 Found 275 hits with Last Name = 'victor' and Initial = 'f'
Found 275 hits with Last Name = 'victor' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86492

(CAS_170713-75-4 | NSC_6324645 | Nociceptin)Show SMILES [#6]-[#6](-[#8])-[#6](-[#7]-[#6](=O)-[#6](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86491

(DiPOA | [8-(3,3-Diphenyl-propyl)-4-oxo-1-phenyl-1,...)Show SMILES OC(=O)CN1CN(c2ccccc2)C2(CCN(CCC(c3ccccc3)c3ccccc3)CC2)C1=O Show InChI InChI=1S/C30H33N3O3/c34-28(35)22-32-23-33(26-14-8-3-9-15-26)30(29(32)36)17-20-31(21-18-30)19-16-27(24-10-4-1-5-11-24)25-12-6-2-7-13-25/h1-15,27H,16-23H2,(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137733
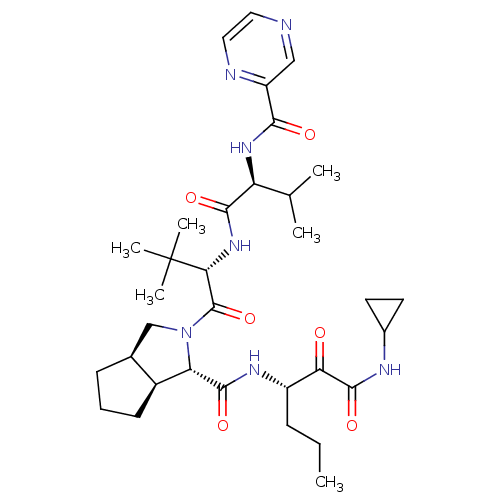
((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22-,24-,25-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86258
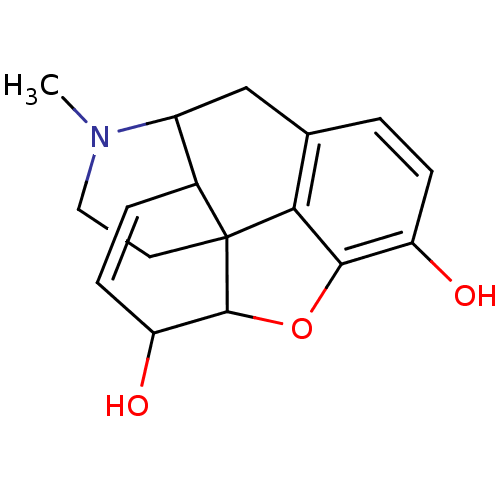
(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50130461
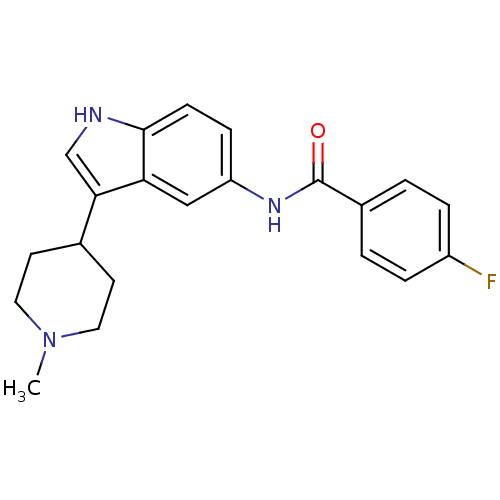
(4-Fluoro-N-[3-(1-methyl-4-piperidinyl)-1H-indol-5-...)Show SMILES CN1CCC(CC1)c1c[nH]c2ccc(NC(=O)c3ccc(F)cc3)cc12 Show InChI InChI=1S/C21H22FN3O/c1-25-10-8-14(9-11-25)19-13-23-20-7-6-17(12-18(19)20)24-21(26)15-2-4-16(22)5-3-15/h2-7,12-14,23H,8-11H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1F receptor by radioligand binding assay |
Bioorg Med Chem Lett 25: 4337-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.042
BindingDB Entry DOI: 10.7270/Q2NC630T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86493
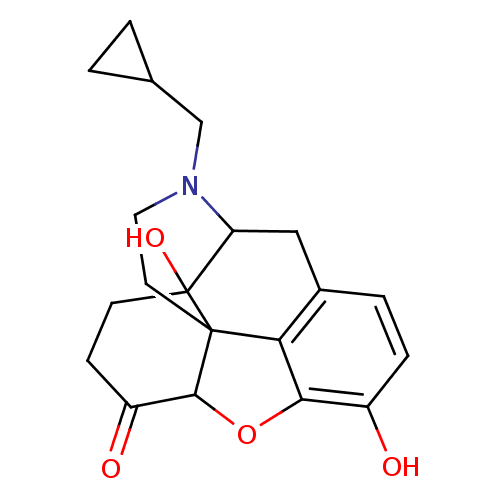
(CAS_27943 | NALTREXONE-HCl | NSC_27943 | Naltrexon...)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(=O)CCC35O |TLB:22:23:7.12.13:5.4.18,THB:24:23:7.12.13:5.4.18| Show InChI InChI=1S/C20H23NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,15,18,22,24H,1-2,5-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM21130
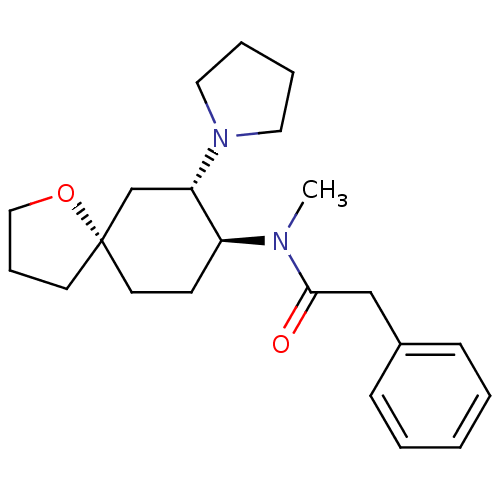
(N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C22H32N2O2/c1-23(21(25)16-18-8-3-2-4-9-18)19-10-12-22(11-7-15-26-22)17-20(19)24-13-5-6-14-24/h2-4,8-9,19-20H,5-7,10-17H2,1H3/t19-,20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137736

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC(C)CC Show InChI InChI=1S/C34H53N7O6/c1-9-12-23(27(42)32(46)37-20(5)10-2)38-31(45)26-22-14-11-13-21(22)18-41(26)33(47)28(34(6,7)8)40-30(44)25(19(3)4)39-29(43)24-17-35-15-16-36-24/h15-17,19-23,25-26,28H,9-14,18H2,1-8H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t20?,21-,22-,23-,25-,26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137730
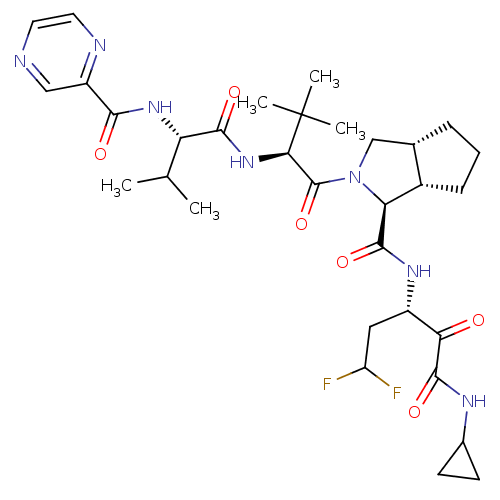
((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CC(C)[C@H](NC(=O)c1cnccn1)C(=O)N[C@H](C(=O)N1C[C@@H]2CCC[C@@H]2[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)C(=O)NC1CC1)C(C)(C)C Show InChI InChI=1S/C32H45F2N7O6/c1-16(2)23(39-27(43)21-14-35-11-12-36-21)28(44)40-26(32(3,4)5)31(47)41-15-17-7-6-8-19(17)24(41)29(45)38-20(13-22(33)34)25(42)30(46)37-18-9-10-18/h11-12,14,16-20,22-24,26H,6-10,13,15H2,1-5H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t17-,19-,20-,23-,24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50119543
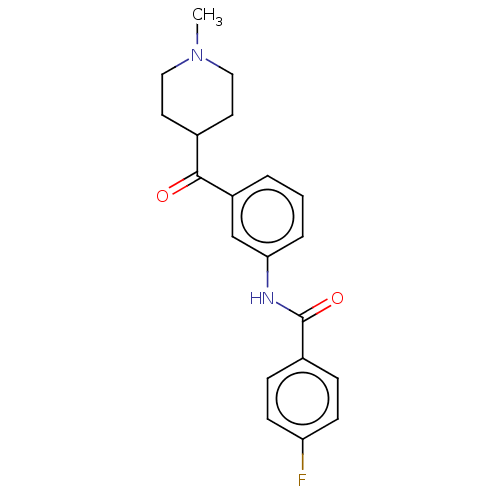
(CHEMBL3617549)Show SMILES CN1CCC(CC1)C(=O)c1cccc(NC(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C20H21FN2O2/c1-23-11-9-14(10-12-23)19(24)16-3-2-4-18(13-16)22-20(25)15-5-7-17(21)8-6-15/h2-8,13-14H,9-12H2,1H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1F receptor by radioligand binding assay |
Bioorg Med Chem Lett 25: 4337-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.042
BindingDB Entry DOI: 10.7270/Q2NC630T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50119542

(CHEMBL3617550)Show SMILES CN1CCC(CC1)C(=O)c1cccc(NC(=O)c2ccc(F)cc2)c1F Show InChI InChI=1S/C20H20F2N2O2/c1-24-11-9-13(10-12-24)19(25)16-3-2-4-17(18(16)22)23-20(26)14-5-7-15(21)8-6-14/h2-8,13H,9-12H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1F receptor by radioligand binding assay |
Bioorg Med Chem Lett 25: 4337-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.042
BindingDB Entry DOI: 10.7270/Q2NC630T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50119532
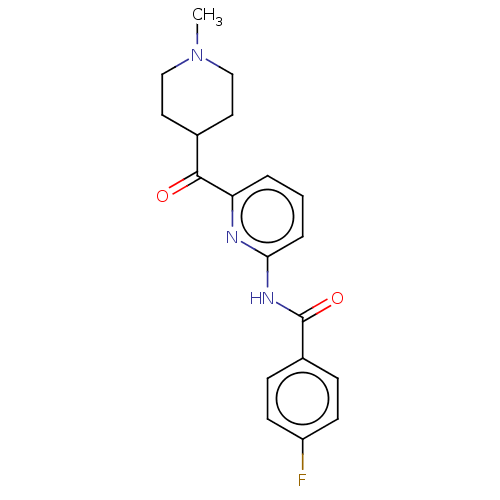
(CHEMBL3617557)Show SMILES CN1CCC(CC1)C(=O)c1cccc(NC(=O)c2ccc(F)cc2)n1 Show InChI InChI=1S/C19H20FN3O2/c1-23-11-9-13(10-12-23)18(24)16-3-2-4-17(21-16)22-19(25)14-5-7-15(20)8-6-14/h2-8,13H,9-12H2,1H3,(H,21,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1F receptor by radioligand binding assay |
Bioorg Med Chem Lett 25: 4337-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.042
BindingDB Entry DOI: 10.7270/Q2NC630T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin L |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137724
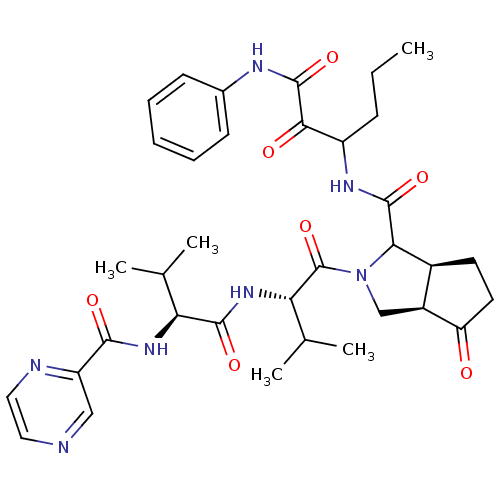
((3aR,5S)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyraz...)Show SMILES CCCC(NC(=O)C1[C@H]2CCC(=O)[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H45N7O7/c1-6-10-24(30(44)34(48)38-21-11-8-7-9-12-21)39-33(47)29-22-13-14-26(43)23(22)18-42(29)35(49)28(20(4)5)41-32(46)27(19(2)3)40-31(45)25-17-36-15-16-37-25/h7-9,11-12,15-17,19-20,22-24,27-29H,6,10,13-14,18H2,1-5H3,(H,38,48)(H,39,47)(H,40,45)(H,41,46)/t22-,23-,24?,27-,28-,29?/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 251-6 (2003)
BindingDB Entry DOI: 10.7270/Q24B30R9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152750
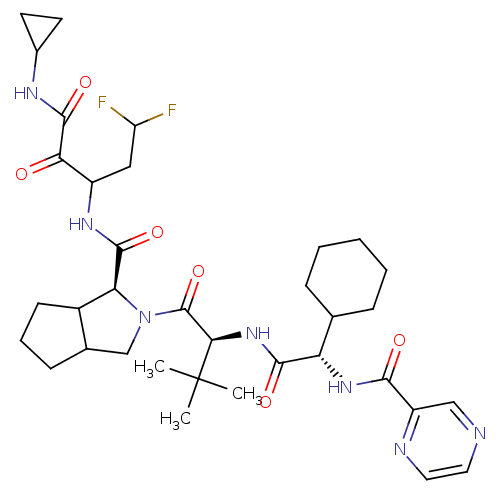
(2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carbonyl...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(=O)N1CC2CCCC2[C@H]1C(=O)NC(CC(F)F)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C35H49F2N7O6/c1-35(2,3)29(43-31(47)26(19-8-5-4-6-9-19)42-30(46)24-17-38-14-15-39-24)34(50)44-18-20-10-7-11-22(20)27(44)32(48)41-23(16-25(36)37)28(45)33(49)40-21-12-13-21/h14-15,17,19-23,25-27,29H,4-13,16,18H2,1-3H3,(H,40,49)(H,41,48)(H,42,46)(H,43,47)/t20?,22?,23?,26-,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50130461

(4-Fluoro-N-[3-(1-methyl-4-piperidinyl)-1H-indol-5-...)Show SMILES CN1CCC(CC1)c1c[nH]c2ccc(NC(=O)c3ccc(F)cc3)cc12 Show InChI InChI=1S/C21H22FN3O/c1-25-10-8-14(9-11-25)19-13-23-20-7-6-17(12-18(19)20)24-21(26)15-2-4-16(22)5-3-15/h2-7,12-14,23H,8-11H2,1H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1A receptor (unknown origin) |
Bioorg Med Chem Lett 25: 4337-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.042
BindingDB Entry DOI: 10.7270/Q2NC630T |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137747
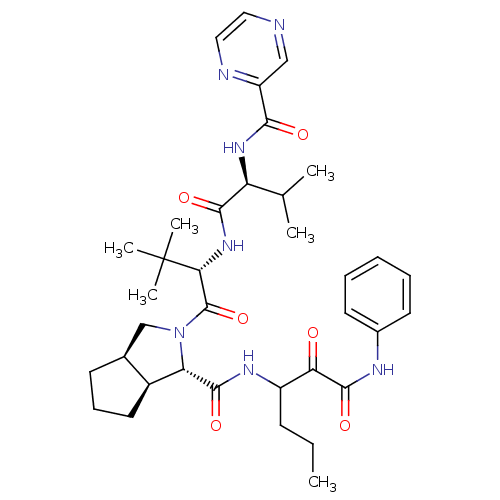
((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H49N7O6/c1-7-12-25(29(44)34(48)39-23-14-9-8-10-15-23)40-33(47)28-24-16-11-13-22(24)20-43(28)35(49)30(36(4,5)6)42-32(46)27(21(2)3)41-31(45)26-19-37-17-18-38-26/h8-10,14-15,17-19,21-22,24-25,27-28,30H,7,11-13,16,20H2,1-6H3,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t22-,24-,25?,27-,28-,30+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 263-6 (2003)
BindingDB Entry DOI: 10.7270/Q2VX0FX5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137732

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C38H53N7O6/c1-8-13-27(31(46)36(50)41-23(4)24-14-10-9-11-15-24)42-35(49)30-26-17-12-16-25(26)21-45(30)37(51)32(38(5,6)7)44-34(48)29(22(2)3)43-33(47)28-20-39-18-19-40-28/h9-11,14-15,18-20,22-23,25-27,29-30,32H,8,12-13,16-17,21H2,1-7H3,(H,41,50)(H,42,49)(H,43,47)(H,44,48)/t23-,25-,26-,27-,29-,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin L |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137730

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CC(C)[C@H](NC(=O)c1cnccn1)C(=O)N[C@H](C(=O)N1C[C@@H]2CCC[C@@H]2[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)C(=O)NC1CC1)C(C)(C)C Show InChI InChI=1S/C32H45F2N7O6/c1-16(2)23(39-27(43)21-14-35-11-12-36-21)28(44)40-26(32(3,4)5)31(47)41-15-17-7-6-8-19(17)24(41)29(45)38-20(13-22(33)34)25(42)30(46)37-18-9-10-18/h11-12,14,16-20,22-24,26H,6-10,13,15H2,1-5H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t17-,19-,20-,23-,24-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin L |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50119540

(CHEMBL3617552)Show SMILES CN1CCC(CC1)C(=O)c1cc(F)cc(NC(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C20H20F2N2O2/c1-24-8-6-13(7-9-24)19(25)15-10-17(22)12-18(11-15)23-20(26)14-2-4-16(21)5-3-14/h2-5,10-13H,6-9H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1F receptor by radioligand binding assay |
Bioorg Med Chem Lett 25: 4337-41 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.042
BindingDB Entry DOI: 10.7270/Q2NC630T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137736

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC(C)CC Show InChI InChI=1S/C34H53N7O6/c1-9-12-23(27(42)32(46)37-20(5)10-2)38-31(45)26-22-14-11-13-21(22)18-41(26)33(47)28(34(6,7)8)40-30(44)25(19(3)4)39-29(43)24-17-35-15-16-36-24/h15-17,19-23,25-26,28H,9-14,18H2,1-8H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t20?,21-,22-,23-,25-,26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin L |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50137732

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C38H53N7O6/c1-8-13-27(31(46)36(50)41-23(4)24-14-10-9-11-15-24)42-35(49)30-26-17-12-16-25(26)21-45(30)37(51)32(38(5,6)7)44-34(48)29(22(2)3)43-33(47)28-20-39-18-19-40-28/h9-11,14-15,18-20,22-23,25-27,29-30,32H,8,12-13,16-17,21H2,1-7H3,(H,41,50)(H,42,49)(H,43,47)(H,44,48)/t23-,25-,26-,27-,29-,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin B |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50137732

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C38H53N7O6/c1-8-13-27(31(46)36(50)41-23(4)24-14-10-9-11-15-24)42-35(49)30-26-17-12-16-25(26)21-45(30)37(51)32(38(5,6)7)44-34(48)29(22(2)3)43-33(47)28-20-39-18-19-40-28/h9-11,14-15,18-20,22-23,25-27,29-30,32H,8,12-13,16-17,21H2,1-7H3,(H,41,50)(H,42,49)(H,43,47)(H,44,48)/t23-,25-,26-,27-,29-,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Chymotrypsin |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137721

(2-(3-{[(1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C38H51N7O8/c1-6-11-26(32(46)36(50)42-27(38(52)53)18-23-12-8-7-9-13-23)41-35(49)31-25-15-10-14-24(25)20-45(31)37(51)30(22(4)5)44-34(48)29(21(2)3)43-33(47)28-19-39-16-17-40-28/h7-9,12-13,16-17,19,21-22,24-27,29-31H,6,10-11,14-15,18,20H2,1-5H3,(H,41,49)(H,42,50)(H,43,47)(H,44,48)(H,52,53)/t24-,25-,26?,27-,29-,30-,31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 251-6 (2003)
BindingDB Entry DOI: 10.7270/Q24B30R9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152754
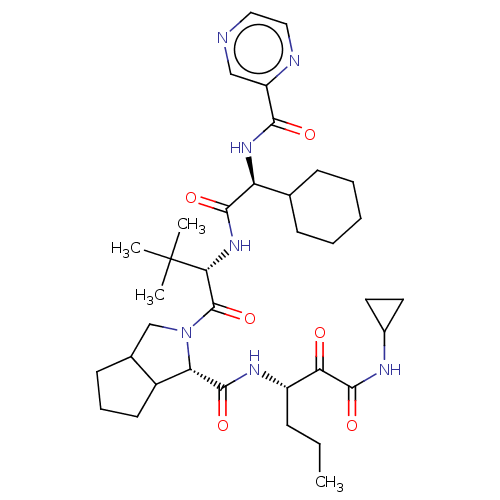
(2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carbonyl...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C36H53N7O6/c1-5-10-25(29(44)34(48)39-23-15-16-23)40-33(47)28-24-14-9-13-22(24)20-43(28)35(49)30(36(2,3)4)42-32(46)27(21-11-7-6-8-12-21)41-31(45)26-19-37-17-18-38-26/h17-19,21-25,27-28,30H,5-16,20H2,1-4H3,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t22?,24?,25?,27-,28-,30+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50137733

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22-,24-,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152753

((S)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[(pyrazi...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)[C@@H](C)CC)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C34H51N7O6/c1-7-10-23(27(42)32(46)37-21-13-14-21)38-31(45)26-22-12-9-11-20(22)18-41(26)33(47)28(34(4,5)6)40-30(44)25(19(3)8-2)39-29(43)24-17-35-15-16-36-24/h15-17,19-23,25-26,28H,7-14,18H2,1-6H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t19?,20?,22?,23?,25-,26-,28+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
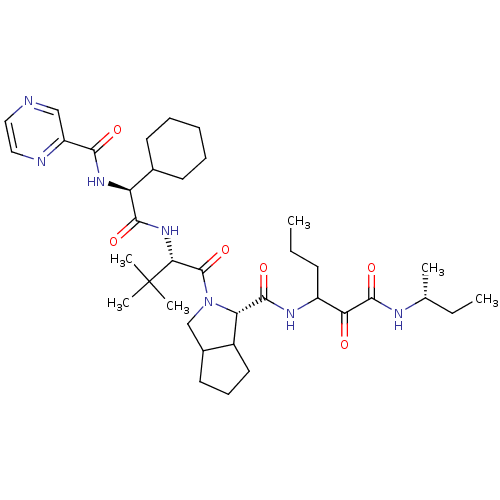
(Hepatitis C virus) | BDBM50152748
((S)-2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carb...)Show SMILES CCCC(NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)N[C@H](C)CC Show InChI InChI=1S/C37H57N7O6/c1-7-13-26(30(45)35(49)40-22(3)8-2)41-34(48)29-25-17-12-16-24(25)21-44(29)36(50)31(37(4,5)6)43-33(47)28(23-14-10-9-11-15-23)42-32(46)27-20-38-18-19-39-27/h18-20,22-26,28-29,31H,7-17,21H2,1-6H3,(H,40,49)(H,41,48)(H,42,46)(H,43,47)/t22-,24?,25?,26?,28+,29+,31-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
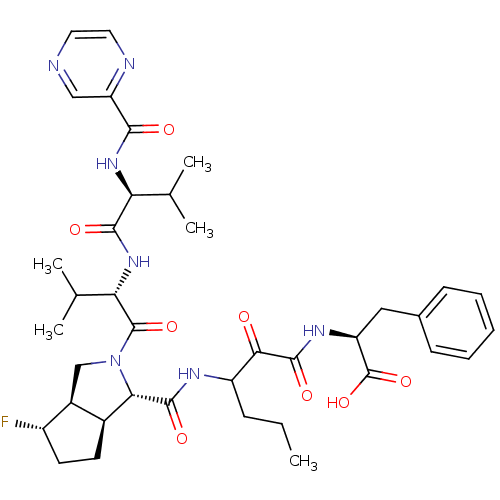
(Human rhinovirus B) | BDBM50137713
((S)-2-((S)-3-{[(1S,5S,6R)-4-Fluoro-2-((S)-3-methyl...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CC[C@H](F)[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C38H50FN7O8/c1-6-10-26(32(47)36(51)43-27(38(53)54)17-22-11-8-7-9-12-22)42-35(50)31-23-13-14-25(39)24(23)19-46(31)37(52)30(21(4)5)45-34(49)29(20(2)3)44-33(48)28-18-40-15-16-41-28/h7-9,11-12,15-16,18,20-21,23-27,29-31H,6,10,13-14,17,19H2,1-5H3,(H,42,50)(H,43,51)(H,44,48)(H,45,49)(H,53,54)/t23-,24-,25-,26?,27-,29-,30-,31-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 251-6 (2003)
BindingDB Entry DOI: 10.7270/Q24B30R9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
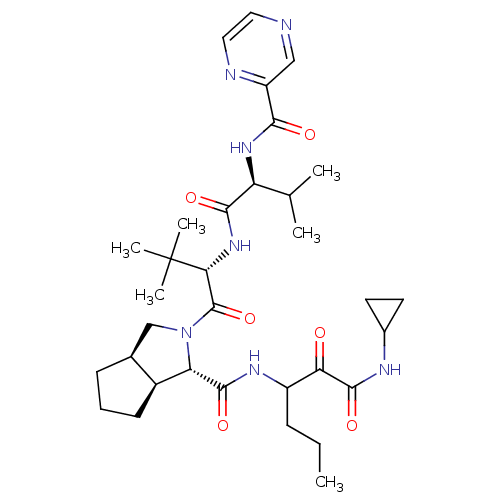
(Human rhinovirus B) | BDBM50137739
((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22?,24-,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 263-6 (2003)
BindingDB Entry DOI: 10.7270/Q2VX0FX5 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137733

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22-,24-,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152751

(2-((S)-2-{(S)-2-Cyclohexyl-2-[((R)-pyrazine-2-carb...)Show SMILES CCCC(NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C41H57N7O6/c1-6-14-30(34(49)39(53)44-25(2)26-15-9-7-10-16-26)45-38(52)33-29-20-13-19-28(29)24-48(33)40(54)35(41(3,4)5)47-37(51)32(27-17-11-8-12-18-27)46-36(50)31-23-42-21-22-43-31/h7,9-10,15-16,21-23,25,27-30,32-33,35H,6,8,11-14,17-20,24H2,1-5H3,(H,44,53)(H,45,52)(H,46,50)(H,47,51)/t25-,28?,29?,30?,32+,33+,35-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
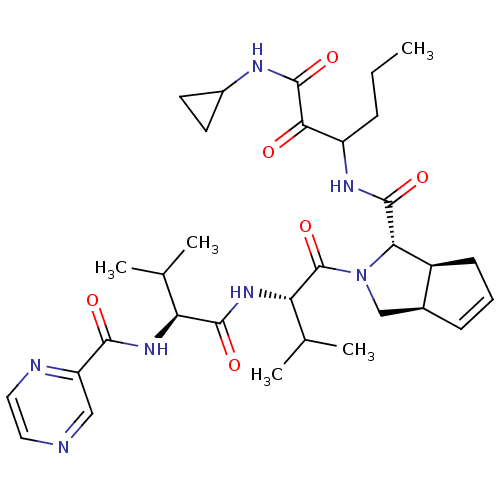
(Human rhinovirus B) | BDBM50137718
((1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyr...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CC=C[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 |c:10| Show InChI InChI=1S/C32H45N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h7,9,13-15,17-22,24-26H,6,8,10-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 251-6 (2003)
BindingDB Entry DOI: 10.7270/Q24B30R9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137738
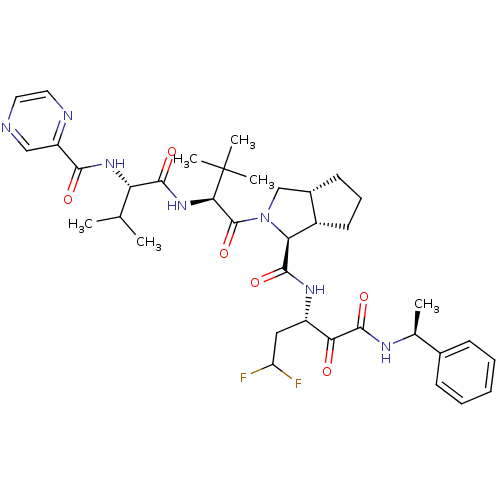
((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CC(C)[C@H](NC(=O)c1cnccn1)C(=O)N[C@H](C(=O)N1C[C@@H]2CCC[C@@H]2[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)C(=O)N[C@@H](C)c1ccccc1)C(C)(C)C Show InChI InChI=1S/C37H49F2N7O6/c1-20(2)28(44-32(48)26-18-40-15-16-41-26)33(49)45-31(37(4,5)6)36(52)46-19-23-13-10-14-24(23)29(46)34(50)43-25(17-27(38)39)30(47)35(51)42-21(3)22-11-8-7-9-12-22/h7-9,11-12,15-16,18,20-21,23-25,27-29,31H,10,13-14,17,19H2,1-6H3,(H,42,51)(H,43,50)(H,44,48)(H,45,49)/t21-,23-,24-,25-,28-,29-,31+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Cytotoxic activity in rat liver Huh-7 cells |
Bioorg Med Chem Lett 14: 263-6 (2003)
BindingDB Entry DOI: 10.7270/Q2VX0FX5 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137720

((1S,3aR,6aS)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(p...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C32H47N7O6/c1-6-8-22(27(40)31(44)35-20-11-12-20)36-30(43)26-21-10-7-9-19(21)16-39(26)32(45)25(18(4)5)38-29(42)24(17(2)3)37-28(41)23-15-33-13-14-34-23/h13-15,17-22,24-26H,6-12,16H2,1-5H3,(H,35,44)(H,36,43)(H,37,41)(H,38,42)/t19-,21-,22?,24-,25-,26-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 251-6 (2003)
BindingDB Entry DOI: 10.7270/Q24B30R9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM86258

(CAS_23552-18-3 | Morphine | NSC_5980)Show SMILES CN1CCC23C4Oc5c2c(CC1C3C=CC4O)ccc5O |c:16,TLB:13:12:1.2.3:10.9.8| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152755
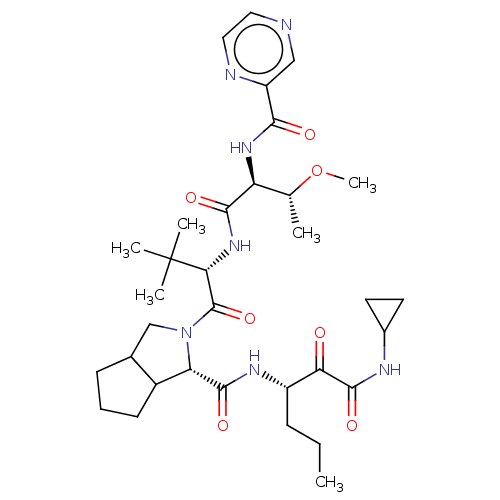
(2-((S)-2-{(S)-3-Methoxy-2-[(pyrazine-2-carbonyl)-a...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)[C@@H](C)OC)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O7/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(3,4)5)39-29(43)24(18(2)47-6)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t18?,19?,21?,22?,24-,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50137733

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22-,24-,25-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin L |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50152752
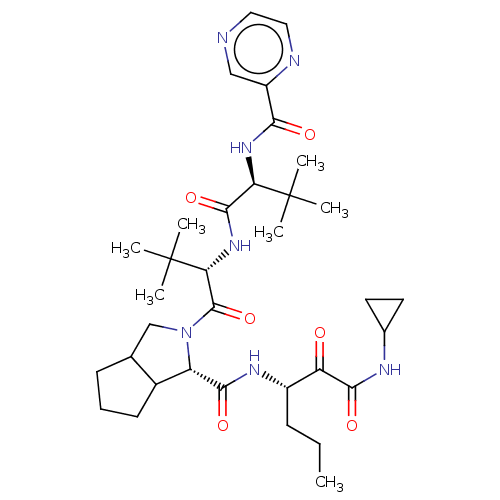
((S)-2-((S)-2-{(S)-3,3-Dimethyl-2-[(pyrazine-2-carb...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C34H51N7O6/c1-8-10-22(25(42)30(45)37-20-13-14-20)38-29(44)24-21-12-9-11-19(21)18-41(24)32(47)27(34(5,6)7)40-31(46)26(33(2,3)4)39-28(43)23-17-35-15-16-36-23/h15-17,19-22,24,26-27H,8-14,18H2,1-7H3,(H,37,45)(H,38,44)(H,39,43)(H,40,46)/t19?,21?,22?,24-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease in the pNA based inhibition assay |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50404057
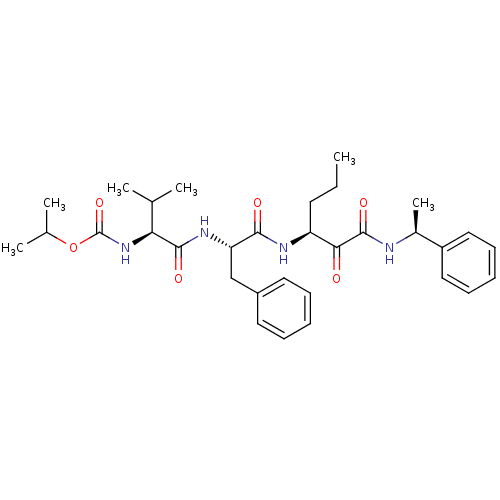
(CHEMBL118534)Show SMILES CCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OC(C)C)C(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C32H44N4O6/c1-7-14-25(28(37)31(40)33-22(6)24-17-12-9-13-18-24)34-29(38)26(19-23-15-10-8-11-16-23)35-30(39)27(20(2)3)36-32(41)42-21(4)5/h8-13,15-18,20-22,25-27H,7,14,19H2,1-6H3,(H,33,40)(H,34,38)(H,35,39)(H,36,41)/t22-,25-,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antiviral activity against human rhinovirus-14 (HRV-14) 3C protease using enzyme assay |
Bioorg Med Chem Lett 13: 3531-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PC33J5 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137722
((S)-2-(3-{[(1S,5S,6R)-4,4-Difluoro-2-((S)-3-methyl...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC(F)(F)[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)C)C(=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C38H49F2N7O8/c1-6-10-25(31(48)35(52)44-26(37(54)55)17-22-11-8-7-9-12-22)43-34(51)30-23-13-14-38(39,40)24(23)19-47(30)36(53)29(21(4)5)46-33(50)28(20(2)3)45-32(49)27-18-41-15-16-42-27/h7-9,11-12,15-16,18,20-21,23-26,28-30H,6,10,13-14,17,19H2,1-5H3,(H,43,51)(H,44,52)(H,45,49)(H,46,50)(H,54,55)/t23-,24-,25?,26-,28-,29-,30-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 251-6 (2003)
BindingDB Entry DOI: 10.7270/Q24B30R9 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137732

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C38H53N7O6/c1-8-13-27(31(46)36(50)41-23(4)24-14-10-9-11-15-24)42-35(49)30-26-17-12-16-25(26)21-45(30)37(51)32(38(5,6)7)44-34(48)29(22(2)3)43-33(47)28-20-39-18-19-40-28/h9-11,14-15,18-20,22-23,25-27,29-30,32H,8,12-13,16-17,21H2,1-7H3,(H,41,50)(H,42,49)(H,43,47)(H,44,48)/t23-,25-,26-,27-,29-,30-,32+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137748
((1S,3aR,6aS)-2-((S)-3,3-Dimethyl-2-{(3S,4S)-3-meth...)Show SMILES CCCC(NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C38H53N7O6/c1-8-13-27(31(46)36(50)41-23(4)24-14-10-9-11-15-24)42-35(49)30-26-17-12-16-25(26)21-45(30)37(51)32(38(5,6)7)44-34(48)29(22(2)3)43-33(47)28-20-39-18-19-40-28/h9-11,14-15,18-20,22-23,25-27,29-30,32H,8,12-13,16-17,21H2,1-7H3,(H,41,50)(H,42,49)(H,43,47)(H,44,48)/t23-,25-,26-,27?,29-,30-,32+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 263-6 (2003)
BindingDB Entry DOI: 10.7270/Q2VX0FX5 |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137730

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CC(C)[C@H](NC(=O)c1cnccn1)C(=O)N[C@H](C(=O)N1C[C@@H]2CCC[C@@H]2[C@H]1C(=O)N[C@@H](CC(F)F)C(=O)C(=O)NC1CC1)C(C)(C)C Show InChI InChI=1S/C32H45F2N7O6/c1-16(2)23(39-27(43)21-14-35-11-12-36-21)28(44)40-26(32(3,4)5)31(47)41-15-17-7-6-8-19(17)24(41)29(45)38-20(13-22(33)34)25(42)30(46)37-18-9-10-18/h11-12,14,16-20,22-24,26H,6-10,13,15H2,1-5H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t17-,19-,20-,23-,24-,26+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137736

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC(C)CC Show InChI InChI=1S/C34H53N7O6/c1-9-12-23(27(42)32(46)37-20(5)10-2)38-31(45)26-22-14-11-13-21(22)18-41(26)33(47)28(34(6,7)8)40-30(44)25(19(3)4)39-29(43)24-17-35-15-16-36-24/h15-17,19-23,25-26,28H,9-14,18H2,1-8H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t20?,21-,22-,23-,25-,26-,28+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Protease using PNA assay in rats |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Human rhinovirus B) | BDBM50137729
((1S,5S,6R)-2-((S)-3-Methyl-2-{(S)-3-methyl-2-[(pyr...)Show SMILES CC(C)[C@H](NC(=O)c1cnccn1)C(=O)N[C@@H](C(C)C)C(=O)N1C[C@@H]2CCC[C@@H]2[C@H]1C(=O)NC(CC(F)F)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C31H43F2N7O6/c1-15(2)23(38-27(42)21-13-34-10-11-35-21)28(43)39-24(16(3)4)31(46)40-14-17-6-5-7-19(17)25(40)29(44)37-20(12-22(32)33)26(41)30(45)36-18-8-9-18/h10-11,13,15-20,22-25H,5-9,12,14H2,1-4H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t17-,19-,20?,23-,24-,25-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against HCV protease using replicon assay in rats |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50137732

((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C38H53N7O6/c1-8-13-27(31(46)36(50)41-23(4)24-14-10-9-11-15-24)42-35(49)30-26-17-12-16-25(26)21-45(30)37(51)32(38(5,6)7)44-34(48)29(22(2)3)43-33(47)28-20-39-18-19-40-28/h9-11,14-15,18-20,22-23,25-27,29-30,32H,8,12-13,16-17,21H2,1-7H3,(H,41,50)(H,42,49)(H,43,47)(H,44,48)/t23-,25-,26-,27-,29-,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against cathepsin K |
Bioorg Med Chem Lett 14: 257-61 (2003)
BindingDB Entry DOI: 10.7270/Q20P0ZDG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data