 Found 422 hits with Last Name = 'tran' and Initial = 'g'
Found 422 hits with Last Name = 'tran' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086
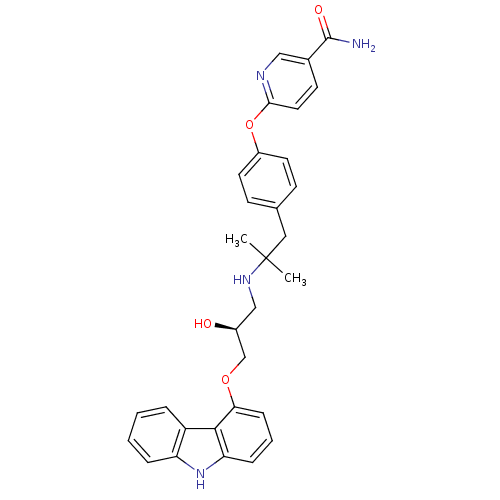
(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086

(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta1 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037076
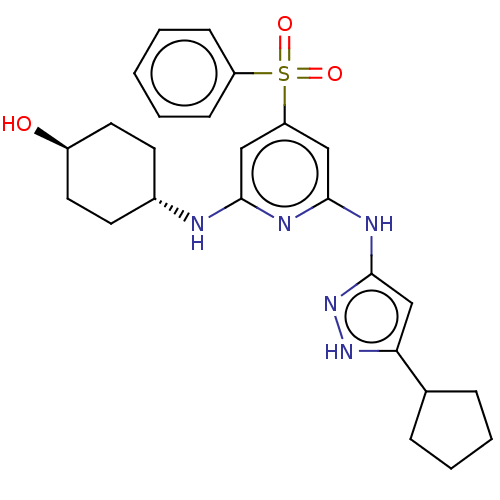
(CHEMBL3355737)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)C2CCCC2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(11.82,-31.11,;11.83,-29.57,;13.16,-28.8,;13.17,-27.26,;11.84,-26.5,;10.5,-27.26,;10.5,-28.8,;11.83,-24.96,;10.5,-24.19,;9.16,-24.96,;7.83,-24.19,;7.83,-22.65,;9.16,-21.88,;9.15,-20.34,;10.49,-19.56,;11.9,-20.19,;12.93,-19.05,;12.15,-17.72,;10.65,-18.04,;14.46,-19.19,;15.24,-20.52,;16.74,-20.2,;16.9,-18.67,;15.49,-18.04,;10.49,-22.64,;6.49,-24.96,;7.25,-26.29,;5.72,-26.28,;5.16,-24.19,;5.17,-22.65,;3.84,-21.88,;2.5,-22.65,;2.51,-24.2,;3.84,-24.96,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037066
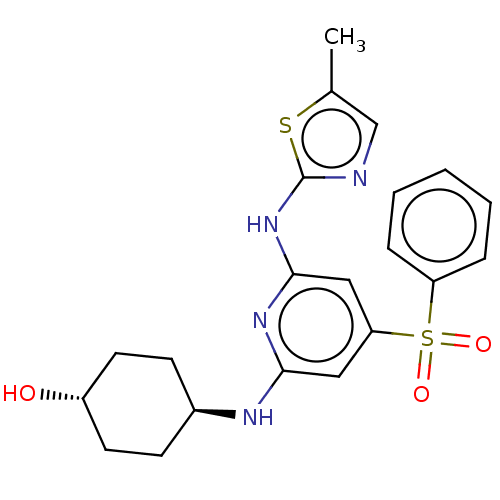
(CHEMBL3355728)Show SMILES Cc1cnc(Nc2cc(cc(N[C@H]3CC[C@H](O)CC3)n2)S(=O)(=O)c2ccccc2)s1 |r,wU:12.11,wD:15.15,(26,-11.56,;24.47,-11.4,;23.69,-10.07,;22.19,-10.39,;22.03,-11.92,;20.69,-12.69,;20.7,-14.23,;19.37,-15,;19.37,-16.55,;20.7,-17.32,;22.04,-16.55,;23.37,-17.31,;23.38,-18.85,;24.71,-19.61,;24.7,-21.15,;23.37,-21.92,;23.37,-23.46,;22.04,-21.15,;22.04,-19.62,;22.03,-15,;18.03,-17.31,;18.79,-18.64,;17.26,-18.64,;16.7,-16.54,;16.71,-15,;15.38,-14.23,;14.04,-15,;14.05,-16.55,;15.38,-17.31,;23.44,-12.55,)| Show InChI InChI=1S/C21H24N4O3S2/c1-14-13-22-21(29-14)25-20-12-18(30(27,28)17-5-3-2-4-6-17)11-19(24-20)23-15-7-9-16(26)10-8-15/h2-6,11-13,15-16,26H,7-10H2,1H3,(H2,22,23,24,25)/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037077
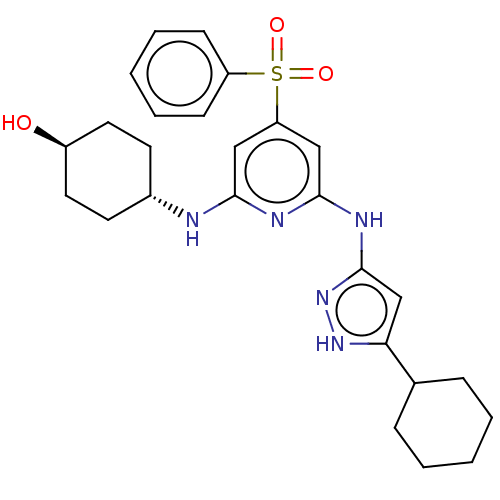
(CHEMBL3355738)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)C2CCCCC2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(26.39,-31.95,;26.39,-30.41,;27.73,-29.64,;27.73,-28.09,;26.4,-27.34,;25.06,-28.1,;25.06,-29.63,;26.4,-25.8,;25.06,-25.03,;23.73,-25.8,;22.39,-25.03,;22.39,-23.48,;23.72,-22.71,;23.72,-21.17,;25.05,-20.4,;26.46,-21.03,;27.49,-19.88,;26.71,-18.55,;25.21,-18.88,;29.01,-20.04,;29.64,-21.44,;31.16,-21.6,;32.07,-20.36,;31.44,-18.95,;29.91,-18.79,;25.06,-23.48,;21.05,-25.79,;21.81,-27.12,;20.28,-27.12,;19.72,-25.03,;19.73,-23.49,;18.4,-22.72,;17.07,-23.49,;17.07,-25.03,;18.4,-25.8,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001786
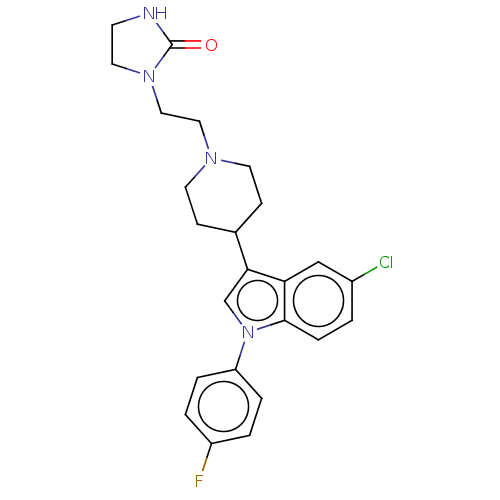
(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50056947

(CHEMBL354210 | N-{2-[4-(1,2,3,4-Tetrahydro-naphtha...)Show InChI InChI=1S/C23H29N3O/c27-23(20-8-2-1-3-9-20)24-13-14-25-15-17-26(18-16-25)22-12-6-10-19-7-4-5-11-21(19)22/h1-5,7-9,11,22H,6,10,12-18H2,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cooperation Pharmaceutique Fran�aise
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor of rat hippocampal membranes |
J Med Chem 40: 952-60 (1997)
Article DOI: 10.1021/jm950759z
BindingDB Entry DOI: 10.7270/Q2HT2Q00 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50056947

(CHEMBL354210 | N-{2-[4-(1,2,3,4-Tetrahydro-naphtha...)Show InChI InChI=1S/C23H29N3O/c27-23(20-8-2-1-3-9-20)24-13-14-25-15-17-26(18-16-25)22-12-6-10-19-7-4-5-11-21(19)22/h1-5,7-9,11,22H,6,10,12-18H2,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cooperation Pharmaceutique Fran�aise
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor of rat hippocampal membranes |
J Med Chem 40: 952-60 (1997)
Article DOI: 10.1021/jm950759z
BindingDB Entry DOI: 10.7270/Q2HT2Q00 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM22867
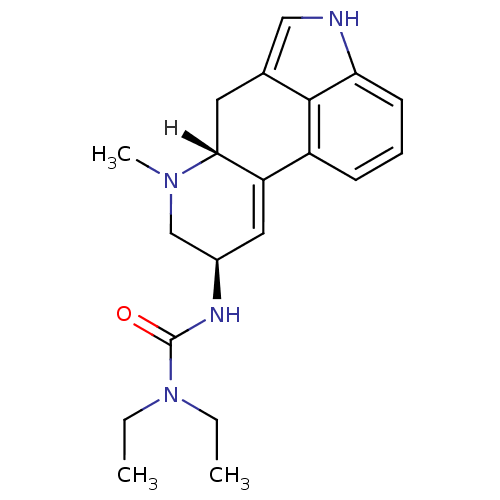
(1,1-diethyl-3-[(8beta)-6-methyl-9,10-didehydroergo...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)NC(=O)N(CC)CC)c34 |r,c:12| Show InChI InChI=1S/C20H26N4O/c1-4-24(5-2)20(25)22-14-10-16-15-7-6-8-17-19(15)13(11-21-17)9-18(16)23(3)12-14/h6-8,10-11,14,18,21H,4-5,9,12H2,1-3H3,(H,22,25)/t14-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury
Curated by PDSP Ki Database
| |
Biochem Pharmacol 45: 1003-9 (1993)
Article DOI: 10.1016/0006-2952(93)90243-p
BindingDB Entry DOI: 10.7270/Q2JH3JPG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cooperation Pharmaceutique Fran�aise
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-hydroxytryptamine 1A receptor |
J Med Chem 40: 952-60 (1997)
Article DOI: 10.1021/jm950759z
BindingDB Entry DOI: 10.7270/Q2HT2Q00 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50443182
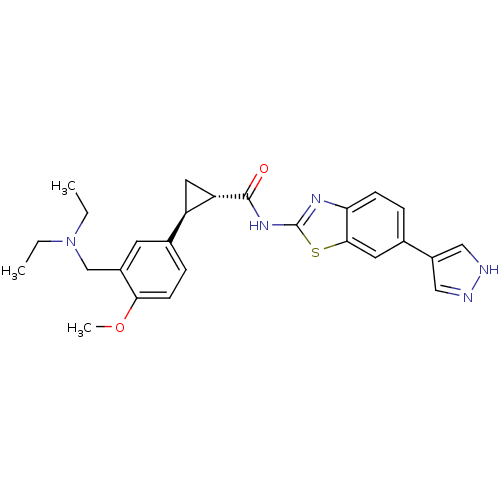
(CHEMBL3086538)Show SMILES CCN(CC)Cc1cc(ccc1OC)[C@H]1C[C@@H]1C(=O)Nc1nc2ccc(cc2s1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C26H29N5O2S/c1-4-31(5-2)15-18-10-17(7-9-23(18)33-3)20-12-21(20)25(32)30-26-29-22-8-6-16(11-24(22)34-26)19-13-27-28-14-19/h6-11,13-14,20-21H,4-5,12,15H2,1-3H3,(H,27,28)(H,29,30,32)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 23: 6331-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.069
BindingDB Entry DOI: 10.7270/Q2GX4D16 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50056944
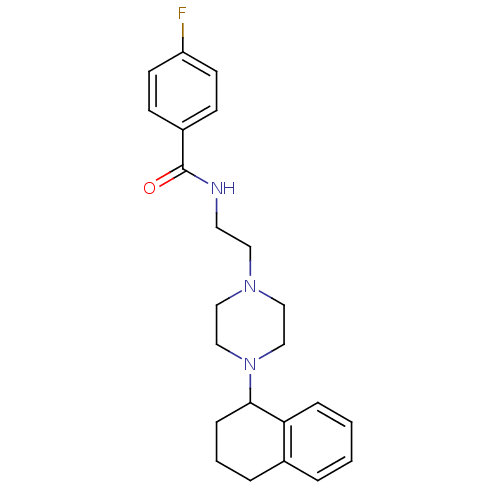
(4-Fluoro-N-{2-[4-(1,2,3,4-tetrahydro-naphthalen-1-...)Show SMILES Fc1ccc(cc1)C(=O)NCCN1CCN(CC1)C1CCCc2ccccc12 Show InChI InChI=1S/C23H28FN3O/c24-20-10-8-19(9-11-20)23(28)25-12-13-26-14-16-27(17-15-26)22-7-3-5-18-4-1-2-6-21(18)22/h1-2,4,6,8-11,22H,3,5,7,12-17H2,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cooperation Pharmaceutique Fran�aise
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor of rat hippocampal membranes |
J Med Chem 40: 952-60 (1997)
Article DOI: 10.1021/jm950759z
BindingDB Entry DOI: 10.7270/Q2HT2Q00 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cooperation Pharmaceutique Fran�aise
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor of rat hippocampal membranes |
J Med Chem 40: 952-60 (1997)
Article DOI: 10.1021/jm950759z
BindingDB Entry DOI: 10.7270/Q2HT2Q00 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50334150
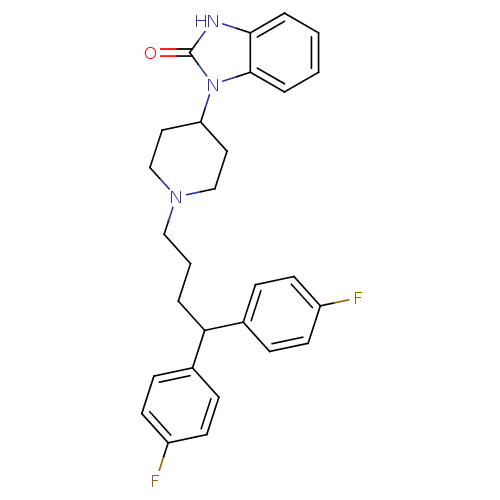
(1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cooperation Pharmaceutique Fran�aise
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Dopamine receptor D2 using [3H]raclopride (1.2 nM) ligand in striatum bovine was determined |
J Med Chem 40: 952-60 (1997)
Article DOI: 10.1021/jm950759z
BindingDB Entry DOI: 10.7270/Q2HT2Q00 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50443181

(CHEMBL3086535)Show SMILES CN(C)Cc1ccc(cc1)C1CC1C(=O)Nc1nc2ccc(cc2s1)-c1cn[nH]c1 Show InChI InChI=1S/C23H23N5OS/c1-28(2)13-14-3-5-15(6-4-14)18-10-19(18)22(29)27-23-26-20-8-7-16(9-21(20)30-23)17-11-24-25-12-17/h3-9,11-12,18-19H,10,13H2,1-2H3,(H,24,25)(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 23: 6331-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.069
BindingDB Entry DOI: 10.7270/Q2GX4D16 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1A adrenergic receptor
(CALF) | BDBM69602
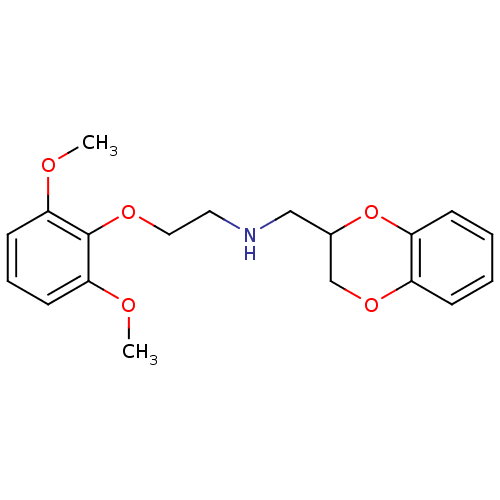
(2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...)Show InChI InChI=1S/C19H23NO5/c1-21-17-8-5-9-18(22-2)19(17)23-11-10-20-12-14-13-24-15-6-3-4-7-16(15)25-14/h3-9,14,20H,10-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cooperation Pharmaceutique Fran�aise
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Alpha-1 adrenergic receptor using [3H]prazosin 0.5 nM ligand in frontal cortex calf was determined |
J Med Chem 40: 952-60 (1997)
Article DOI: 10.1021/jm950759z
BindingDB Entry DOI: 10.7270/Q2HT2Q00 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50443180

(CHEMBL3086536)Show SMILES CN(C)Cc1ccc(cc1)[C@H]1C[C@@H]1C(=O)Nc1nc2ccc(cc2s1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C23H23N5OS/c1-28(2)13-14-3-5-15(6-4-14)18-10-19(18)22(29)27-23-26-20-8-7-16(9-21(20)30-23)17-11-24-25-12-17/h3-9,11-12,18-19H,10,13H2,1-2H3,(H,24,25)(H,26,27,29)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 23: 6331-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.069
BindingDB Entry DOI: 10.7270/Q2GX4D16 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50005118
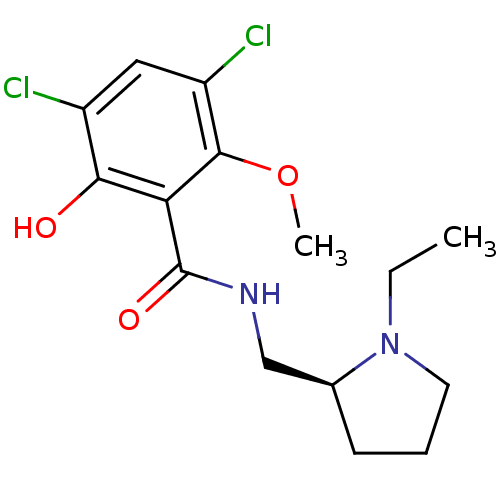
((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...)Show InChI InChI=1S/C15H20Cl2N2O3/c1-3-19-6-4-5-9(19)8-18-15(21)12-13(20)10(16)7-11(17)14(12)22-2/h7,9,20H,3-6,8H2,1-2H3,(H,18,21)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037070

(CHEMBL3355732)Show SMILES COc1ccc(cc1)-c1cc(Nc2cc(cc(N[C@H]3CC[C@H](O)CC3)n2)S(=O)(=O)c2ccccc2)n[nH]1 |r,wU:18.18,wD:21.22,(12.08,-20.69,;11.44,-19.28,;9.91,-19.13,;9.01,-20.38,;7.48,-20.22,;6.85,-18.81,;7.74,-17.57,;9.27,-17.72,;5.32,-18.66,;4.29,-19.8,;2.88,-19.17,;1.55,-19.95,;1.55,-21.49,;.22,-22.26,;.22,-23.8,;1.55,-24.57,;2.89,-23.8,;4.23,-24.57,;4.23,-26.11,;5.56,-26.87,;5.56,-28.41,;4.22,-29.18,;4.22,-30.72,;2.89,-28.41,;2.89,-26.87,;2.89,-22.25,;-1.12,-24.57,;-.36,-25.9,;-1.9,-25.89,;-2.45,-23.8,;-2.45,-22.26,;-3.78,-21.49,;-5.12,-22.26,;-5.1,-23.81,;-3.78,-24.57,;3.04,-17.65,;4.54,-17.32,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50002338
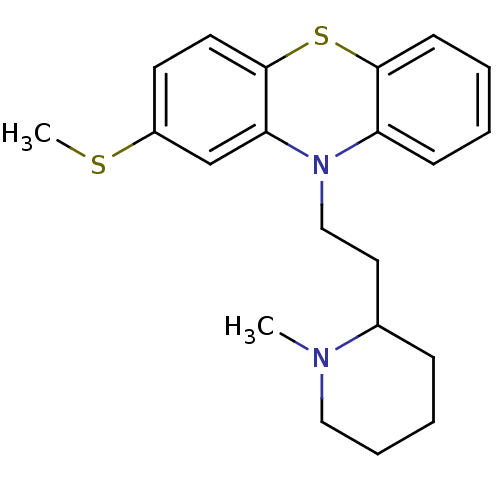
((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50415362
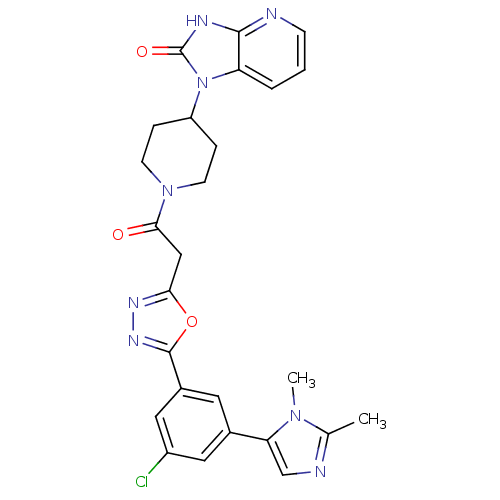
(CHEMBL601857)Show SMILES Cc1ncc(-c2cc(Cl)cc(c2)-c2nnc(CC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)o2)n1C Show InChI InChI=1S/C26H25ClN8O3/c1-15-29-14-21(33(15)2)16-10-17(12-18(27)11-16)25-32-31-22(38-25)13-23(36)34-8-5-19(6-9-34)35-20-4-3-7-28-24(20)30-26(35)37/h3-4,7,10-12,14,19H,5-6,8-9,13H2,1-2H3,(H,28,30,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... |
Bioorg Med Chem Lett 20: 1368-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.012
BindingDB Entry DOI: 10.7270/Q23T9JG4 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50001786

(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037074

(CHEMBL3355736)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)C2CCC2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(26.41,-16.6,;26.41,-15.06,;27.75,-14.29,;27.75,-12.74,;26.42,-11.99,;25.08,-12.75,;25.08,-14.28,;26.42,-10.45,;25.08,-9.68,;23.75,-10.45,;22.41,-9.68,;22.41,-8.14,;23.74,-7.36,;23.74,-5.82,;25.07,-5.05,;26.48,-5.68,;27.51,-4.53,;26.74,-3.2,;25.23,-3.53,;29.04,-4.69,;30.01,-5.89,;31.2,-4.92,;30.23,-3.72,;25.08,-8.13,;21.07,-10.45,;21.83,-11.78,;20.3,-11.77,;19.74,-9.68,;19.75,-8.14,;18.42,-7.37,;17.09,-8.14,;17.09,-9.68,;18.42,-10.45,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037071
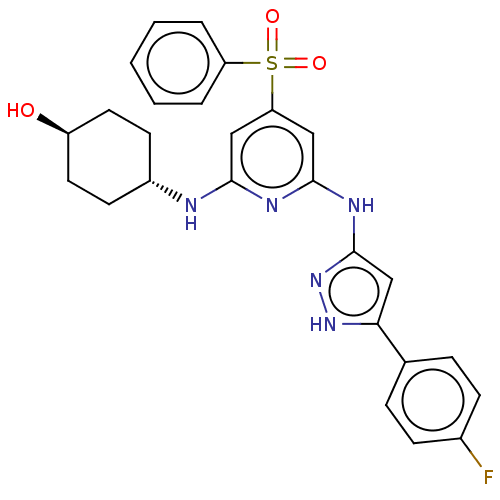
(CHEMBL3355733)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)-c2ccc(F)cc2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(22.41,-30.72,;22.41,-29.18,;23.75,-28.41,;23.75,-26.87,;22.42,-26.11,;21.08,-26.87,;21.08,-28.41,;22.42,-24.57,;21.08,-23.8,;19.75,-24.57,;18.41,-23.8,;18.41,-22.26,;19.74,-21.49,;19.74,-19.95,;21.07,-19.17,;22.48,-19.8,;23.51,-18.66,;22.74,-17.32,;21.23,-17.65,;25.04,-18.81,;25.67,-20.22,;27.2,-20.38,;28.1,-19.13,;29.63,-19.28,;27.46,-17.72,;25.94,-17.57,;21.08,-22.25,;17.07,-24.57,;17.83,-25.9,;16.3,-25.89,;15.74,-23.8,;15.75,-22.26,;14.42,-21.49,;13.09,-22.26,;13.09,-23.81,;14.42,-24.57,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50005118

((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...)Show InChI InChI=1S/C15H20Cl2N2O3/c1-3-19-6-4-5-9(19)8-18-15(21)12-13(20)10(16)7-11(17)14(12)22-2/h7,9,20H,3-6,8H2,1-2H3,(H,18,21)/t9-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cooperation Pharmaceutique Fran�aise
Curated by ChEMBL
| Assay Description
Binding affinity against human 5-hydroxytryptamine 1A receptor |
J Med Chem 40: 952-60 (1997)
Article DOI: 10.1021/jm950759z
BindingDB Entry DOI: 10.7270/Q2HT2Q00 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50443178
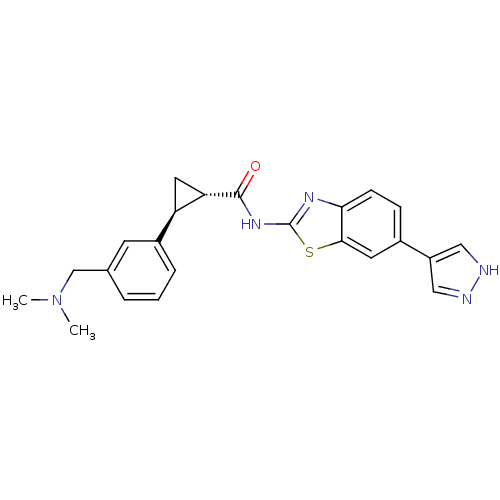
(CHEMBL3086537)Show SMILES CN(C)Cc1cccc(c1)[C@H]1C[C@@H]1C(=O)Nc1nc2ccc(cc2s1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C23H23N5OS/c1-28(2)13-14-4-3-5-16(8-14)18-10-19(18)22(29)27-23-26-20-7-6-15(9-21(20)30-23)17-11-24-25-12-17/h3-9,11-12,18-19H,10,13H2,1-2H3,(H,24,25)(H,26,27,29)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 23: 6331-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.069
BindingDB Entry DOI: 10.7270/Q2GX4D16 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50002338

((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037068
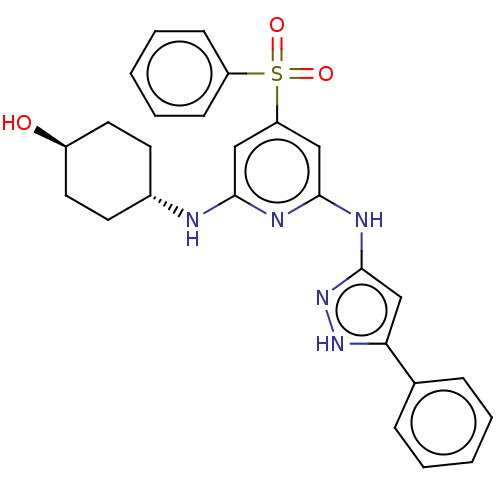
(CHEMBL3355730)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1cc(cc(Nc2cc([nH]n2)-c2ccccc2)n1)S(=O)(=O)c1ccccc1 |r,wU:4.7,wD:1.0,(6.57,-15.01,;6.58,-13.47,;7.91,-12.7,;7.92,-11.16,;6.59,-10.4,;5.25,-11.16,;5.25,-12.7,;6.58,-8.86,;5.25,-8.09,;3.91,-8.86,;2.58,-8.09,;2.58,-6.55,;3.91,-5.78,;3.9,-4.24,;5.23,-3.46,;6.65,-4.09,;7.68,-2.95,;6.9,-1.62,;5.4,-1.94,;9.21,-3.1,;9.83,-4.51,;11.37,-4.67,;12.27,-3.42,;11.63,-2.01,;10.1,-1.86,;5.24,-6.54,;1.24,-8.86,;2,-10.19,;.47,-10.18,;-.1,-8.09,;-.09,-6.55,;-1.42,-5.78,;-2.76,-6.55,;-2.75,-8.1,;-1.42,-8.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM11638

(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50443177
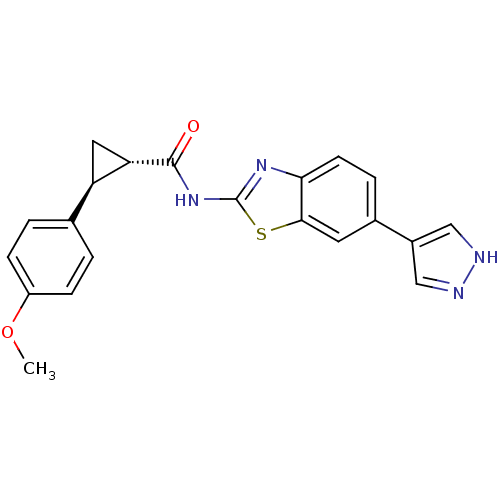
(CHEMBL3086533)Show SMILES COc1ccc(cc1)[C@H]1C[C@@H]1C(=O)Nc1nc2ccc(cc2s1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C21H18N4O2S/c1-27-15-5-2-12(3-6-15)16-9-17(16)20(26)25-21-24-18-7-4-13(8-19(18)28-21)14-10-22-23-11-14/h2-8,10-11,16-17H,9H2,1H3,(H,22,23)(H,24,25,26)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Inhibition of full length GST-tagged ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 23: 6331-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.069
BindingDB Entry DOI: 10.7270/Q2GX4D16 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50415365
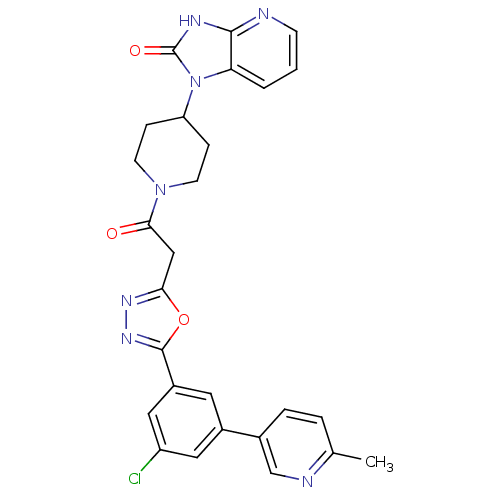
(CHEMBL609757)Show SMILES Cc1ccc(cn1)-c1cc(Cl)cc(c1)-c1nnc(CC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)o1 Show InChI InChI=1S/C27H24ClN7O3/c1-16-4-5-17(15-30-16)18-11-19(13-20(28)12-18)26-33-32-23(38-26)14-24(36)34-9-6-21(7-10-34)35-22-3-2-8-29-25(22)31-27(35)37/h2-5,8,11-13,15,21H,6-7,9-10,14H2,1H3,(H,29,31,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... |
Bioorg Med Chem Lett 20: 1368-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.012
BindingDB Entry DOI: 10.7270/Q23T9JG4 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50415354
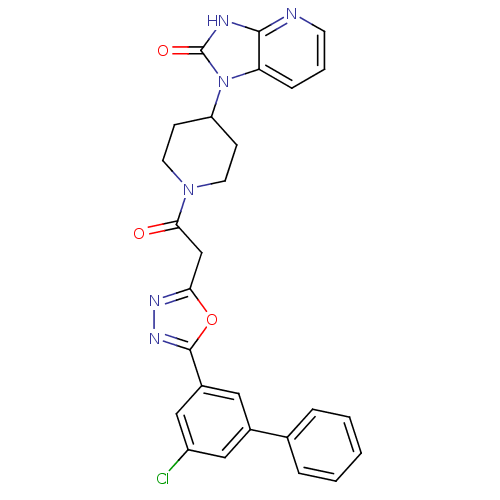
(CHEMBL600836)Show SMILES Clc1cc(cc(c1)-c1ccccc1)-c1nnc(CC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)o1 Show InChI InChI=1S/C27H23ClN6O3/c28-20-14-18(17-5-2-1-3-6-17)13-19(15-20)26-32-31-23(37-26)16-24(35)33-11-8-21(9-12-33)34-22-7-4-10-29-25(22)30-27(34)36/h1-7,10,13-15,21H,8-9,11-12,16H2,(H,29,30,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... |
Bioorg Med Chem Lett 20: 1368-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.012
BindingDB Entry DOI: 10.7270/Q23T9JG4 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Reading
Curated by PDSP Ki Database
| |
Pharmacol Rev 53: 119-33 (2001)
BindingDB Entry DOI: 10.7270/Q2BG2MJX |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50415360
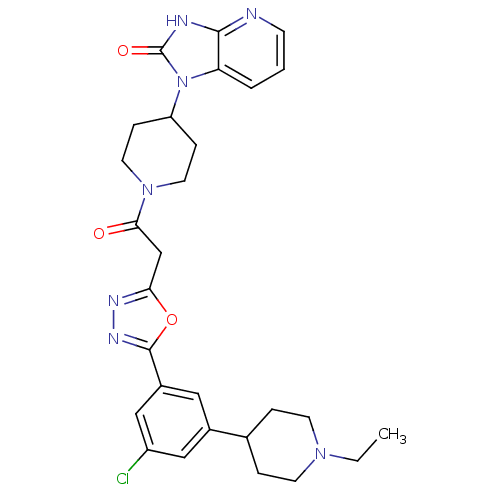
(CHEMBL610062)Show SMILES CCN1CCC(CC1)c1cc(Cl)cc(c1)-c1nnc(CC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)o1 Show InChI InChI=1S/C28H32ClN7O3/c1-2-34-10-5-18(6-11-34)19-14-20(16-21(29)15-19)27-33-32-24(39-27)17-25(37)35-12-7-22(8-13-35)36-23-4-3-9-30-26(23)31-28(36)38/h3-4,9,14-16,18,22H,2,5-8,10-13,17H2,1H3,(H,30,31,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... |
Bioorg Med Chem Lett 20: 1368-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.012
BindingDB Entry DOI: 10.7270/Q23T9JG4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury
Curated by PDSP Ki Database
| |
Biochem Pharmacol 45: 1003-9 (1993)
Article DOI: 10.1016/0006-2952(93)90243-p
BindingDB Entry DOI: 10.7270/Q2JH3JPG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM31005
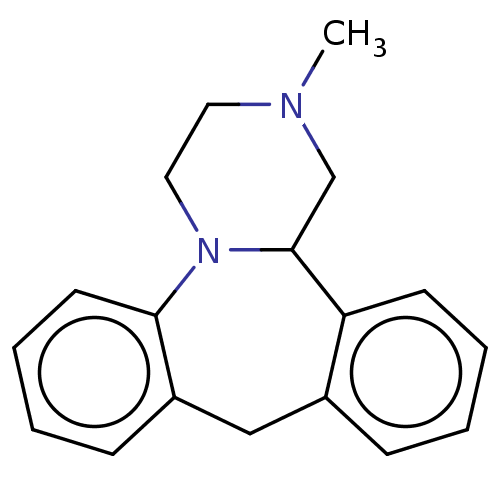
(2-methyl-1,2,3,4,10,14b-hexahydrodibenzo[c,f]pyraz...)Show InChI InChI=1S/C18H20N2.ClH/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19;/h2-9,18H,10-13H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cooperation Pharmaceutique Fran�aise
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards 5-hydroxytryptamine 1C receptor using [3H]mesulergine (1.2 nM) ligand in choroid Plexus pig was determined |
J Med Chem 40: 952-60 (1997)
Article DOI: 10.1021/jm950759z
BindingDB Entry DOI: 10.7270/Q2HT2Q00 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50005127
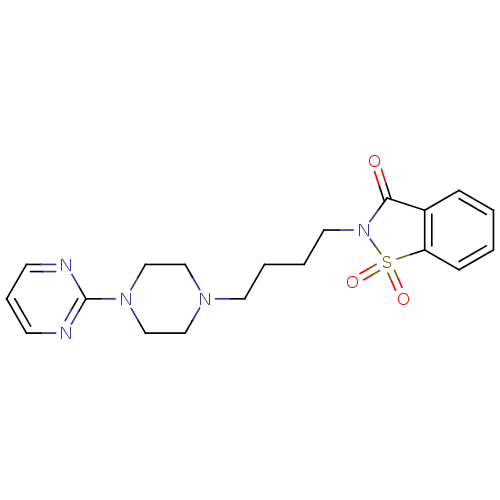
(1,1-Dioxo-2-[4-(4-pyrimidin-2-yl-piperazin-1-yl)-b...)Show SMILES O=C1N(CCCCN2CCN(CC2)c2ncccn2)S(=O)(=O)c2ccccc12 Show InChI InChI=1S/C19H23N5O3S/c25-18-16-6-1-2-7-17(16)28(26,27)24(18)11-4-3-10-22-12-14-23(15-13-22)19-20-8-5-9-21-19/h1-2,5-9H,3-4,10-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury
Curated by PDSP Ki Database
| |
Biochem Pharmacol 45: 1003-9 (1993)
Article DOI: 10.1016/0006-2952(93)90243-p
BindingDB Entry DOI: 10.7270/Q2JH3JPG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Canterbury
Curated by PDSP Ki Database
| |
Biochem Pharmacol 45: 1003-9 (1993)
Article DOI: 10.1016/0006-2952(93)90243-p
BindingDB Entry DOI: 10.7270/Q2JH3JPG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50415359
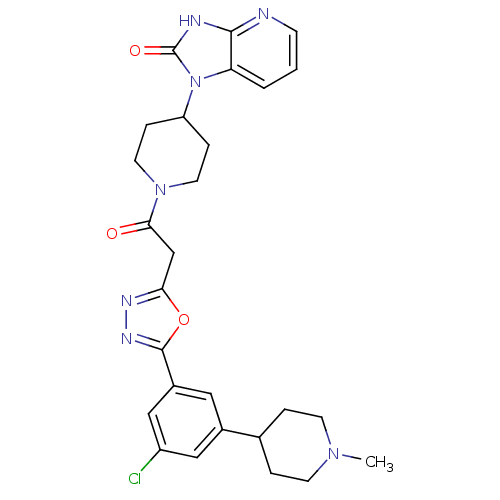
(CHEMBL601854)Show SMILES CN1CCC(CC1)c1cc(Cl)cc(c1)-c1nnc(CC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)o1 Show InChI InChI=1S/C27H30ClN7O3/c1-33-9-4-17(5-10-33)18-13-19(15-20(28)14-18)26-32-31-23(38-26)16-24(36)34-11-6-21(7-12-34)35-22-3-2-8-29-25(22)30-27(35)37/h2-3,8,13-15,17,21H,4-7,9-12,16H2,1H3,(H,29,30,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... |
Bioorg Med Chem Lett 20: 1368-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.012
BindingDB Entry DOI: 10.7270/Q23T9JG4 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50415352

(CHEMBL600636)Show SMILES FC(F)(F)c1cc(Cl)cc(c1)-c1nnc(CC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)o1 Show InChI InChI=1S/C22H18ClF3N6O3/c23-14-9-12(8-13(10-14)22(24,25)26)20-30-29-17(35-20)11-18(33)31-6-3-15(4-7-31)32-16-2-1-5-27-19(16)28-21(32)34/h1-2,5,8-10,15H,3-4,6-7,11H2,(H,27,28,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... |
Bioorg Med Chem Lett 20: 1368-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.012
BindingDB Entry DOI: 10.7270/Q23T9JG4 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50415361
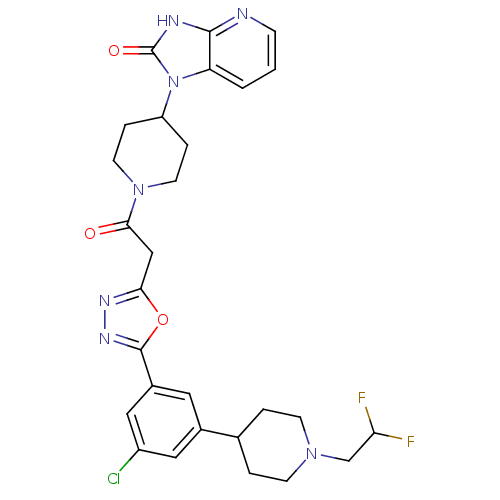
(CHEMBL592649)Show SMILES FC(F)CN1CCC(CC1)c1cc(Cl)cc(c1)-c1nnc(CC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)o1 Show InChI InChI=1S/C28H30ClF2N7O3/c29-20-13-18(17-3-8-36(9-4-17)16-23(30)31)12-19(14-20)27-35-34-24(41-27)15-25(39)37-10-5-21(6-11-37)38-22-2-1-7-32-26(22)33-28(38)40/h1-2,7,12-14,17,21,23H,3-6,8-11,15-16H2,(H,32,33,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... |
Bioorg Med Chem Lett 20: 1368-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.012
BindingDB Entry DOI: 10.7270/Q23T9JG4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50056945

(4-Chloro-N-{2-[4-(1,2,3,4-tetrahydro-naphthalen-1-...)Show SMILES Clc1ccc(cc1)C(=O)NCCN1CCN(CC1)C1CCCc2ccccc12 Show InChI InChI=1S/C23H28ClN3O/c24-20-10-8-19(9-11-20)23(28)25-12-13-26-14-16-27(17-15-26)22-7-3-5-18-4-1-2-6-21(18)22/h1-2,4,6,8-11,22H,3,5,7,12-17H2,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cooperation Pharmaceutique Fran�aise
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor of rat hippocampal membranes |
J Med Chem 40: 952-60 (1997)
Article DOI: 10.1021/jm950759z
BindingDB Entry DOI: 10.7270/Q2HT2Q00 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50037063
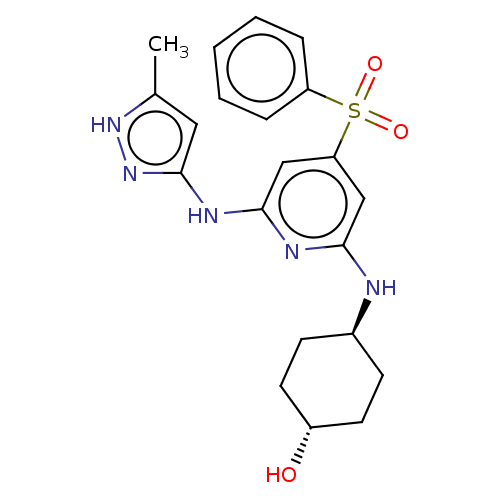
(CHEMBL3355726)Show SMILES Cc1cc(Nc2cc(cc(N[C@H]3CC[C@H](O)CC3)n2)S(=O)(=O)c2ccccc2)n[nH]1 |r,wU:11.10,wD:14.14,(15.1,-16.36,;13.56,-16.21,;12.54,-17.35,;11.12,-16.72,;9.79,-17.5,;9.8,-19.04,;8.47,-19.81,;8.46,-21.35,;9.8,-22.12,;11.14,-21.35,;12.47,-22.12,;12.47,-23.66,;13.81,-24.42,;13.8,-25.96,;12.47,-26.73,;12.46,-28.27,;11.13,-25.96,;11.14,-24.42,;11.13,-19.8,;7.12,-22.12,;7.89,-23.45,;6.35,-23.44,;5.8,-21.35,;5.81,-19.81,;4.48,-19.04,;3.14,-19.81,;3.14,-21.36,;4.48,-22.12,;11.28,-15.2,;12.79,-14.88,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50442147
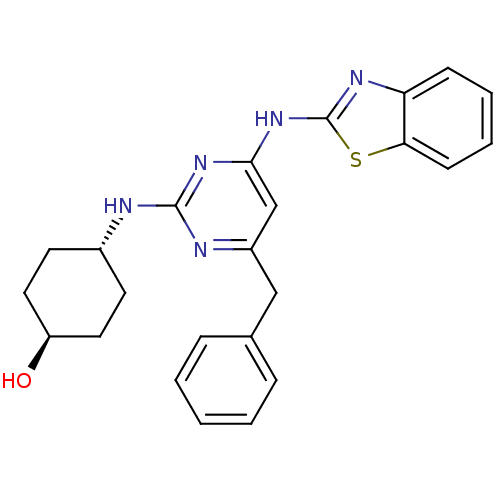
(CHEMBL2441269)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nc(Cc2ccccc2)cc(Nc2nc3ccccc3s2)n1 |r,wU:1.0,wD:4.7,(34.33,-8.73,;33,-9.52,;33.02,-11.06,;31.7,-11.84,;30.36,-11.09,;30.34,-9.56,;31.66,-8.77,;29.03,-11.88,;27.69,-11.14,;26.37,-11.93,;25.03,-11.18,;23.69,-11.97,;22.36,-11.2,;22.37,-9.66,;21.03,-8.89,;19.69,-9.66,;19.69,-11.2,;21.03,-11.97,;25,-9.64,;26.32,-8.85,;26.29,-7.31,;27.5,-6.37,;27.47,-4.83,;28.92,-4.33,;29.52,-2.9,;31.04,-2.71,;31.98,-3.93,;31.38,-5.35,;29.86,-5.55,;28.98,-6.82,;27.67,-9.6,)| Show InChI InChI=1S/C24H25N5OS/c30-19-12-10-17(11-13-19)25-23-26-18(14-16-6-2-1-3-7-16)15-22(28-23)29-24-27-20-8-4-5-9-21(20)31-24/h1-9,15,17,19,30H,10-14H2,(H2,25,26,27,28,29)/t17-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec UK Ltd
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged full-length ITK (unknown origin) using Ac-EFPIYDFLPAKKK-NH2 as substrate after 35 mins by LC/MS analysis |
Bioorg Med Chem Lett 24: 5818-23 (2014)
Article DOI: 10.1016/j.bmcl.2014.10.020
BindingDB Entry DOI: 10.7270/Q2NK3GNT |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50415358
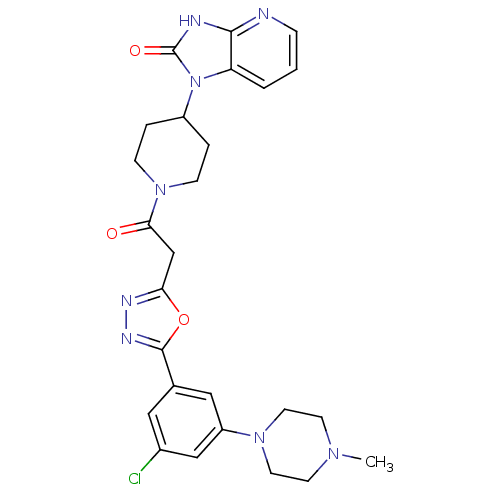
(CHEMBL601639)Show SMILES CN1CCN(CC1)c1cc(Cl)cc(c1)-c1nnc(CC(=O)N2CCC(CC2)n2c3cccnc3[nH]c2=O)o1 Show InChI InChI=1S/C26H29ClN8O3/c1-32-9-11-33(12-10-32)20-14-17(13-18(27)15-20)25-31-30-22(38-25)16-23(36)34-7-4-19(5-8-34)35-21-3-2-6-28-24(21)29-26(35)37/h2-3,6,13-15,19H,4-5,7-12,16H2,1H3,(H,28,29,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant CGRP receptor expressed in HEK293 cells assessed as inhibition of CGRP-stimulated increase of intracellular ... |
Bioorg Med Chem Lett 20: 1368-72 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.012
BindingDB Entry DOI: 10.7270/Q23T9JG4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data