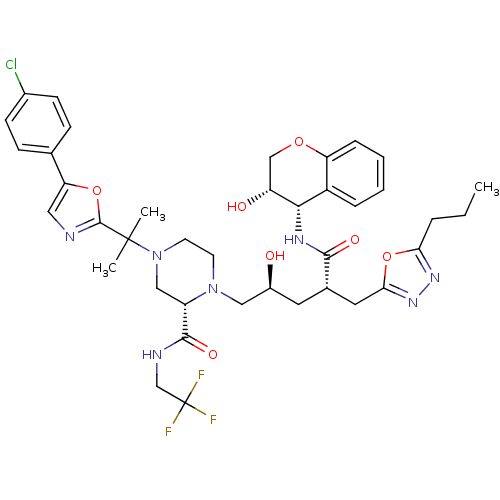 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9109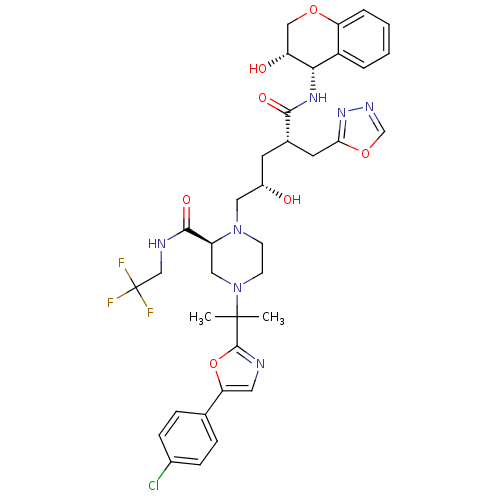 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9115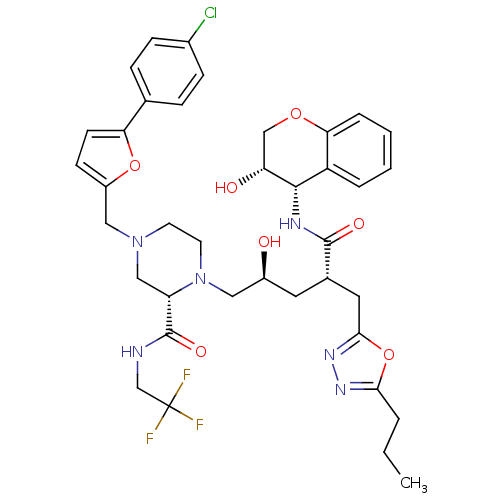 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9114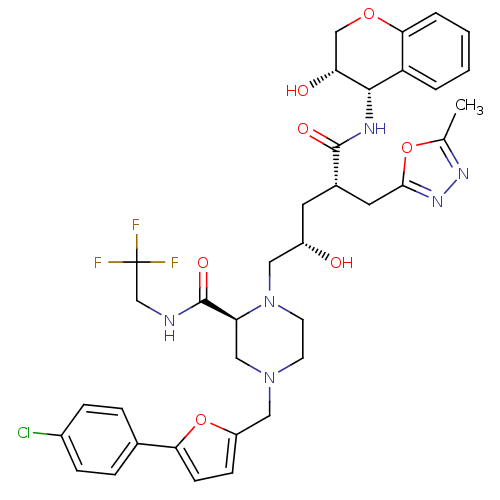 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9107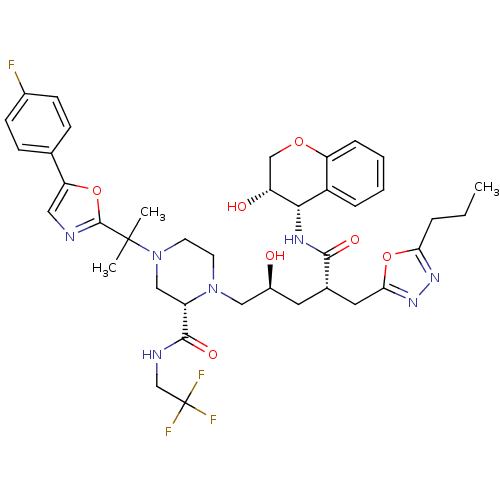 ((2S)-4-{2-[5-(4-fluorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9110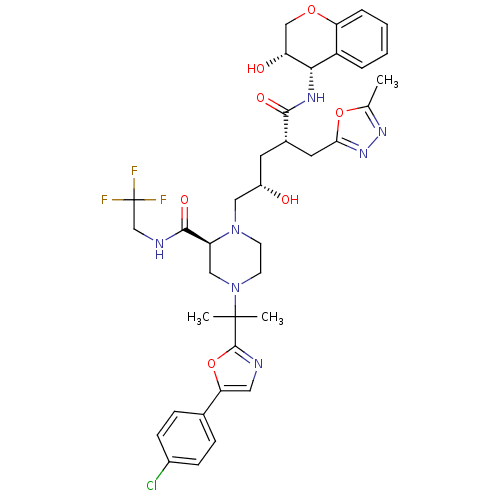 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9116 ((2S)-4-{[5-(4-chlorophenyl)furan-2-yl]methyl}-1-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9108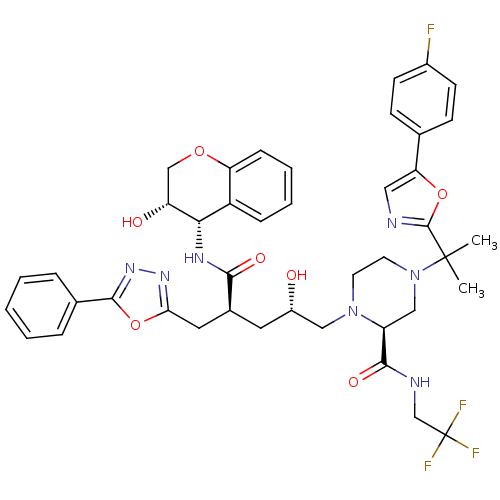 ((2S)-4-{2-[5-(4-fluorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9121 ((2S)-1-[(2S,4R)-2-hydroxy-4-{[(3S,4S)-3-hydroxy-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 4027-30 (2003) Article DOI: 10.1016/j.bmcl.2003.08.049 BindingDB Entry DOI: 10.7270/Q2Z60M7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9091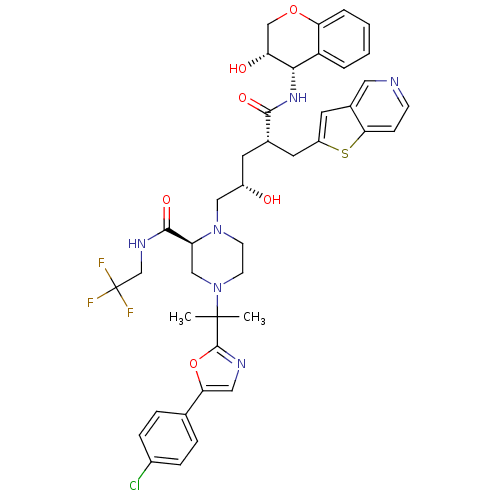 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9099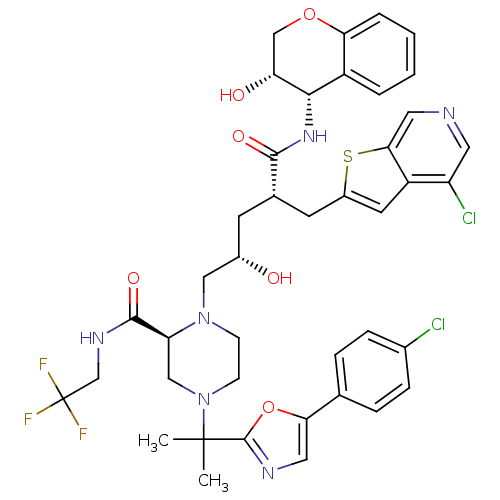 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9151 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 2573-6 (2003) Article DOI: 10.1016/s0960-894x(03)00474-8 BindingDB Entry DOI: 10.7270/Q2TD9VJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9159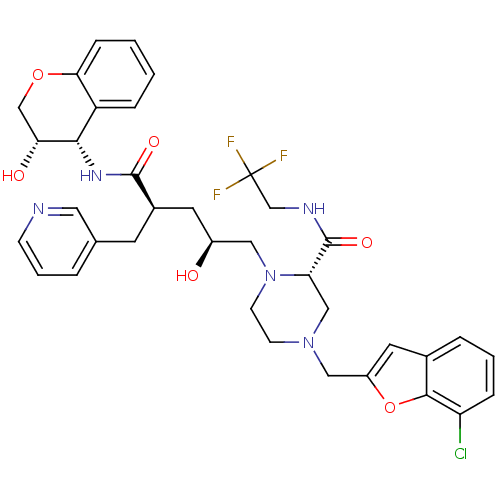 ((2S)-4-[(7-chloro-1-benzofuran-2-yl)methyl]-1-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 3323-6 (2003) Article DOI: 10.1016/s0960-894x(03)00680-2 BindingDB Entry DOI: 10.7270/Q2K072GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9138 ((2S)-4-{[1-(3,4-dichlorophenyl)-1H-pyrrol-3-yl]met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 4027-30 (2003) Article DOI: 10.1016/j.bmcl.2003.08.049 BindingDB Entry DOI: 10.7270/Q2Z60M7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9139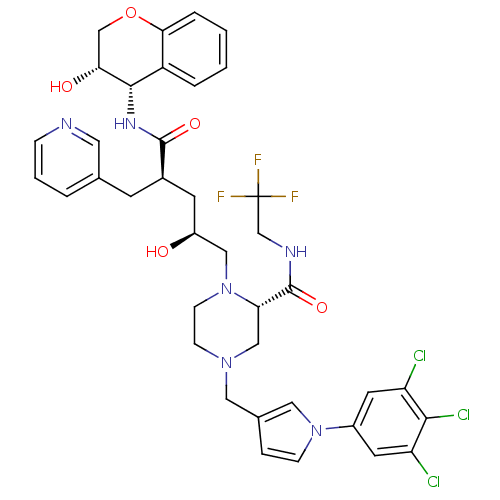 ((2S)-1-[(2S,4R)-2-hydroxy-4-{[(3S,4S)-3-hydroxy-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 4027-30 (2003) Article DOI: 10.1016/j.bmcl.2003.08.049 BindingDB Entry DOI: 10.7270/Q2Z60M7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9152 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 2573-6 (2003) Article DOI: 10.1016/s0960-894x(03)00474-8 BindingDB Entry DOI: 10.7270/Q2TD9VJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9098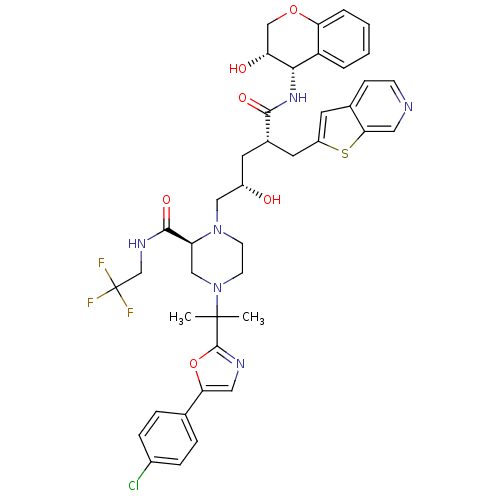 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9097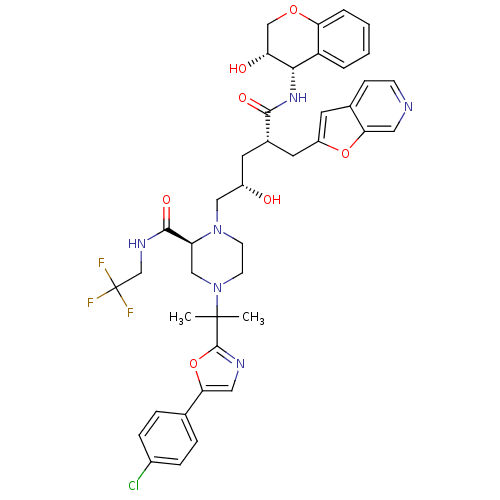 ((2S)-4-{2-[5-(4-chlorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 15: 5311-4 (2005) Article DOI: 10.1016/j.bmcl.2005.08.072 BindingDB Entry DOI: 10.7270/Q26Q1VF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9105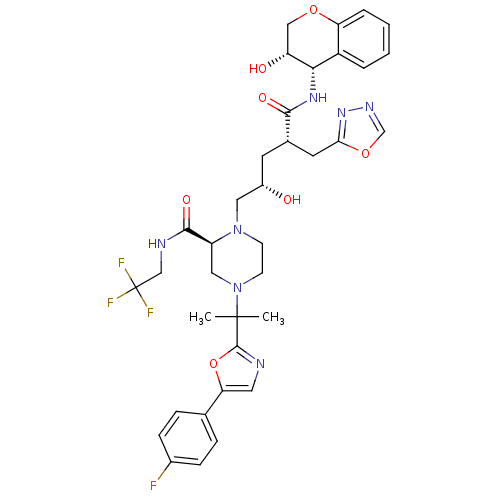 ((2S)-4-{2-[5-(4-fluorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9106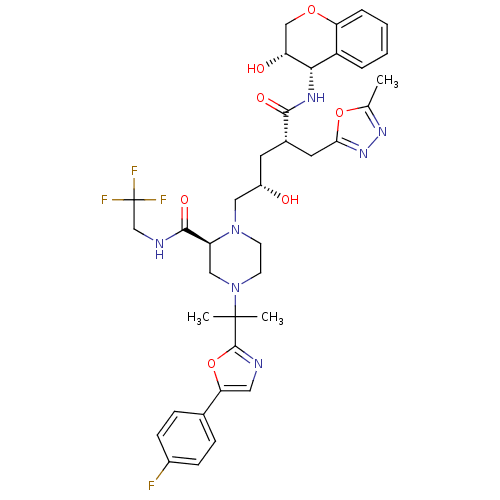 ((2S)-4-{2-[5-(4-fluorophenyl)-1,3-oxazol-2-yl]prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 14: 4651-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.092 BindingDB Entry DOI: 10.7270/Q22Z13QX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127974 ((R)-3-[(2S,4R)-2-Hydroxy-4-((3S,4S)-3-hydroxy-chro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of wild type HIV protease | Bioorg Med Chem Lett 13: 1821-4 (2003) BindingDB Entry DOI: 10.7270/Q27S7N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,Q496K] (Human immunodeficiency virus type 1) | BDBM9144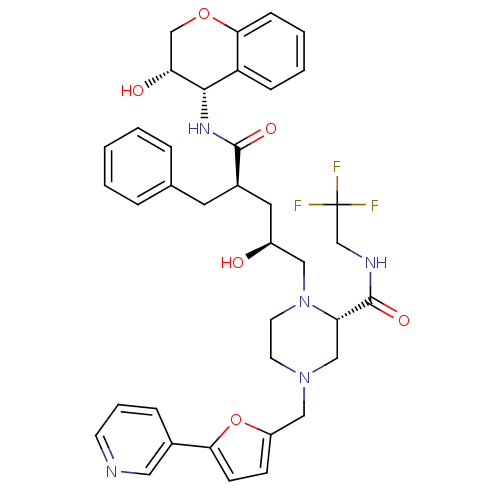 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(3S,4S)-3-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 2573-6 (2003) Article DOI: 10.1016/s0960-894x(03)00474-8 BindingDB Entry DOI: 10.7270/Q2TD9VJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2767 total ) | Next | Last >> |