

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50381952 (CHEMBL2022933) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50381961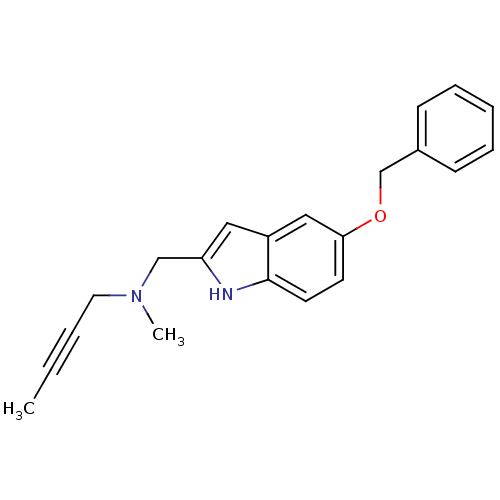 (CHEMBL2022928) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50381960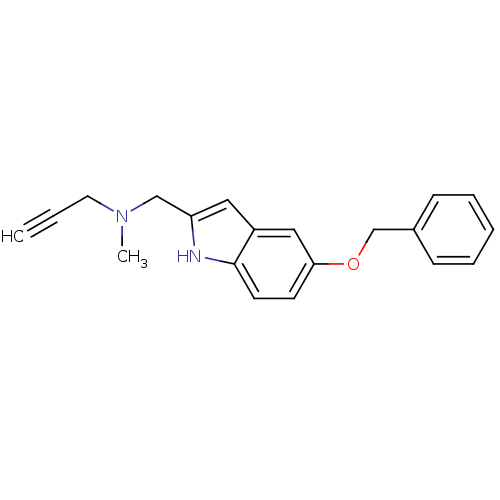 (CHEMBL2022927) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-B in rat liver homogenates using [14C]-phenylethylamine as substrate preincubated for 30 mins by liquid scintillation... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50381960 (CHEMBL2022927) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-A in rat liver homogenates using [14C]-(5-hydroxy-triptamine) as substrate preincubated for 30 mins by liquid scintil... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333148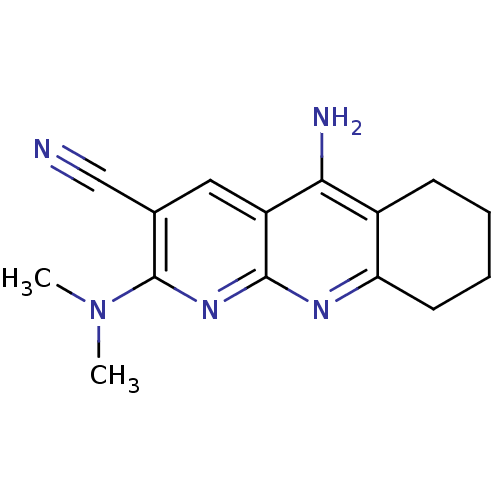 (5-Amino-2-(dimethylamino)-6,7,8,9-tetrahydrobenzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50381959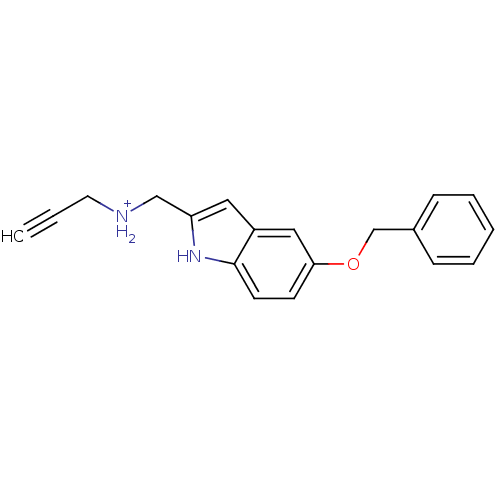 (CHEMBL2021938) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-B in rat liver homogenates using [14C]-phenylethylamine as substrate preincubated for 30 mins by liquid scintillation... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50381952 (CHEMBL2022933) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333150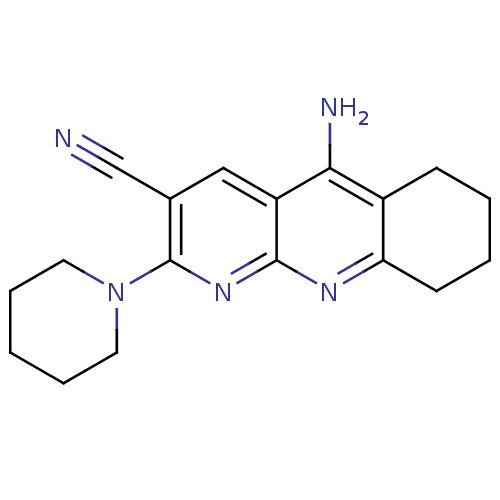 (5-Amino-2-piperidin-1-yl-6,7,8,9-tetrahydrobenzo[1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50381964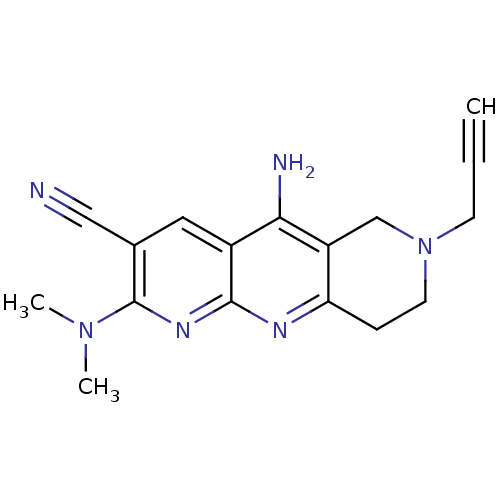 (CHEMBL2022932) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333151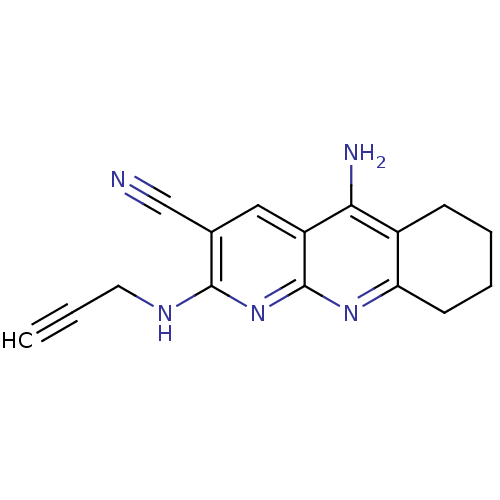 (5-Amino-2-(prop-2-yn-1-ylamino)-6,7,8,9-tetrahydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50381958 (CHEMBL2022926) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-B in rat liver homogenates using [14C]-phenylethylamine as substrate preincubated for 30 mins by liquid scintillation... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50381961 (CHEMBL2022928) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-B in rat liver homogenates using [14C]-phenylethylamine as substrate preincubated for 30 mins by liquid scintillation... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-A in rat liver homogenates using [14C]-(5-hydroxy-triptamine) as substrate preincubated for 30 mins by liquid scintil... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333149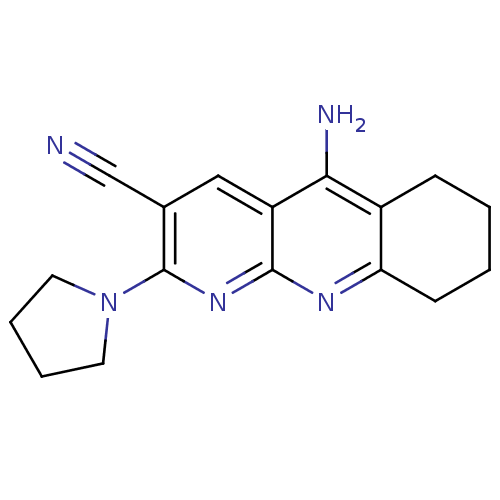 (5-Amino-2-pyrrolidin-1-yl-6,7,8,9-tetrahydrobenzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333154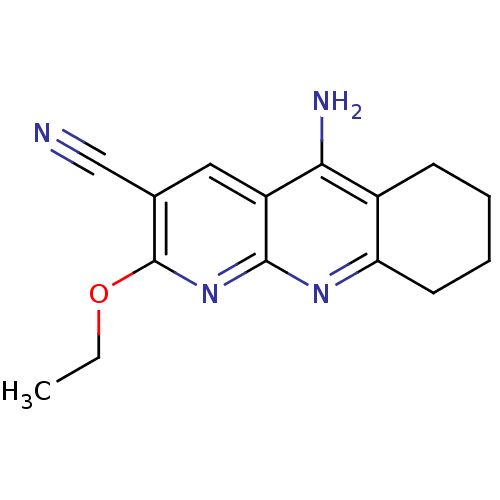 (5-Amino-2-(ethyloxy)-6,7,8,9-tetrahydrobenzo[1,8-b...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333153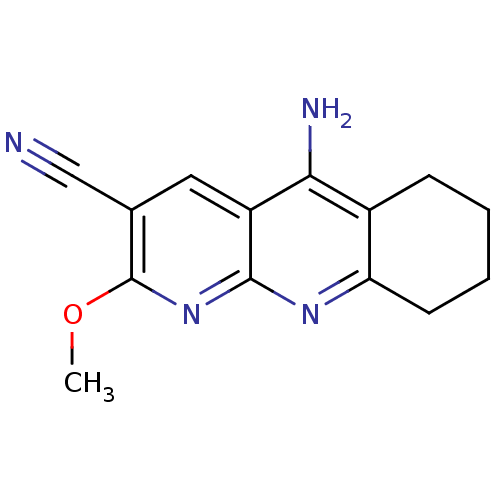 (5-Amino-2-(methyloxy)-6,7,8,9-tetrahydrobenzo[1,8-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50359390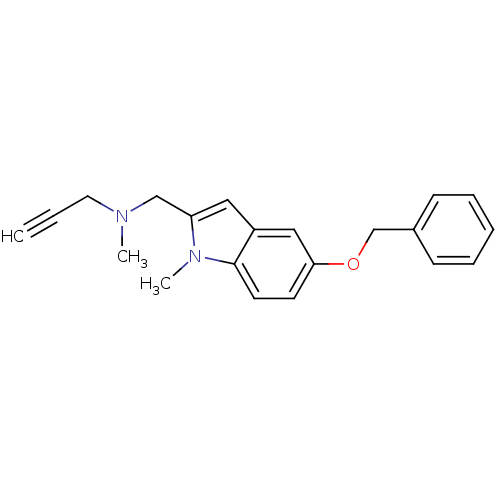 (CHEMBL1929418 | CHEMBL2022924) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-B in rat liver homogenates using [14C]-phenylethylamine as substrate preincubated for 30 mins by liquid scintillation... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50381957 (CHEMBL2022925) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-B in rat liver homogenates using [14C]-phenylethylamine as substrate preincubated for 30 mins by liquid scintillation... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50381954 (CHEMBL2022934) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333155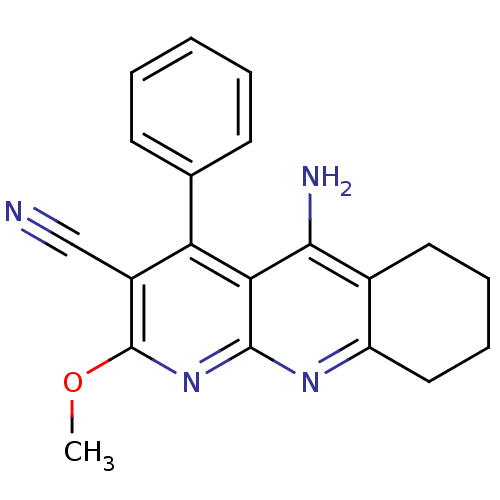 (5-Amino-2-methoxy-4-phenyl-6,7,8,9-tetrahydrobenzo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333152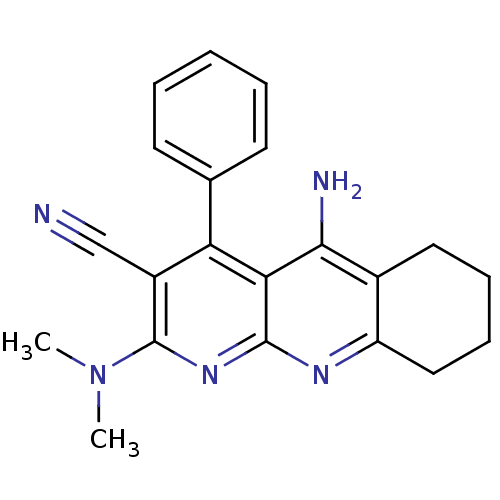 (5-Amino-2-(dimethylamino)-4-phenyl-6,7,8,9-tetrahy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50359390 (CHEMBL1929418 | CHEMBL2022924) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-A in rat liver homogenates using [14C]-(5-hydroxy-triptamine) as substrate preincubated for 30 mins by liquid scintil... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333148 (5-Amino-2-(dimethylamino)-6,7,8,9-tetrahydrobenzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-B in rat liver homogenates using [14C]-phenylethylamine as substrate preincubated for 30 mins by liquid scintillation... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50381961 (CHEMBL2022928) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-A in rat liver homogenates using [14C]-(5-hydroxy-triptamine) as substrate preincubated for 30 mins by liquid scintil... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50381957 (CHEMBL2022925) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-A in rat liver homogenates using [14C]-(5-hydroxy-triptamine) as substrate preincubated for 30 mins by liquid scintil... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333149 (5-Amino-2-pyrrolidin-1-yl-6,7,8,9-tetrahydrobenzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50381958 (CHEMBL2022926) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-A in rat liver homogenates using [14C]-(5-hydroxy-triptamine) as substrate preincubated for 30 mins by liquid scintil... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333159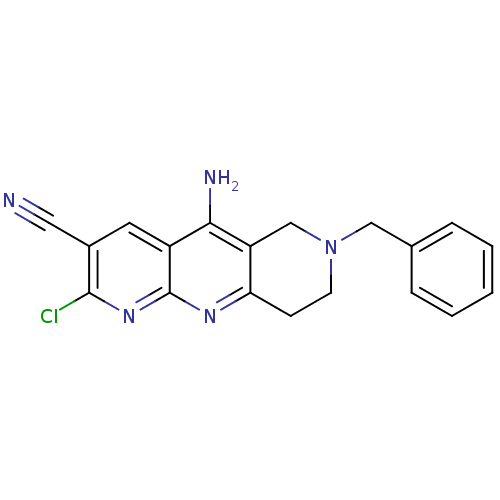 (5-Amino-7-benzyl-2-chloro-6,7,8,9-tetrahydropyrido...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333158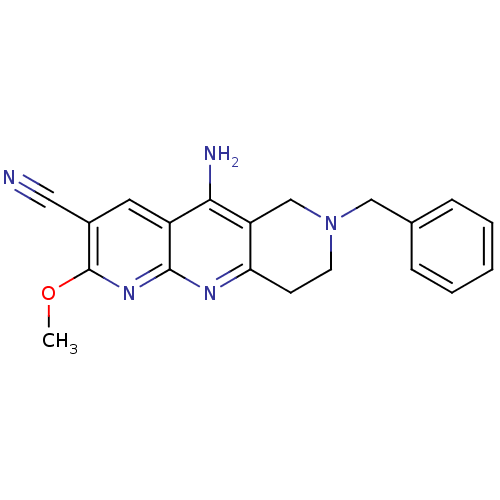 (5-Amino-7-benzyl-2-methoxy-6,7,8,9-tetrahydropyrid...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50381963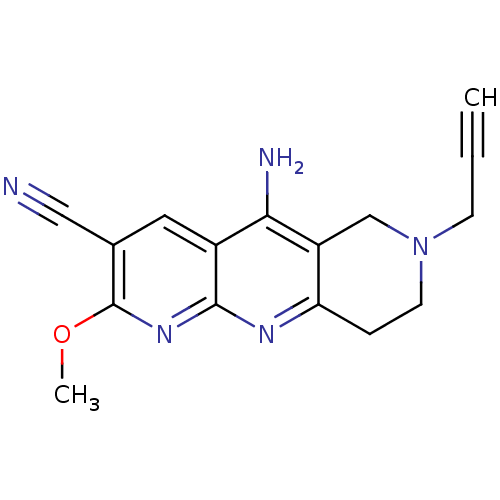 (CHEMBL2022931) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50381952 (CHEMBL2022933) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333154 (5-Amino-2-(ethyloxy)-6,7,8,9-tetrahydrobenzo[1,8-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333150 (5-Amino-2-piperidin-1-yl-6,7,8,9-tetrahydrobenzo[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50381959 (CHEMBL2021938) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-A in rat liver homogenates using [14C]-(5-hydroxy-triptamine) as substrate preincubated for 30 mins by liquid scintil... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50381962 (CHEMBL2022930) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50381953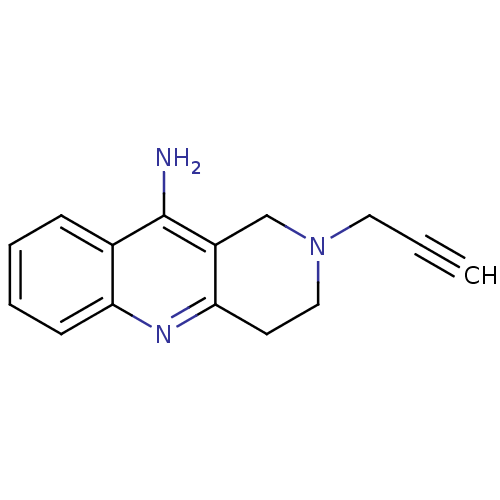 (CHEMBL2022929) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333151 (5-Amino-2-(prop-2-yn-1-ylamino)-6,7,8,9-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333153 (5-Amino-2-(methyloxy)-6,7,8,9-tetrahydrobenzo[1,8-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50333151 (5-Amino-2-(prop-2-yn-1-ylamino)-6,7,8,9-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50333150 (5-Amino-2-piperidin-1-yl-6,7,8,9-tetrahydrobenzo[1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50333152 (5-Amino-2-(dimethylamino)-4-phenyl-6,7,8,9-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocytes AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 105 total ) | Next | Last >> |