 Found 221 hits with Last Name = 'thomson' and Initial = 'f'
Found 221 hits with Last Name = 'thomson' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418366

(CHEMBL1774023)Show SMILES Clc1ccc(C[C@@H](NC(=O)Cc2ccc(Cl)cc2)C(=O)N[C@@H]2C[C@@H]3CC[C@H](C2)N3)cc1 |r| Show InChI InChI=1S/C24H27Cl2N3O2/c25-17-5-1-15(2-6-17)11-22(29-23(30)12-16-3-7-18(26)8-4-16)24(31)28-21-13-19-9-10-20(14-21)27-19/h1-8,19-22,27H,9-14H2,(H,28,31)(H,29,30)/t19-,20+,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418365
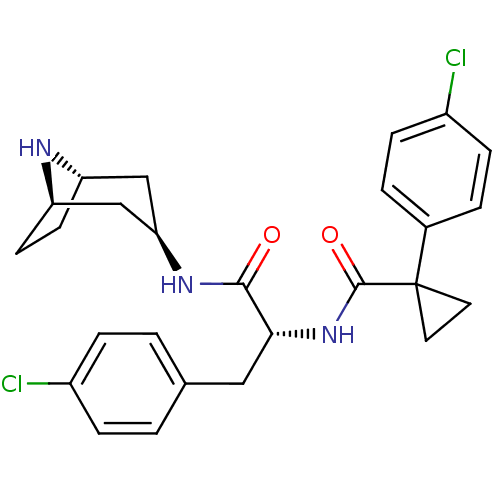
(CHEMBL1774024)Show SMILES Clc1ccc(C[C@@H](NC(=O)C2(CC2)c2ccc(Cl)cc2)C(=O)N[C@@H]2C[C@@H]3CC[C@H](C2)N3)cc1 |r| Show InChI InChI=1S/C26H29Cl2N3O2/c27-18-5-1-16(2-6-18)13-23(24(32)30-22-14-20-9-10-21(15-22)29-20)31-25(33)26(11-12-26)17-3-7-19(28)8-4-17/h1-8,20-23,29H,9-15H2,(H,30,32)(H,31,33)/t20-,21+,22+,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by whole cell binding assay |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in CHO cells by whole cell binding assay |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416116

(CHEMBL1084315)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CCCNCC2)c2ccccc12 Show InChI InChI=1S/C25H27N3O4S/c1-32-23-11-12-24(20-9-3-2-8-19(20)23)33(30,31)28-17-21(18-7-4-5-10-22(18)28)25(29)27-15-6-13-26-14-16-27/h2-5,7-12,21,26H,6,13-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418372

(CHEMBL1774022)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1 |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C27H31Cl2N3O2/c1-32-22-10-11-23(32)16-21(15-22)30-25(33)24(14-17-2-6-19(28)7-3-17)31-26(34)27(12-13-27)18-4-8-20(29)9-5-18/h2-9,21-24H,10-16H2,1H3,(H,30,33)(H,31,34)/t21-,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418373

(CHEMBL1774017)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C25H29Cl2N3O2/c1-30-21-10-11-22(30)15-20(14-21)28-25(32)23(12-16-2-6-18(26)7-3-16)29-24(31)13-17-4-8-19(27)9-5-17/h2-9,20-23H,10-15H2,1H3,(H,28,32)(H,29,31)/t20-,21+,22-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418397
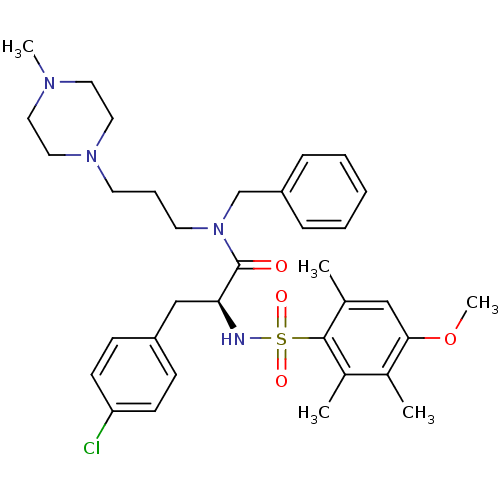
(CHEMBL1773857)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N[C@@H](Cc1ccc(Cl)cc1)C(=O)N(CCCN1CCN(C)CC1)Cc1ccccc1 |r| Show InChI InChI=1S/C34H45ClN4O4S/c1-25-22-32(43-5)26(2)27(3)33(25)44(41,42)36-31(23-28-12-14-30(35)15-13-28)34(40)39(24-29-10-7-6-8-11-29)17-9-16-38-20-18-37(4)19-21-38/h6-8,10-15,22,31,36H,9,16-21,23-24H2,1-5H3/t31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418367

(CHEMBL1774021)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C(C)(C)c1ccc(Cl)cc1 |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C27H33Cl2N3O2/c1-27(2,18-6-10-20(29)11-7-18)26(34)31-24(14-17-4-8-19(28)9-5-17)25(33)30-21-15-22-12-13-23(16-21)32(22)3/h4-11,21-24H,12-16H2,1-3H3,(H,30,33)(H,31,34)/t21-,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50418365

(CHEMBL1774024)Show SMILES Clc1ccc(C[C@@H](NC(=O)C2(CC2)c2ccc(Cl)cc2)C(=O)N[C@@H]2C[C@@H]3CC[C@H](C2)N3)cc1 |r| Show InChI InChI=1S/C26H29Cl2N3O2/c27-18-5-1-16(2-6-18)13-23(24(32)30-22-14-20-9-10-21(15-22)29-20)31-25(33)26(11-12-26)17-3-7-19(28)8-4-17/h1-8,20-23,29H,9-15H2,(H,30,32)(H,31,33)/t20-,21+,22+,23-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418377

(CHEMBL1774012)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)C(Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)c(Cl)c1 |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C25H28Cl3N3O2/c1-31-19-7-8-20(31)14-18(13-19)29-25(33)23(11-15-2-5-17(26)6-3-15)30-24(32)12-16-4-9-21(27)22(28)10-16/h2-6,9-10,18-20,23H,7-8,11-14H2,1H3,(H,29,33)(H,30,32)/t18-,19+,20-,23? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416120
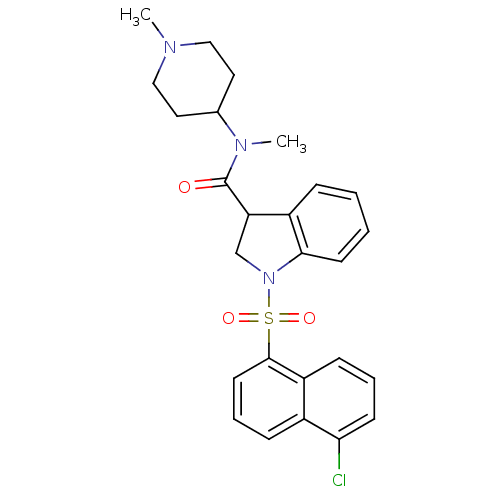
(CHEMBL1082484)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc2c(Cl)cccc12 Show InChI InChI=1S/C26H28ClN3O3S/c1-28-15-13-18(14-16-28)29(2)26(31)22-17-30(24-11-4-3-7-20(22)24)34(32,33)25-12-6-8-19-21(25)9-5-10-23(19)27/h3-12,18,22H,13-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418384
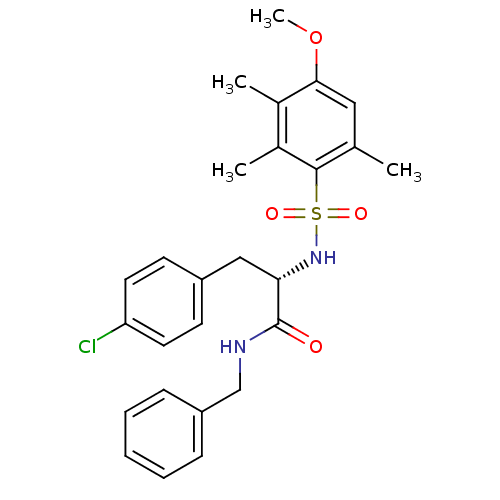
(CHEMBL1774000)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N[C@@H](Cc1ccc(Cl)cc1)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C26H29ClN2O4S/c1-17-14-24(33-4)18(2)19(3)25(17)34(31,32)29-23(15-20-10-12-22(27)13-11-20)26(30)28-16-21-8-6-5-7-9-21/h5-14,23,29H,15-16H2,1-4H3,(H,28,30)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418369
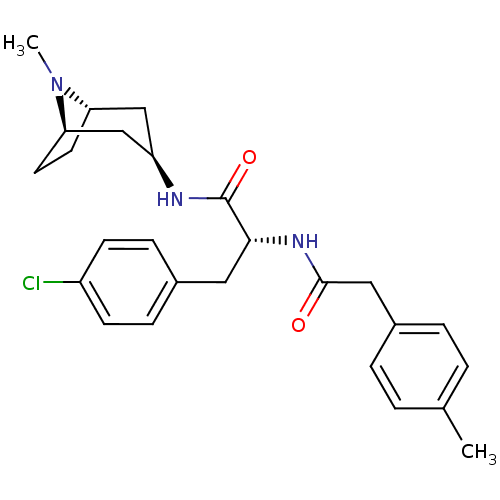
(CHEMBL1774019)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(C)cc1 |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C26H32ClN3O2/c1-17-3-5-19(6-4-17)14-25(31)29-24(13-18-7-9-20(27)10-8-18)26(32)28-21-15-22-11-12-23(16-21)30(22)2/h3-10,21-24H,11-16H2,1-2H3,(H,28,32)(H,29,31)/t21-,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416114
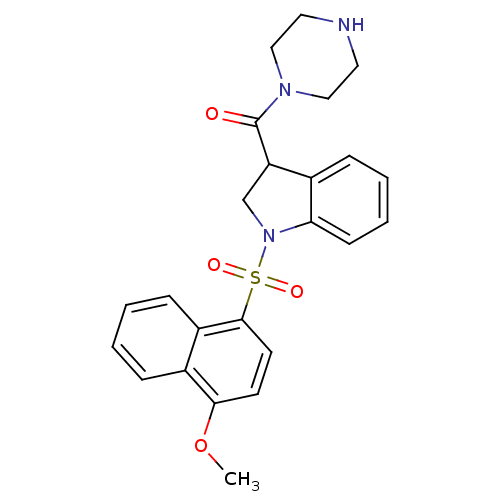
(CHEMBL1085608)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CCNCC2)c2ccccc12 Show InChI InChI=1S/C24H25N3O4S/c1-31-22-10-11-23(19-8-3-2-7-18(19)22)32(29,30)27-16-20(17-6-4-5-9-21(17)27)24(28)26-14-12-25-13-15-26/h2-11,20,25H,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416108
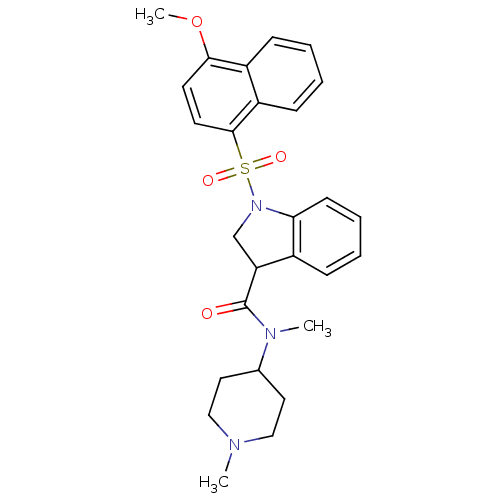
(CHEMBL1082818)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N(C)C2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C27H31N3O4S/c1-28-16-14-19(15-17-28)29(2)27(31)23-18-30(24-11-7-6-8-20(23)24)35(32,33)26-13-12-25(34-3)21-9-4-5-10-22(21)26/h4-13,19,23H,14-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418399

(CHEMBL1774001)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N[C@@H](Cc1ccc(Cl)cc1)C(=O)NCCN1CCN(C)CC1 |r| Show InChI InChI=1S/C26H37ClN4O4S/c1-18-16-24(35-5)19(2)20(3)25(18)36(33,34)29-23(17-21-6-8-22(27)9-7-21)26(32)28-10-11-31-14-12-30(4)13-15-31/h6-9,16,23,29H,10-15,17H2,1-5H3,(H,28,32)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418386

(CHEMBL1773998)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N[C@@H](Cc1ccc(Cl)cc1)C(=O)N(CCCN1CCN(C)CC1)C(C)C |r| Show InChI InChI=1S/C30H45ClN4O4S/c1-21(2)35(14-8-13-34-17-15-33(6)16-18-34)30(36)27(20-25-9-11-26(31)12-10-25)32-40(37,38)29-22(3)19-28(39-7)23(4)24(29)5/h9-12,19,21,27,32H,8,13-18,20H2,1-7H3/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418395

(CHEMBL1773859)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N[C@@H](Cc1ccccc1)C(=O)N(CCCN1CCN(C)CC1)Cc1ccccc1 |r| Show InChI InChI=1S/C34H46N4O4S/c1-26-23-32(42-5)27(2)28(3)33(26)43(40,41)35-31(24-29-13-8-6-9-14-29)34(39)38(25-30-15-10-7-11-16-30)18-12-17-37-21-19-36(4)20-22-37/h6-11,13-16,23,31,35H,12,17-22,24-25H2,1-5H3/t31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418368

(CHEMBL1774020)Show SMILES COc1ccc(CC(=O)N[C@H](Cc2ccc(Cl)cc2)C(=O)N[C@@H]2C[C@@H]3CC[C@H](C2)N3C)cc1 |r,TLB:30:29:26.25:22.28.23| Show InChI InChI=1S/C26H32ClN3O3/c1-30-21-9-10-22(30)16-20(15-21)28-26(32)24(13-17-3-7-19(27)8-4-17)29-25(31)14-18-5-11-23(33-2)12-6-18/h3-8,11-12,20-22,24H,9-10,13-16H2,1-2H3,(H,28,32)(H,29,31)/t20-,21+,22-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416117
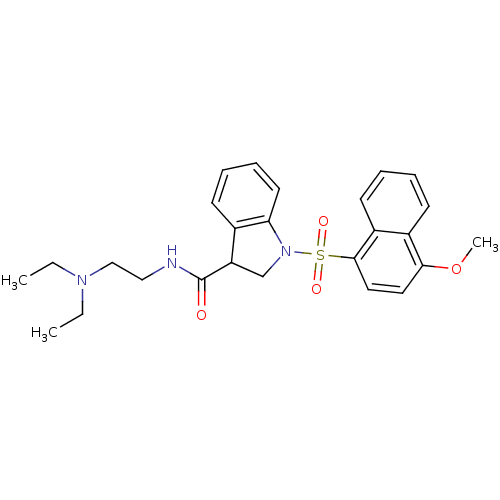
(CHEMBL1084316)Show SMILES CCN(CC)CCNC(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC)c2ccccc12 Show InChI InChI=1S/C26H31N3O4S/c1-4-28(5-2)17-16-27-26(30)22-18-29(23-13-9-8-10-19(22)23)34(31,32)25-15-14-24(33-3)20-11-6-7-12-21(20)25/h6-15,22H,4-5,16-18H2,1-3H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416131
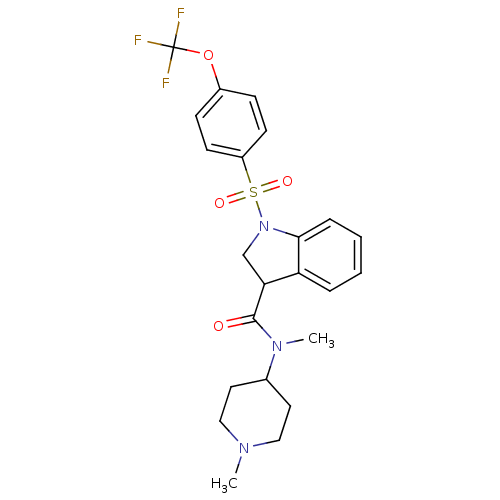
(CHEMBL1085965)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H26F3N3O4S/c1-27-13-11-16(12-14-27)28(2)22(30)20-15-29(21-6-4-3-5-19(20)21)34(31,32)18-9-7-17(8-10-18)33-23(24,25)26/h3-10,16,20H,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50418366

(CHEMBL1774023)Show SMILES Clc1ccc(C[C@@H](NC(=O)Cc2ccc(Cl)cc2)C(=O)N[C@@H]2C[C@@H]3CC[C@H](C2)N3)cc1 |r| Show InChI InChI=1S/C24H27Cl2N3O2/c25-17-5-1-15(2-6-17)11-22(29-23(30)12-16-3-7-18(26)8-4-16)24(31)28-21-13-19-9-10-20(14-21)27-19/h1-8,19-22,27H,9-14H2,(H,28,31)(H,29,30)/t19-,20+,21+,22-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human vasopressin V1a receptor at 5 uM |
Bioorg Med Chem Lett 21: 1871-5 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.081
BindingDB Entry DOI: 10.7270/Q2BR8SG4 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418373

(CHEMBL1774017)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1 |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C25H29Cl2N3O2/c1-30-21-10-11-22(30)15-20(14-21)28-25(32)23(12-16-2-6-18(26)7-3-16)29-24(31)13-17-4-8-19(27)9-5-17/h2-9,20-23H,10-15H2,1H3,(H,28,32)(H,29,31)/t20-,21+,22-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418387
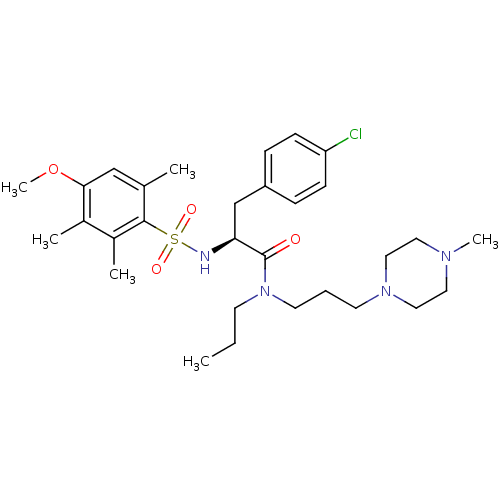
(CHEMBL1773997)Show SMILES CCCN(CCCN1CCN(C)CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NS(=O)(=O)c1c(C)cc(OC)c(C)c1C |r| Show InChI InChI=1S/C30H45ClN4O4S/c1-7-13-35(15-8-14-34-18-16-33(5)17-19-34)30(36)27(21-25-9-11-26(31)12-10-25)32-40(37,38)29-22(2)20-28(39-6)23(3)24(29)4/h9-12,20,27,32H,7-8,13-19,21H2,1-6H3/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416122
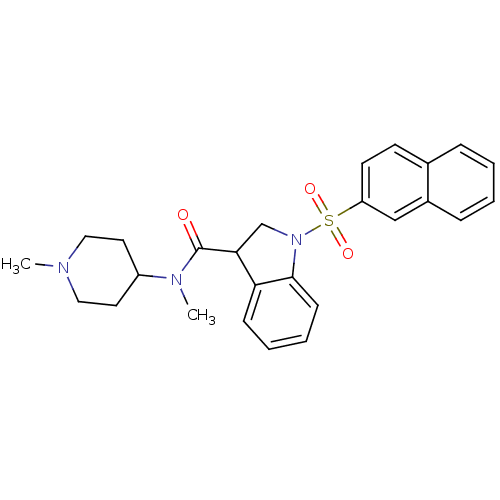
(CHEMBL1085351)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C26H29N3O3S/c1-27-15-13-21(14-16-27)28(2)26(30)24-18-29(25-10-6-5-9-23(24)25)33(31,32)22-12-11-19-7-3-4-8-20(19)17-22/h3-12,17,21,24H,13-16,18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416109
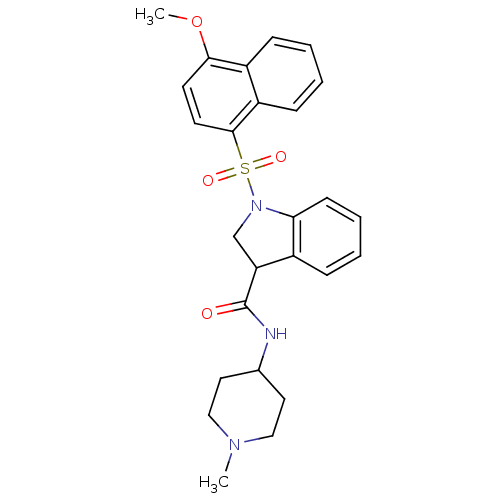
(CHEMBL1085835)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NC2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C26H29N3O4S/c1-28-15-13-18(14-16-28)27-26(30)22-17-29(23-10-6-5-7-19(22)23)34(31,32)25-12-11-24(33-2)20-8-3-4-9-21(20)25/h3-12,18,22H,13-17H2,1-2H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416130
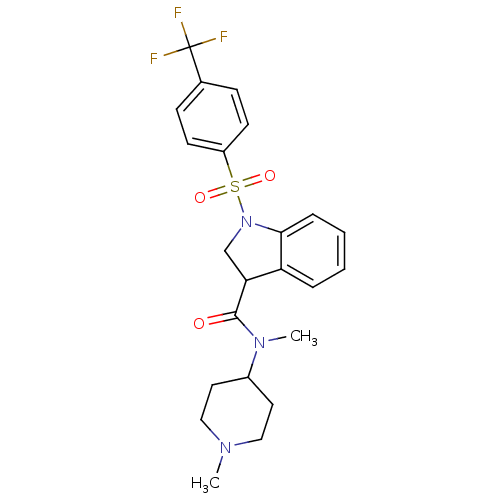
(CHEMBL1083909)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H26F3N3O3S/c1-27-13-11-17(12-14-27)28(2)22(30)20-15-29(21-6-4-3-5-19(20)21)33(31,32)18-9-7-16(8-10-18)23(24,25)26/h3-10,17,20H,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416132
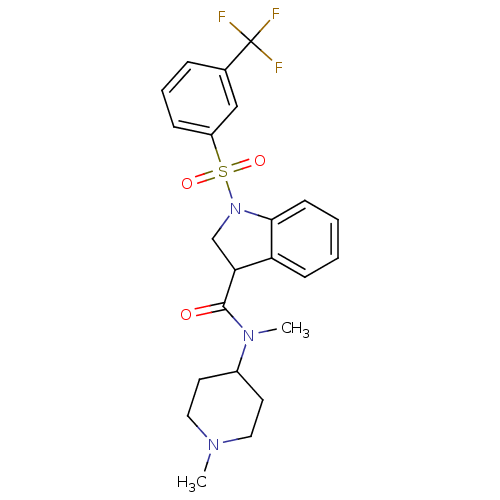
(CHEMBL1083297)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H26F3N3O3S/c1-27-12-10-17(11-13-27)28(2)22(30)20-15-29(21-9-4-3-8-19(20)21)33(31,32)18-7-5-6-16(14-18)23(24,25)26/h3-9,14,17,20H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418370
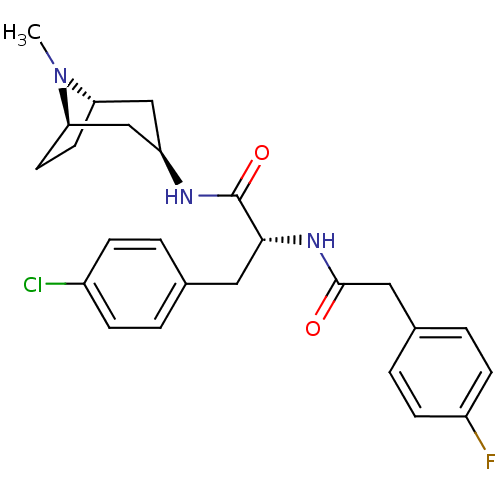
(CHEMBL1774018)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(F)cc1 |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C25H29ClFN3O2/c1-30-21-10-11-22(30)15-20(14-21)28-25(32)23(12-16-2-6-18(26)7-3-16)29-24(31)13-17-4-8-19(27)9-5-17/h2-9,20-23H,10-15H2,1H3,(H,28,32)(H,29,31)/t20-,21+,22-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418402
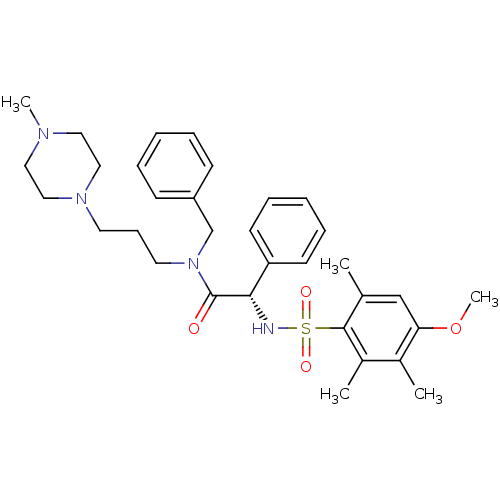
(CHEMBL1773080)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N[C@H](C(=O)N(CCCN1CCN(C)CC1)Cc1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C33H44N4O4S/c1-25-23-30(41-5)26(2)27(3)32(25)42(39,40)34-31(29-15-10-7-11-16-29)33(38)37(24-28-13-8-6-9-14-28)18-12-17-36-21-19-35(4)20-22-36/h6-11,13-16,23,31,34H,12,17-22,24H2,1-5H3/t31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416136
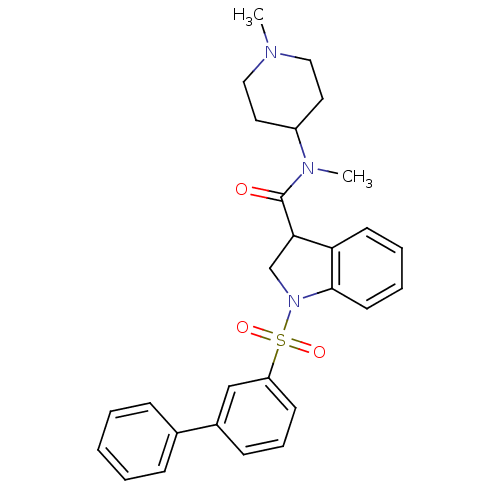
(CHEMBL1084483)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C28H31N3O3S/c1-29-17-15-23(16-18-29)30(2)28(32)26-20-31(27-14-7-6-13-25(26)27)35(33,34)24-12-8-11-22(19-24)21-9-4-3-5-10-21/h3-14,19,23,26H,15-18,20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416113
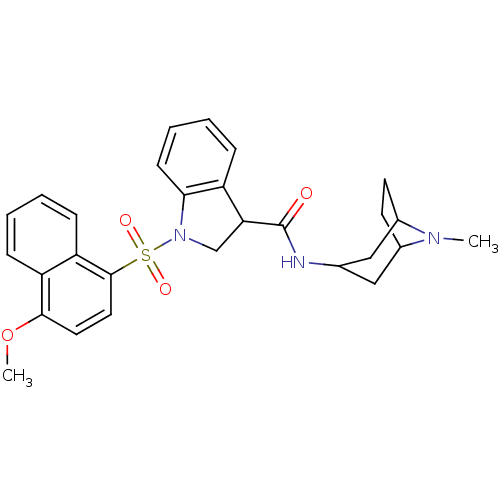
(CHEMBL1082494)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NC2CC3CCC(C2)N3C)c2ccccc12 |TLB:20:21:24.25:28| Show InChI InChI=1S/C28H31N3O4S/c1-30-19-11-12-20(30)16-18(15-19)29-28(32)24-17-31(25-10-6-5-7-21(24)25)36(33,34)27-14-13-26(35-2)22-8-3-4-9-23(22)27/h3-10,13-14,18-20,24H,11-12,15-17H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416118
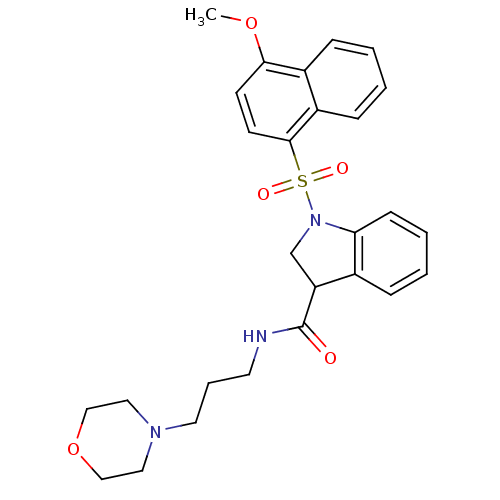
(CHEMBL1083122)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NCCCN2CCOCC2)c2ccccc12 Show InChI InChI=1S/C27H31N3O5S/c1-34-25-11-12-26(22-9-3-2-8-21(22)25)36(32,33)30-19-23(20-7-4-5-10-24(20)30)27(31)28-13-6-14-29-15-17-35-18-16-29/h2-5,7-12,23H,6,13-19H2,1H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416121
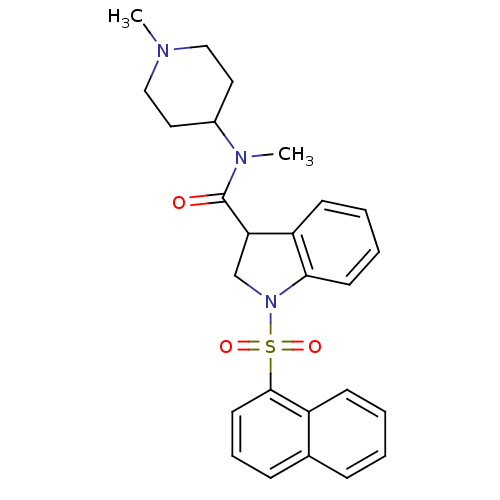
(CHEMBL1082485)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C26H29N3O3S/c1-27-16-14-20(15-17-27)28(2)26(30)23-18-29(24-12-6-5-11-22(23)24)33(31,32)25-13-7-9-19-8-3-4-10-21(19)25/h3-13,20,23H,14-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416123
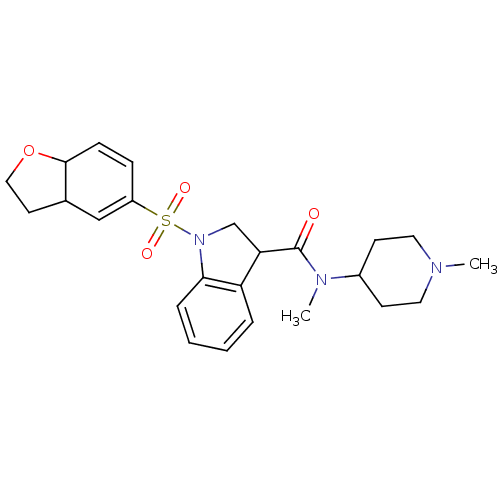
(CHEMBL1085352)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)C1=CC2CCOC2C=C1 |c:34,t:26| Show InChI InChI=1S/C24H31N3O4S/c1-25-12-9-18(10-13-25)26(2)24(28)21-16-27(22-6-4-3-5-20(21)22)32(29,30)19-7-8-23-17(15-19)11-14-31-23/h3-8,15,17-18,21,23H,9-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418398
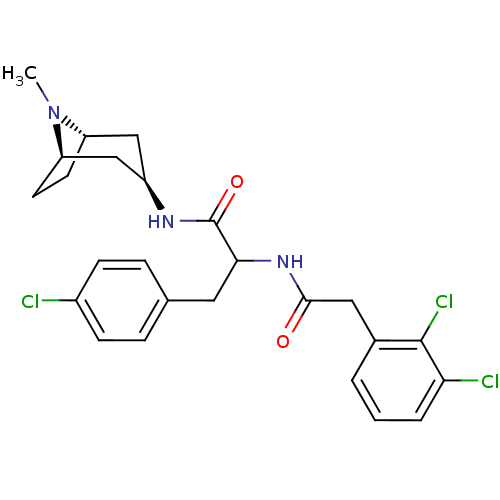
(CHEMBL1774011)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)C(Cc1ccc(Cl)cc1)NC(=O)Cc1cccc(Cl)c1Cl |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C25H28Cl3N3O2/c1-31-19-9-10-20(31)14-18(13-19)29-25(33)22(11-15-5-7-17(26)8-6-15)30-23(32)12-16-3-2-4-21(27)24(16)28/h2-8,18-20,22H,9-14H2,1H3,(H,29,33)(H,30,32)/t18-,19+,20-,22? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418376
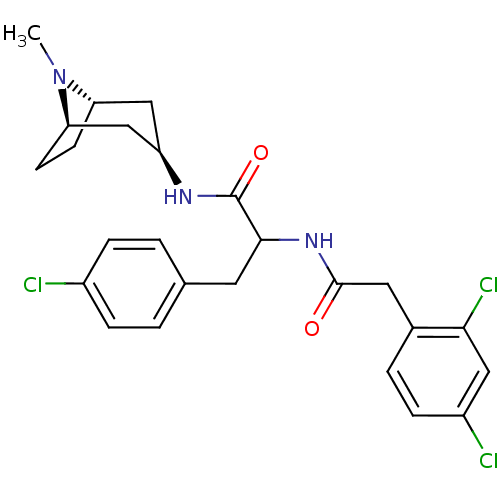
(CHEMBL1774013)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)C(Cc1ccc(Cl)cc1)NC(=O)Cc1ccc(Cl)cc1Cl |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C25H28Cl3N3O2/c1-31-20-8-9-21(31)14-19(13-20)29-25(33)23(10-15-2-5-17(26)6-3-15)30-24(32)11-16-4-7-18(27)12-22(16)28/h2-7,12,19-21,23H,8-11,13-14H2,1H3,(H,29,33)(H,30,32)/t19-,20+,21-,23? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418375
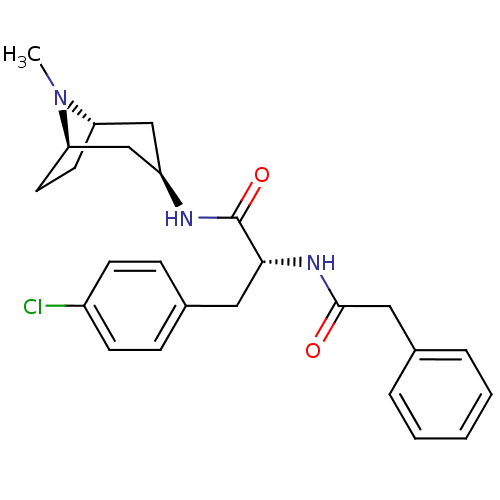
(CHEMBL1774014)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)Cc1ccccc1 |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C25H30ClN3O2/c1-29-21-11-12-22(29)16-20(15-21)27-25(31)23(13-18-7-9-19(26)10-8-18)28-24(30)14-17-5-3-2-4-6-17/h2-10,20-23H,11-16H2,1H3,(H,27,31)(H,28,30)/t20-,21+,22-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418380
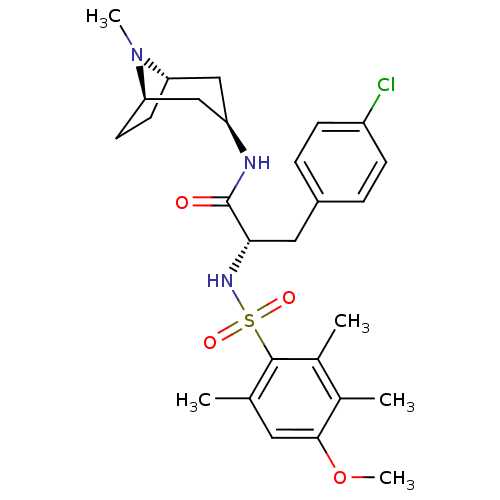
(CHEMBL1774005)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N[C@@H](Cc1ccc(Cl)cc1)C(=O)N[C@@H]1C[C@@H]2CC[C@H](C1)N2C |r,TLB:35:34:31.30:27.33.28| Show InChI InChI=1S/C27H36ClN3O4S/c1-16-12-25(35-5)17(2)18(3)26(16)36(33,34)30-24(13-19-6-8-20(28)9-7-19)27(32)29-21-14-22-10-11-23(15-21)31(22)4/h6-9,12,21-24,30H,10-11,13-15H2,1-5H3,(H,29,32)/t21-,22+,23-,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416124
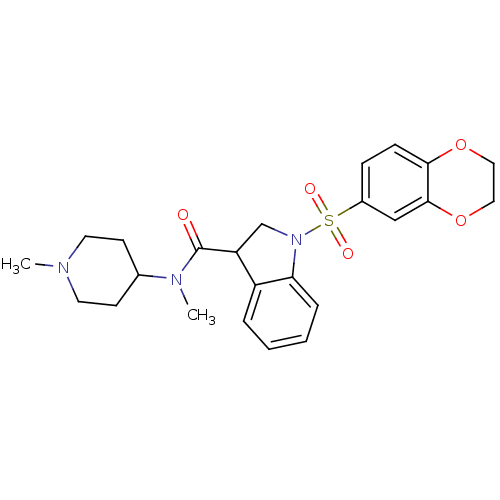
(CHEMBL1085353)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc2OCCOc2c1 Show InChI InChI=1S/C24H29N3O5S/c1-25-11-9-17(10-12-25)26(2)24(28)20-16-27(21-6-4-3-5-19(20)21)33(29,30)18-7-8-22-23(15-18)32-14-13-31-22/h3-8,15,17,20H,9-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416110

(CHEMBL1085836)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)NC2CCN(Cc3ccccc3)CC2)c2ccccc12 Show InChI InChI=1S/C32H33N3O4S/c1-39-30-15-16-31(27-13-6-5-12-26(27)30)40(37,38)35-22-28(25-11-7-8-14-29(25)35)32(36)33-24-17-19-34(20-18-24)21-23-9-3-2-4-10-23/h2-16,24,28H,17-22H2,1H3,(H,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418396
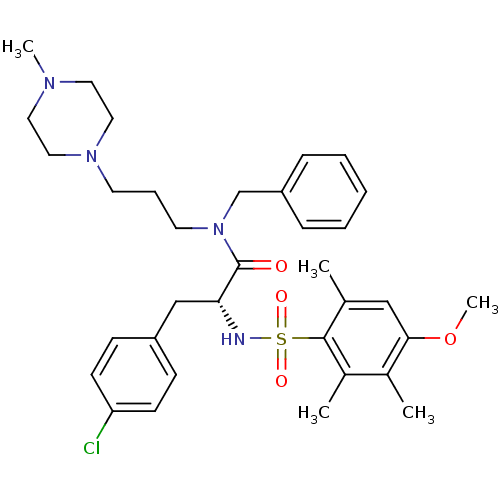
(CHEMBL1773858)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N[C@H](Cc1ccc(Cl)cc1)C(=O)N(CCCN1CCN(C)CC1)Cc1ccccc1 |r| Show InChI InChI=1S/C34H45ClN4O4S/c1-25-22-32(43-5)26(2)27(3)33(25)44(41,42)36-31(23-28-12-14-30(35)15-13-28)34(40)39(24-29-10-7-6-8-11-29)17-9-16-38-20-18-37(4)19-21-38/h6-8,10-15,22,31,36H,9,16-21,23-24H2,1-5H3/t31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50418372

(CHEMBL1774022)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1 |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C27H31Cl2N3O2/c1-32-22-10-11-23(32)16-21(15-22)30-25(33)24(14-17-2-6-19(28)7-3-17)31-26(34)27(12-13-27)18-4-8-20(29)9-5-18/h2-9,21-24H,10-16H2,1H3,(H,30,33)(H,31,34)/t21-,22+,23-,24-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from rat V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418378
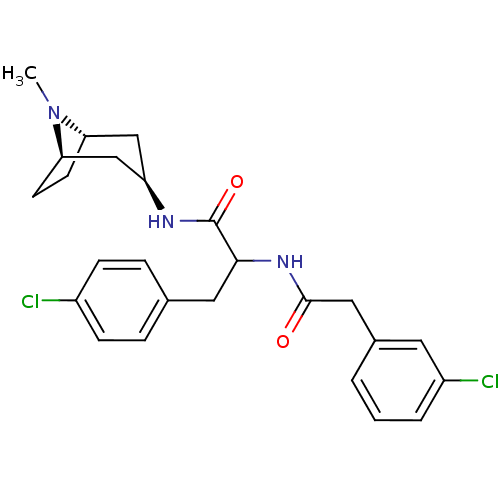
(CHEMBL1774009)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)NC(=O)C(Cc1ccc(Cl)cc1)NC(=O)Cc1cccc(Cl)c1 |r,TLB:0:1:4.3:7.6.8| Show InChI InChI=1S/C25H29Cl2N3O2/c1-30-21-9-10-22(30)15-20(14-21)28-25(32)23(12-16-5-7-18(26)8-6-16)29-24(31)13-17-3-2-4-19(27)11-17/h2-8,11,20-23H,9-10,12-15H2,1H3,(H,28,32)(H,29,31)/t20-,21+,22-,23? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50418400
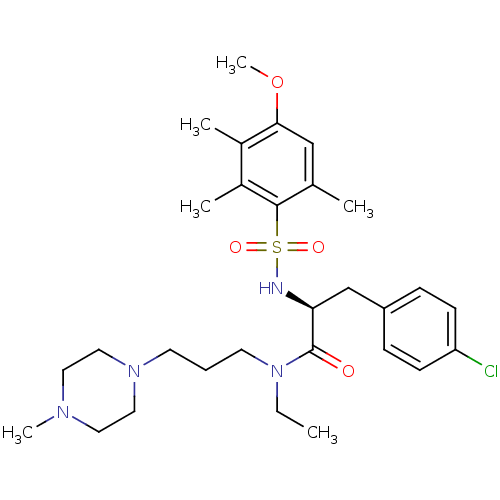
(CHEMBL1773996)Show SMILES CCN(CCCN1CCN(C)CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NS(=O)(=O)c1c(C)cc(OC)c(C)c1C |r| Show InChI InChI=1S/C29H43ClN4O4S/c1-7-34(14-8-13-33-17-15-32(5)16-18-33)29(35)26(20-24-9-11-25(30)12-10-24)31-39(36,37)28-21(2)19-27(38-6)22(3)23(28)4/h9-12,19,26,31H,7-8,13-18,20H2,1-6H3/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]AVP from human V1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3163-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.096
BindingDB Entry DOI: 10.7270/Q2K64KB0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50345085

((2S)-N-(3-(1-aminoisoquinolin-6-yl)-1-oxo-1-(piper...)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N[C@@H](Cc1ccccc1)C(=O)NC(Cc1ccc2c(N)nccc2c1)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C36H43N5O5S/c1-23-19-32(46-4)24(2)25(3)33(23)47(44,45)40-30(21-26-11-7-5-8-12-26)35(42)39-31(36(43)41-17-9-6-10-18-41)22-27-13-14-29-28(20-27)15-16-38-34(29)37/h5,7-8,11-16,19-20,30-31,40H,6,9-10,17-18,21-22H2,1-4H3,(H2,37,38)(H,39,42)/t30-,31?/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V1B receptor expressed in CHO cells |
Bioorg Med Chem Lett 21: 3603-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.104
BindingDB Entry DOI: 10.7270/Q2833SCQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416127
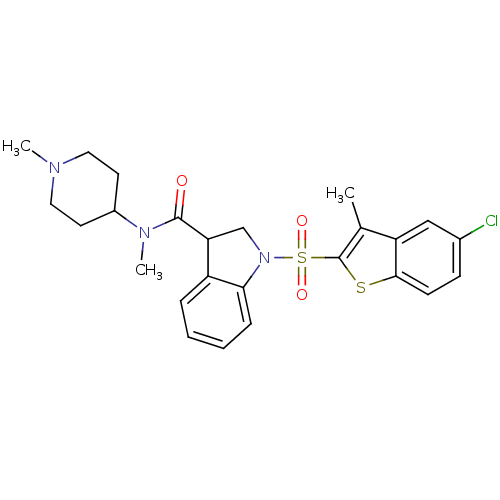
(CHEMBL1082765)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1sc2ccc(Cl)cc2c1C Show InChI InChI=1S/C25H28ClN3O3S2/c1-16-20-14-17(26)8-9-23(20)33-25(16)34(31,32)29-15-21(19-6-4-5-7-22(19)29)24(30)28(3)18-10-12-27(2)13-11-18/h4-9,14,18,21H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 63.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data