

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092228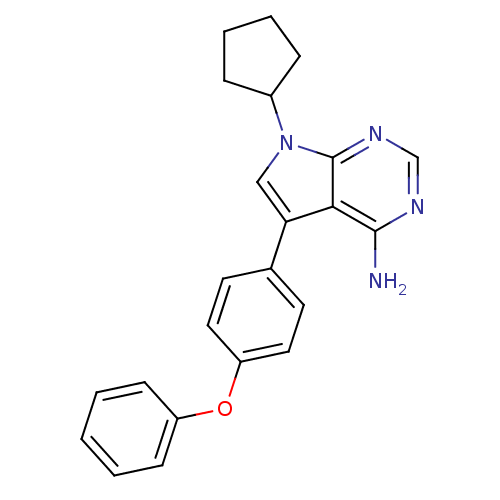 (4-Amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092249 (4-[4-(4-Amino-7-tert-butyl-7H-pyrrolo[2,3-d]pyrimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092233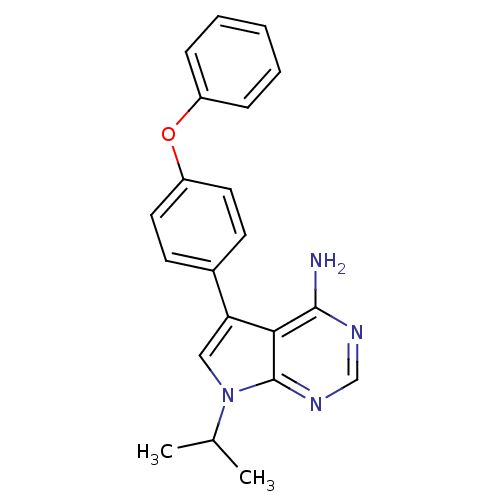 (7-Isopropyl-5-(4-phenoxy-phenyl)-7H-pyrrolo[2,3-d]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092241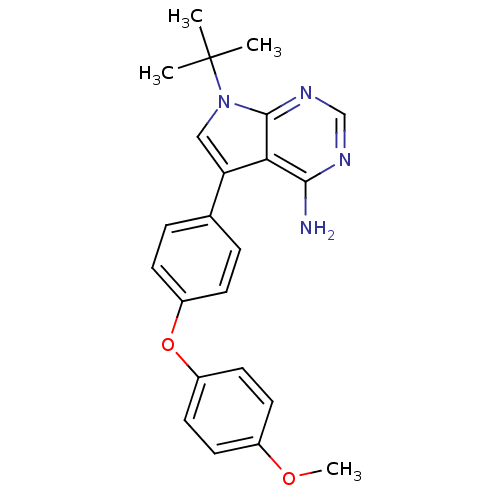 (7-tert-Butyl-5-[4-(4-methoxy-phenoxy)-phenyl]-7H-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092225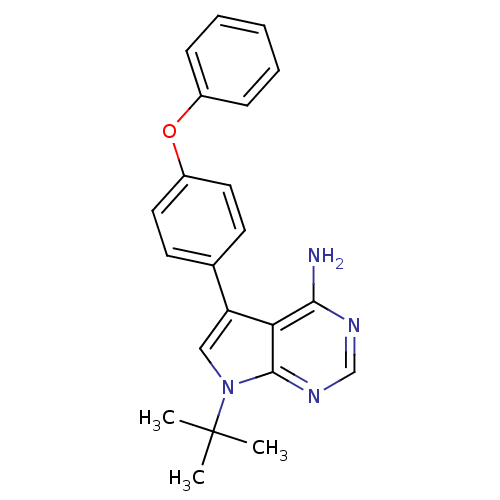 (7-tert-Butyl-5-(4-phenoxy-phenyl)-7H-pyrrolo[2,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 uM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092228 (4-Amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092243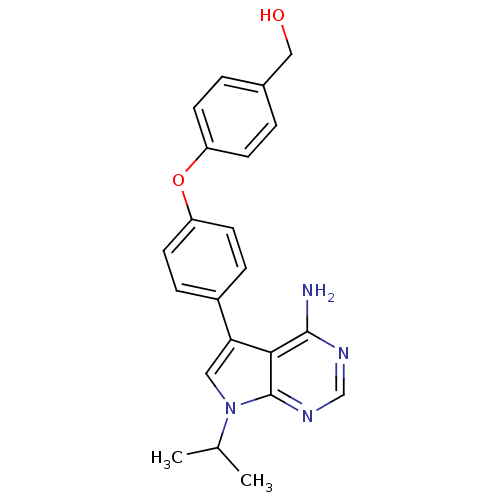 (CHEMBL70034 | {4-[4-(4-Amino-7-isopropyl-7H-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 uM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092236 (CHEMBL71541 | {2-[4-(4-Amino-7-isopropyl-7H-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092244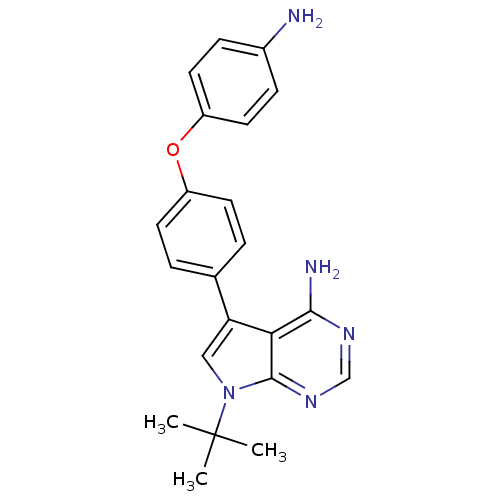 (5-[4-(4-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 uM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092254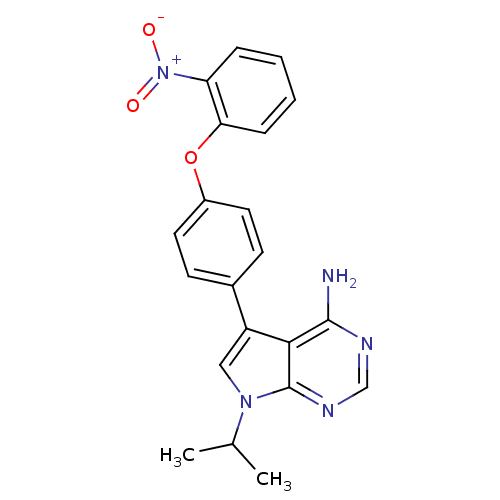 (7-Isopropyl-5-[4-(2-nitro-phenoxy)-phenyl]-7H-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092240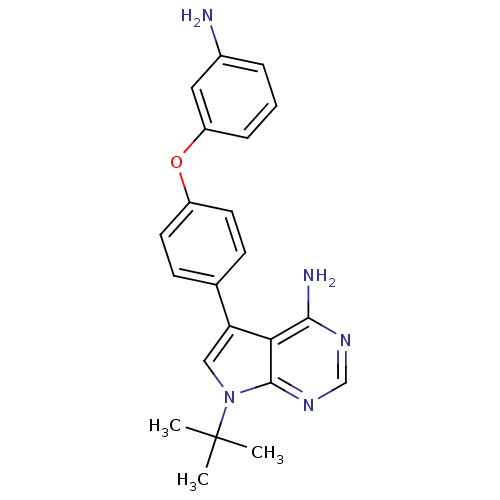 (5-[4-(3-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092256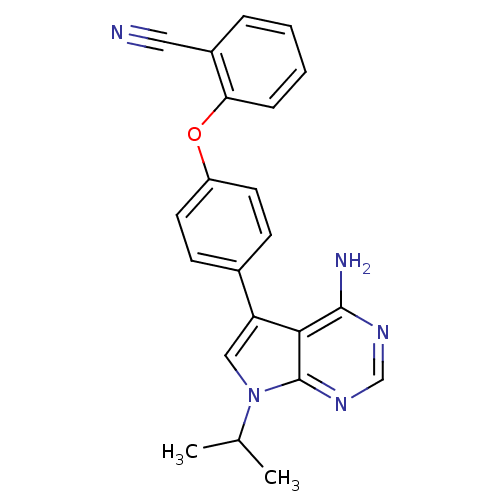 (2-[4-(4-Amino-7-isopropyl-7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 uM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092235 (5-[4-(2-Amino-phenoxy)-phenyl]-7-isopropyl-7H-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092239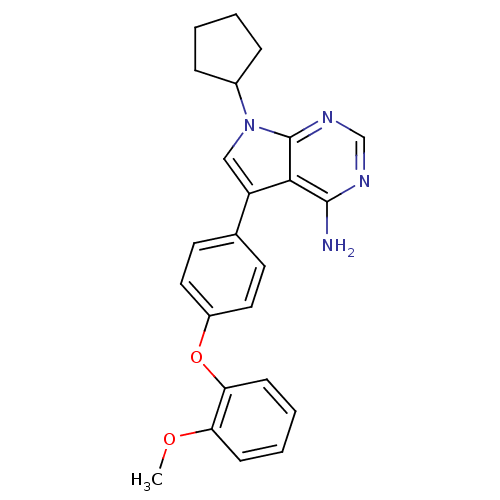 (7-Cyclopentyl-5-[4-(2-methoxy-phenoxy)-phenyl]-7H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092234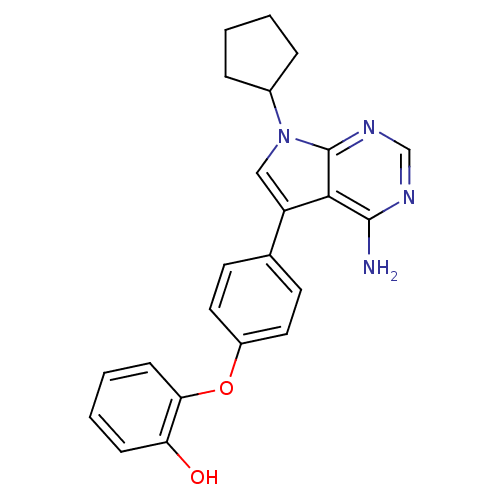 (2-[4-(4-Amino-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092233 (7-Isopropyl-5-(4-phenoxy-phenyl)-7H-pyrrolo[2,3-d]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092252 (6-Chloro-7-cyclopentyl-5-(4-phenoxy-phenyl)-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092225 (7-tert-Butyl-5-(4-phenoxy-phenyl)-7H-pyrrolo[2,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092240 (5-[4-(3-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092249 (4-[4-(4-Amino-7-tert-butyl-7H-pyrrolo[2,3-d]pyrimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092250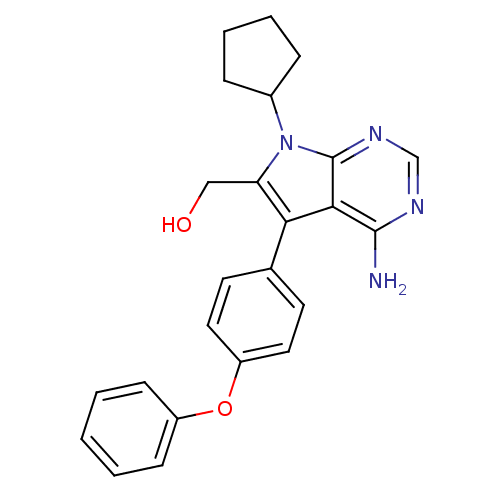 (CHEMBL302279 | [4-Amino-7-cyclopentyl-5-(4-phenoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092244 (5-[4-(4-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092239 (7-Cyclopentyl-5-[4-(2-methoxy-phenoxy)-phenyl]-7H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092246 (4-Amino-7-cyclopentyl-5-(4-phenoxy-phenyl)-7H-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50092243 (CHEMBL70034 | {4-[4-(4-Amino-7-isopropyl-7H-pyrrol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against vascular endothelial growth factor receptor 2 (VEGFR2) at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092245 (4-Amino-7-cyclopentyl-5-(4-phenoxy-phenyl)-7H-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092237 (6-Bromo-7-cyclopentyl-5-(4-phenoxy-phenyl)-7H-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092254 (7-Isopropyl-5-[4-(2-nitro-phenoxy)-phenyl]-7H-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092255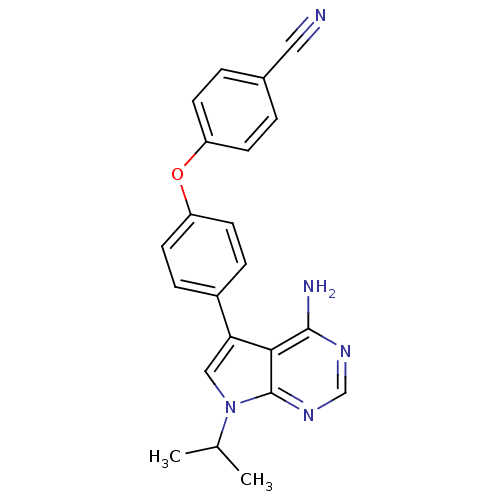 (4-[4-(4-Amino-7-isopropyl-7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092252 (6-Chloro-7-cyclopentyl-5-(4-phenoxy-phenyl)-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092241 (7-tert-Butyl-5-[4-(4-methoxy-phenoxy)-phenyl]-7H-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50092234 (2-[4-(4-Amino-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against vascular endothelial growth factor receptor 2 (VEGFR2) at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50092228 (4-Amino-5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50092255 (4-[4-(4-Amino-7-isopropyl-7H-pyrrolo[2,3-d]pyrimid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against vascular endothelial growth factor receptor 2 (VEGFR2) at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50092251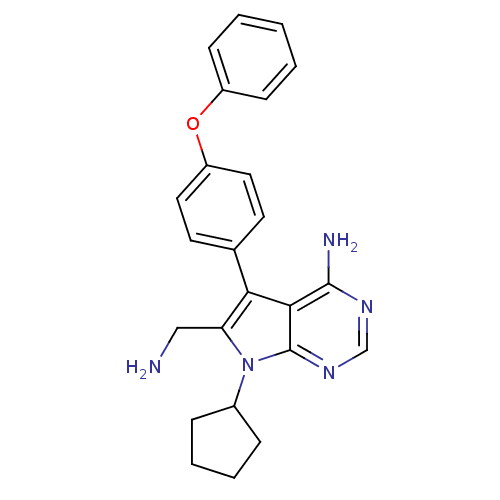 (6-Aminomethyl-7-cyclopentyl-5-(4-phenoxy-phenyl)-7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against tie-2 at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092257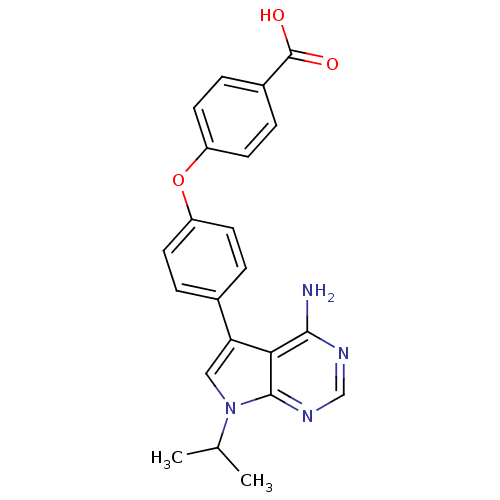 (4-[4-(4-Amino-7-isopropyl-7H-pyrrolo[2,3-d]pyrimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092251 (6-Aminomethyl-7-cyclopentyl-5-(4-phenoxy-phenyl)-7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50092236 (CHEMBL71541 | {2-[4-(4-Amino-7-isopropyl-7H-pyrrol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against vascular endothelial growth factor receptor 2 (VEGFR2) at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092235 (5-[4-(2-Amino-phenoxy)-phenyl]-7-isopropyl-7H-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50092256 (2-[4-(4-Amino-7-isopropyl-7H-pyrrolo[2,3-d]pyrimid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against vascular endothelial growth factor receptor 2 (VEGFR2) at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50092236 (CHEMBL71541 | {2-[4-(4-Amino-7-isopropyl-7H-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against tie-2 at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50092256 (2-[4-(4-Amino-7-isopropyl-7H-pyrrolo[2,3-d]pyrimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 239 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against tie-2 at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50092243 (CHEMBL70034 | {4-[4-(4-Amino-7-isopropyl-7H-pyrrol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against tie-2 at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092238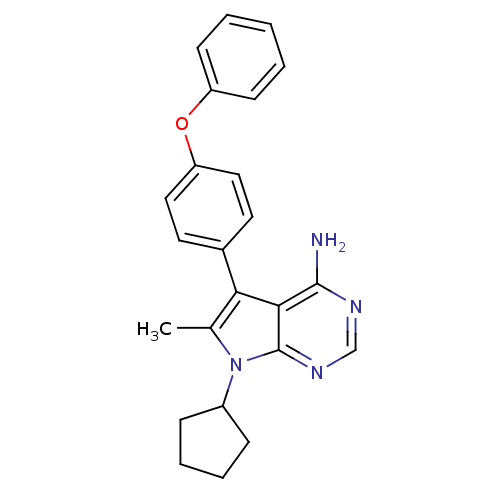 (7-Cyclopentyl-6-methyl-5-(4-phenoxy-phenyl)-7H-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092258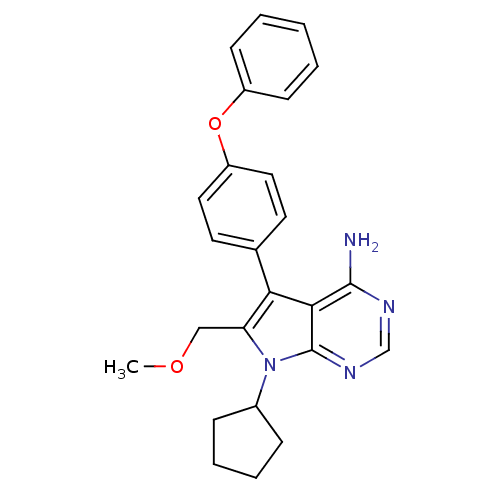 (7-Cyclopentyl-6-methoxymethyl-5-(4-phenoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092234 (2-[4-(4-Amino-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50092250 (CHEMBL302279 | [4-Amino-7-cyclopentyl-5-(4-phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against tie-2 at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50092240 (5-[4-(3-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against vascular endothelial growth factor receptor 2 (VEGFR2) at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50092235 (5-[4-(2-Amino-phenoxy)-phenyl]-7-isopropyl-7H-pyrr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against tie-2 at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50092246 (4-Amino-7-cyclopentyl-5-(4-phenoxy-phenyl)-7H-pyrr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against tie-2 at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 102 total ) | Next | Last >> |