 Found 163 hits with Last Name = 'welsh' and Initial = 'km'
Found 163 hits with Last Name = 'welsh' and Initial = 'km' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046660

(2-[4-(2-Amino-6-methyl-4-oxo-3,4-dihydro-quinazoli...)Show SMILES Cc1ccc2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H20N4O6S/c1-10-2-7-13-16(19(29)25-21(22)24-13)17(10)32-12-5-3-11(4-6-12)18(28)23-14(20(30)31)8-9-15(26)27/h2-7,14H,8-9H2,1H3,(H,23,28)(H,26,27)(H,30,31)(H3,22,24,25,29)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for TS activity against human Thymidylate synthase by tight binding kinetics |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046665
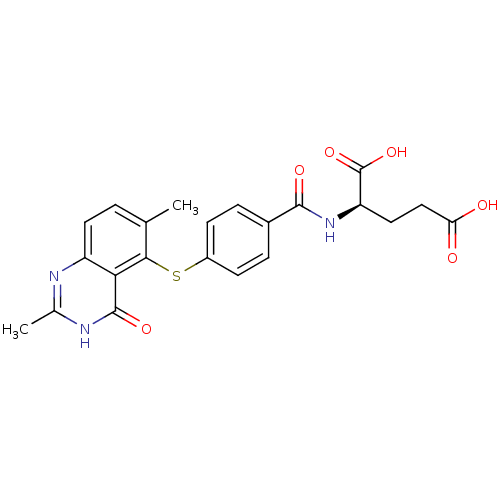
(2-[4-(2,6-Dimethyl-4-oxo-3,4-dihydro-quinazolin-5-...)Show SMILES Cc1nc2ccc(C)c(Sc3ccc(cc3)C(=O)N[C@H](CCC(O)=O)C(O)=O)c2c(=O)[nH]1 Show InChI InChI=1S/C22H21N3O6S/c1-11-3-8-15-18(21(29)24-12(2)23-15)19(11)32-14-6-4-13(5-7-14)20(28)25-16(22(30)31)9-10-17(26)27/h3-8,16H,9-10H2,1-2H3,(H,25,28)(H,26,27)(H,30,31)(H,23,24,29)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was tested for TS activity against human Thymidylate synthase by tight binding kinetics |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50035018
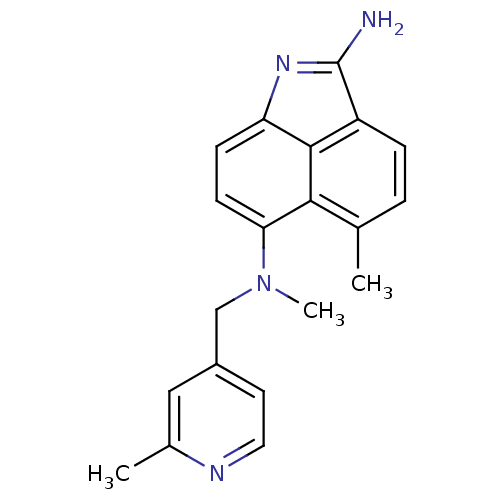
(5,N*6*-Dimethyl-N*6*-(2-methyl-pyridin-4-ylmethyl)...)Show SMILES CN(Cc1ccnc(C)c1)c1ccc2N=C(N)c3ccc(C)c1c23 |t:15| Show InChI InChI=1S/C20H20N4/c1-12-4-5-15-19-16(23-20(15)21)6-7-17(18(12)19)24(3)11-14-8-9-22-13(2)10-14/h4-10H,11H2,1-3H3,(H2,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human thymidylate synthase |
J Med Chem 38: 1892-903 (1995)
BindingDB Entry DOI: 10.7270/Q2X929B2 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005335
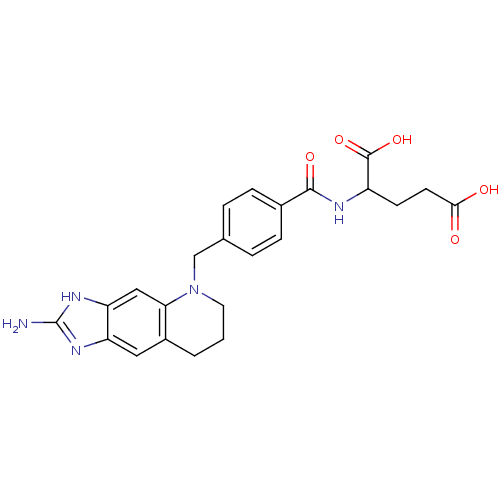
(2-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)C(=O)NC(CCC(O)=O)C(O)=O)c3cc2[nH]1 Show InChI InChI=1S/C23H25N5O5/c24-23-26-17-10-15-2-1-9-28(19(15)11-18(17)27-23)12-13-3-5-14(6-4-13)21(31)25-16(22(32)33)7-8-20(29)30/h3-6,10-11,16H,1-2,7-9,12H2,(H,25,31)(H,29,30)(H,32,33)(H3,24,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005329
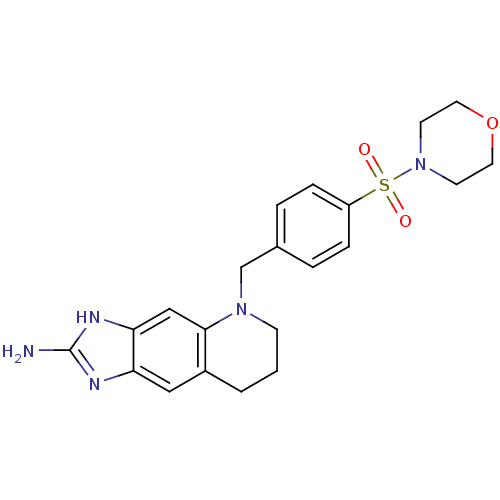
(5-[4-(Morpholine-4-sulfonyl)-benzyl]-5,6,7,8-tetra...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)N4CCOCC4)c3cc2[nH]1 Show InChI InChI=1S/C21H25N5O3S/c22-21-23-18-12-16-2-1-7-25(20(16)13-19(18)24-21)14-15-3-5-17(6-4-15)30(27,28)26-8-10-29-11-9-26/h3-6,12-13H,1-2,7-11,14H2,(H3,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005324

(4-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)c4ccc(O)cc4)c3cc2[nH]1 Show InChI InChI=1S/C23H22N4O3S/c24-23-25-20-12-16-2-1-11-27(22(16)13-21(20)26-23)14-15-3-7-18(8-4-15)31(29,30)19-9-5-17(28)6-10-19/h3-10,12-13,28H,1-2,11,14H2,(H3,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50035012

(5,N*6*-Dimethyl-N*6*-pyridazin-4-ylmethyl-benzo[cd...)Show SMILES CN(Cc1ccnnc1)c1ccc2N=C(N)c3ccc(C)c1c23 |t:14| Show InChI InChI=1S/C18H17N5/c1-11-3-4-13-17-14(22-18(13)19)5-6-15(16(11)17)23(2)10-12-7-8-20-21-9-12/h3-9H,10H2,1-2H3,(H2,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human thymidylate synthase |
J Med Chem 38: 1892-903 (1995)
BindingDB Entry DOI: 10.7270/Q2X929B2 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046646
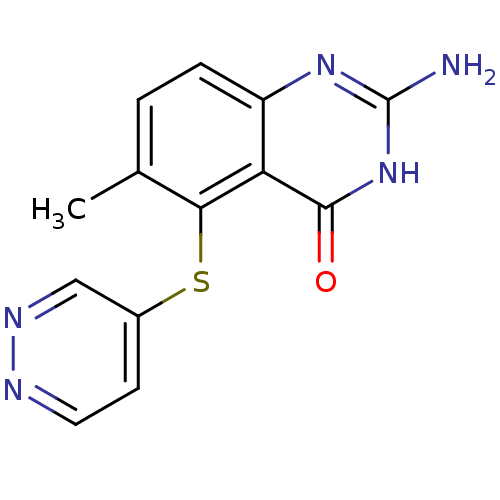
(2-Amino-6-methyl-5-(pyridazin-4-ylsulfanyl)-3H-qui...)Show InChI InChI=1S/C13H11N5OS/c1-7-2-3-9-10(12(19)18-13(14)17-9)11(7)20-8-4-5-15-16-6-8/h2-6H,1H3,(H3,14,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005335

(2-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)C(=O)NC(CCC(O)=O)C(O)=O)c3cc2[nH]1 Show InChI InChI=1S/C23H25N5O5/c24-23-26-17-10-15-2-1-9-28(19(15)11-18(17)27-23)12-13-3-5-14(6-4-13)21(31)25-16(22(32)33)7-8-20(29)30/h3-6,10-11,16H,1-2,7-9,12H2,(H,25,31)(H,29,30)(H,32,33)(H3,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005329

(5-[4-(Morpholine-4-sulfonyl)-benzyl]-5,6,7,8-tetra...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)N4CCOCC4)c3cc2[nH]1 Show InChI InChI=1S/C21H25N5O3S/c22-21-23-18-12-16-2-1-7-25(20(16)13-19(18)24-21)14-15-3-5-17(6-4-15)30(27,28)26-8-10-29-11-9-26/h3-6,12-13H,1-2,7-11,14H2,(H3,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50011244

(6-{[(4-Benzenesulfonyl-phenyl)-prop-2-ynyl-amino]-...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H21N3O3S/c1-3-15-28(17-19-9-14-24-23(16-19)25(29)27-18(2)26-24)20-10-12-22(13-11-20)32(30,31)21-7-5-4-6-8-21/h1,4-14,16H,15,17H2,2H3,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50011244

(6-{[(4-Benzenesulfonyl-phenyl)-prop-2-ynyl-amino]-...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H21N3O3S/c1-3-15-28(17-19-9-14-24-23(16-19)25(29)27-18(2)26-24)20-10-12-22(13-11-20)32(30,31)21-7-5-4-6-8-21/h1,4-14,16H,15,17H2,2H3,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005330
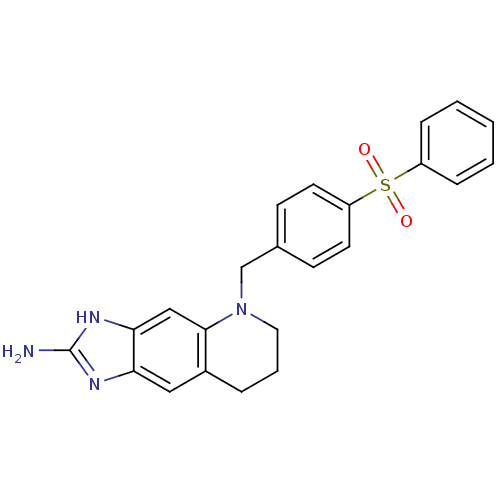
(5-(4-Benzenesulfonyl-benzyl)-5,6,7,8-tetrahydro-1H...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)c4ccccc4)c3cc2[nH]1 Show InChI InChI=1S/C23H22N4O2S/c24-23-25-20-13-17-5-4-12-27(22(17)14-21(20)26-23)15-16-8-10-19(11-9-16)30(28,29)18-6-2-1-3-7-18/h1-3,6-11,13-14H,4-5,12,15H2,(H3,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50035013

(5,N*2*,N*6*-Trimethyl-N*6*-pyridin-4-ylmethyl-benz...)Show SMILES CNC1=Nc2ccc(N(C)Cc3ccncc3)c3c(C)ccc1c23 |t:2| Show InChI InChI=1S/C20H20N4/c1-13-4-5-15-19-16(23-20(15)21-2)6-7-17(18(13)19)24(3)12-14-8-10-22-11-9-14/h4-11H,12H2,1-3H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human thymidylate synthase |
J Med Chem 38: 1892-903 (1995)
BindingDB Entry DOI: 10.7270/Q2X929B2 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50035006
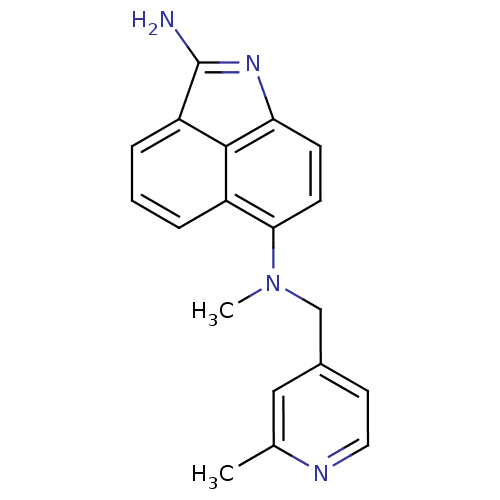
(CHEMBL434980 | N*6*-Methyl-N*6*-(2-methyl-pyridin-...)Show SMILES CN(Cc1ccnc(C)c1)c1ccc2N=C(N)c3cccc1c23 |t:15| Show InChI InChI=1S/C19H18N4/c1-12-10-13(8-9-21-12)11-23(2)17-7-6-16-18-14(17)4-3-5-15(18)19(20)22-16/h3-10H,11H2,1-2H3,(H2,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human thymidylate synthase |
J Med Chem 38: 1892-903 (1995)
BindingDB Entry DOI: 10.7270/Q2X929B2 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046657
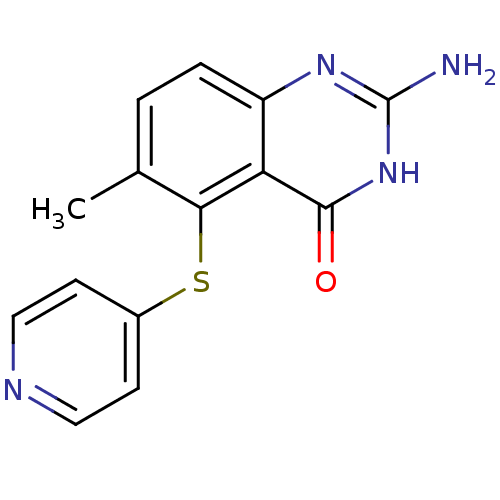
(2-Amino-6-methyl-5-(pyridin-4-ylsulfanyl)-3H-quina...)Show InChI InChI=1S/C14H12N4OS/c1-8-2-3-10-11(13(19)18-14(15)17-10)12(8)20-9-4-6-16-7-5-9/h2-7H,1H3,(H3,15,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046657

(2-Amino-6-methyl-5-(pyridin-4-ylsulfanyl)-3H-quina...)Show InChI InChI=1S/C14H12N4OS/c1-8-2-3-10-11(13(19)18-14(15)17-10)12(8)20-9-4-6-16-7-5-9/h2-7H,1H3,(H3,15,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046653

(2-Amino-6-chloro-5-(pyridin-4-ylsulfanyl)-3H-quina...)Show InChI InChI=1S/C13H9ClN4OS/c14-8-1-2-9-10(12(19)18-13(15)17-9)11(8)20-7-3-5-16-6-4-7/h1-6H,(H3,15,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50035009
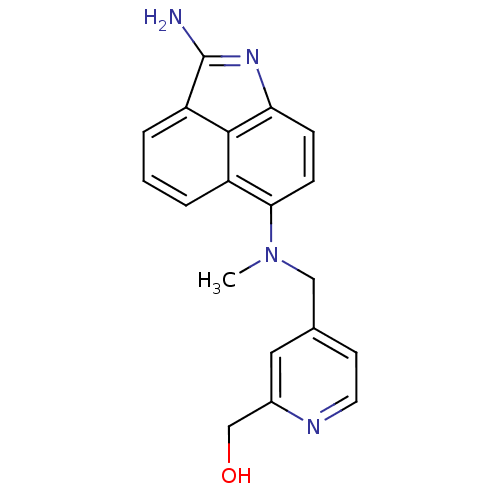
((4-{[(2-Amino-benzo[cd]indol-6-yl)-methyl-amino]-m...)Show SMILES CN(Cc1ccnc(CO)c1)c1ccc2N=C(N)c3cccc1c23 |t:16| Show InChI InChI=1S/C19H18N4O/c1-23(10-12-7-8-21-13(9-12)11-24)17-6-5-16-18-14(17)3-2-4-15(18)19(20)22-16/h2-9,24H,10-11H2,1H3,(H2,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human thymidylate synthase |
J Med Chem 38: 1892-903 (1995)
BindingDB Entry DOI: 10.7270/Q2X929B2 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056521
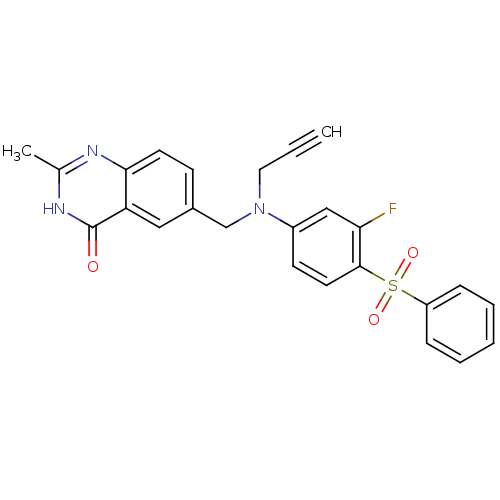
(6-{[(4-Benzenesulfonyl-3-fluoro-phenyl)-prop-2-yny...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(c(F)c3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20FN3O3S/c1-3-13-29(16-18-9-11-23-21(14-18)25(30)28-17(2)27-23)19-10-12-24(22(26)15-19)33(31,32)20-7-5-4-6-8-20/h1,4-12,14-15H,13,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005324

(4-[4-(2-Amino-1,6,7,8-tetrahydro-imidazo[4,5-g]qui...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)c4ccc(O)cc4)c3cc2[nH]1 Show InChI InChI=1S/C23H22N4O3S/c24-23-25-20-12-16-2-1-11-27(22(16)13-21(20)26-23)14-15-3-7-18(8-4-15)31(29,30)19-9-5-17(28)6-10-19/h3-10,12-13,28H,1-2,11,14H2,(H3,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046653

(2-Amino-6-chloro-5-(pyridin-4-ylsulfanyl)-3H-quina...)Show InChI InChI=1S/C13H9ClN4OS/c14-8-1-2-9-10(12(19)18-13(15)17-9)11(8)20-7-3-5-16-6-4-7/h1-6H,(H3,15,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056516
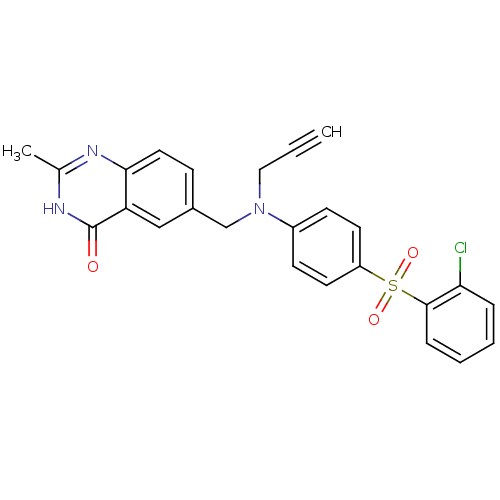
(6-({[4-(2-Chloro-benzenesulfonyl)-phenyl]-prop-2-y...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)c3ccccc3Cl)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20ClN3O3S/c1-3-14-29(16-18-8-13-23-21(15-18)25(30)28-17(2)27-23)19-9-11-20(12-10-19)33(31,32)24-7-5-4-6-22(24)26/h1,4-13,15H,14,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056519

(6-{[(4-Benzenesulfonyl-3-chloro-phenyl)-prop-2-yny...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(c(Cl)c3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20ClN3O3S/c1-3-13-29(16-18-9-11-23-21(14-18)25(30)28-17(2)27-23)19-10-12-24(22(26)15-19)33(31,32)20-7-5-4-6-8-20/h1,4-12,14-15H,13,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005332
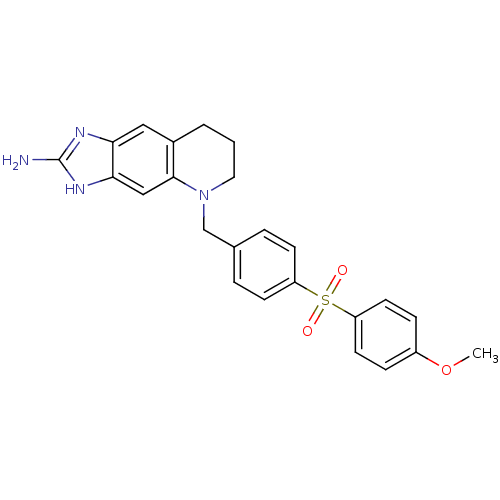
(5-[4-(4-Methoxy-benzenesulfonyl)-benzyl]-5,6,7,8-t...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(CN2CCCc3cc4nc(N)[nH]c4cc23)cc1 Show InChI InChI=1S/C24H24N4O3S/c1-31-18-6-10-20(11-7-18)32(29,30)19-8-4-16(5-9-19)15-28-12-2-3-17-13-21-22(14-23(17)28)27-24(25)26-21/h4-11,13-14H,2-3,12,15H2,1H3,(H3,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046654
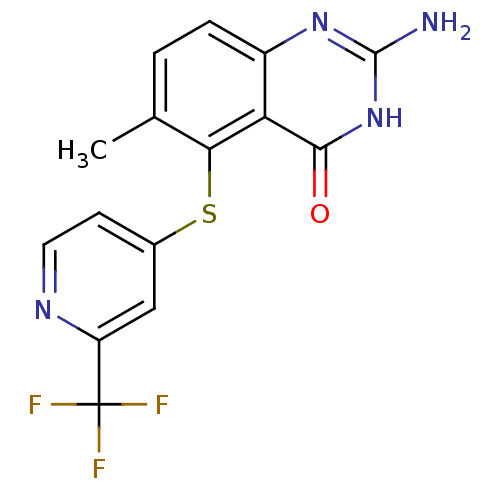
(2-Amino-6-methyl-5-(2-trifluoromethyl-pyridin-4-yl...)Show SMILES Cc1ccc2nc(N)[nH]c(=O)c2c1Sc1ccnc(c1)C(F)(F)F Show InChI InChI=1S/C15H11F3N4OS/c1-7-2-3-9-11(13(23)22-14(19)21-9)12(7)24-8-4-5-20-10(6-8)15(16,17)18/h2-6H,1H3,(H3,19,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50011244

(6-{[(4-Benzenesulfonyl-phenyl)-prop-2-ynyl-amino]-...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H21N3O3S/c1-3-15-28(17-19-9-14-24-23(16-19)25(29)27-18(2)26-24)20-10-12-22(13-11-20)32(30,31)21-7-5-4-6-8-21/h1,4-14,16H,15,17H2,2H3,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant thymidylate synthase in E. coli |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50035005
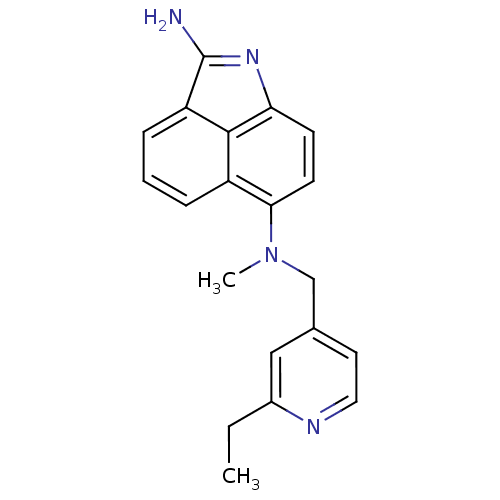
(CHEMBL56611 | N*6*-(2-Ethyl-pyridin-4-ylmethyl)-N*...)Show SMILES CCc1cc(CN(C)c2ccc3N=C(N)c4cccc2c34)ccn1 |t:12| Show InChI InChI=1S/C20H20N4/c1-3-14-11-13(9-10-22-14)12-24(2)18-8-7-17-19-15(18)5-4-6-16(19)20(21)23-17/h4-11H,3,12H2,1-2H3,(H2,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human thymidylate synthase |
J Med Chem 38: 1892-903 (1995)
BindingDB Entry DOI: 10.7270/Q2X929B2 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056520

(2-Methyl-6-({prop-2-ynyl-[4-(toluene-2-sulfonyl)-p...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)c3ccccc3C)cc2c(=O)[nH]1 Show InChI InChI=1S/C26H23N3O3S/c1-4-15-29(17-20-9-14-24-23(16-20)26(30)28-19(3)27-24)21-10-12-22(13-11-21)33(31,32)25-8-6-5-7-18(25)2/h1,5-14,16H,15,17H2,2-3H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056518
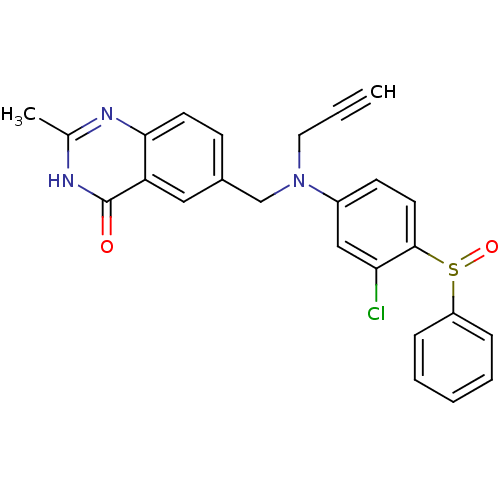
(6-{[(4-Benzenesulfinyl-3-chloro-phenyl)-prop-2-yny...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(c(Cl)c3)S(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20ClN3O2S/c1-3-13-29(16-18-9-11-23-21(14-18)25(30)28-17(2)27-23)19-10-12-24(22(26)15-19)32(31)20-7-5-4-6-8-20/h1,4-12,14-15H,13,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056518

(6-{[(4-Benzenesulfinyl-3-chloro-phenyl)-prop-2-yny...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(c(Cl)c3)S(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20ClN3O2S/c1-3-13-29(16-18-9-11-23-21(14-18)25(30)28-17(2)27-23)19-10-12-24(22(26)15-19)32(31)20-7-5-4-6-8-20/h1,4-12,14-15H,13,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046654

(2-Amino-6-methyl-5-(2-trifluoromethyl-pyridin-4-yl...)Show SMILES Cc1ccc2nc(N)[nH]c(=O)c2c1Sc1ccnc(c1)C(F)(F)F Show InChI InChI=1S/C15H11F3N4OS/c1-7-2-3-9-11(13(23)22-14(19)21-9)12(7)24-8-4-5-20-10(6-8)15(16,17)18/h2-6H,1H3,(H3,19,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50035007
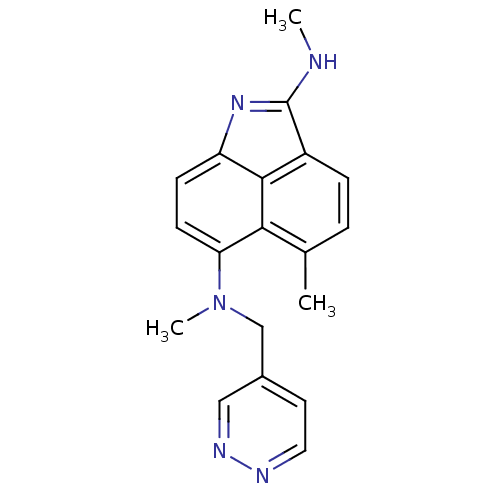
(5,N*2*,N*6*-Trimethyl-N*6*-pyridazin-4-ylmethyl-be...)Show SMILES CNC1=Nc2ccc(N(C)Cc3ccnnc3)c3c(C)ccc1c23 |t:2| Show InChI InChI=1S/C19H19N5/c1-12-4-5-14-18-15(23-19(14)20-2)6-7-16(17(12)18)24(3)11-13-8-9-21-22-10-13/h4-10H,11H2,1-3H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human thymidylate synthase |
J Med Chem 38: 1892-903 (1995)
BindingDB Entry DOI: 10.7270/Q2X929B2 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046651
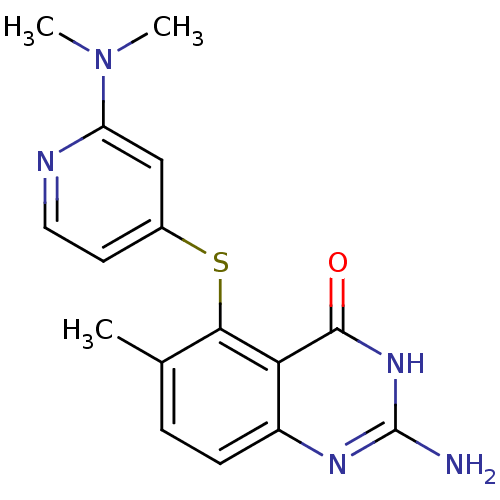
(2-Amino-5-(2-dimethylamino-pyridin-4-ylsulfanyl)-6...)Show SMILES CN(C)c1cc(Sc2c(C)ccc3nc(N)[nH]c(=O)c23)ccn1 Show InChI InChI=1S/C16H17N5OS/c1-9-4-5-11-13(15(22)20-16(17)19-11)14(9)23-10-6-7-18-12(8-10)21(2)3/h4-8H,1-3H3,(H3,17,19,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056516

(6-({[4-(2-Chloro-benzenesulfonyl)-phenyl]-prop-2-y...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)c3ccccc3Cl)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20ClN3O3S/c1-3-14-29(16-18-8-13-23-21(15-18)25(30)28-17(2)27-23)19-9-11-20(12-10-19)33(31,32)24-7-5-4-6-22(24)26/h1,4-13,15H,14,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant thymidylate synthase in E. coli |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056516

(6-({[4-(2-Chloro-benzenesulfonyl)-phenyl]-prop-2-y...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)(=O)c3ccccc3Cl)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20ClN3O3S/c1-3-14-29(16-18-8-13-23-21(15-18)25(30)28-17(2)27-23)19-9-11-20(12-10-19)33(31,32)24-7-5-4-6-22(24)26/h1,4-13,15H,14,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant thymidylate synthase in E. coli |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056519

(6-{[(4-Benzenesulfonyl-3-chloro-phenyl)-prop-2-yny...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(c(Cl)c3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20ClN3O3S/c1-3-13-29(16-18-9-11-23-21(14-18)25(30)28-17(2)27-23)19-10-12-24(22(26)15-19)33(31,32)20-7-5-4-6-8-20/h1,4-12,14-15H,13,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant thymidylate synthase in E. coli |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056519

(6-{[(4-Benzenesulfonyl-3-chloro-phenyl)-prop-2-yny...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(c(Cl)c3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20ClN3O3S/c1-3-13-29(16-18-9-11-23-21(14-18)25(30)28-17(2)27-23)19-10-12-24(22(26)15-19)33(31,32)20-7-5-4-6-8-20/h1,4-12,14-15H,13,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant thymidylate synthase in E. coli |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50012245

(6-{[(4-Benzenesulfonyl-3-trifluoromethyl-phenyl)-p...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(c(c3)C(F)(F)F)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C26H20F3N3O3S/c1-3-13-32(16-18-9-11-23-21(14-18)25(33)31-17(2)30-23)19-10-12-24(22(15-19)26(27,28)29)36(34,35)20-7-5-4-6-8-20/h1,4-12,14-15H,13,16H2,2H3,(H,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant thymidylate synthase in E. coli |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50005322
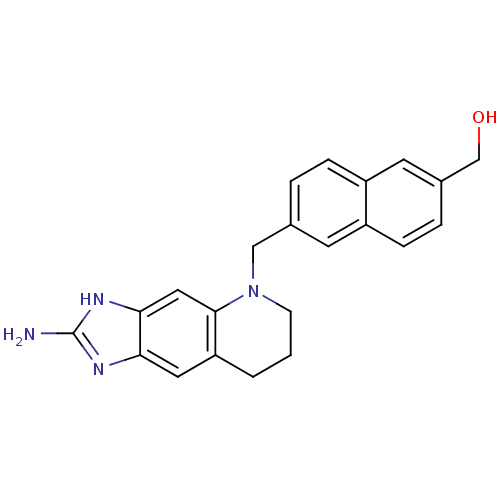
(CHEMBL353813 | [6-(2-Amino-1,6,7,8-tetrahydro-imid...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc5cc(CO)ccc5c4)c3cc2[nH]1 Show InChI InChI=1S/C22H22N4O/c23-22-24-19-10-18-2-1-7-26(21(18)11-20(19)25-22)12-14-3-5-17-9-15(13-27)4-6-16(17)8-14/h3-6,8-11,27H,1-2,7,12-13H2,(H3,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50046656
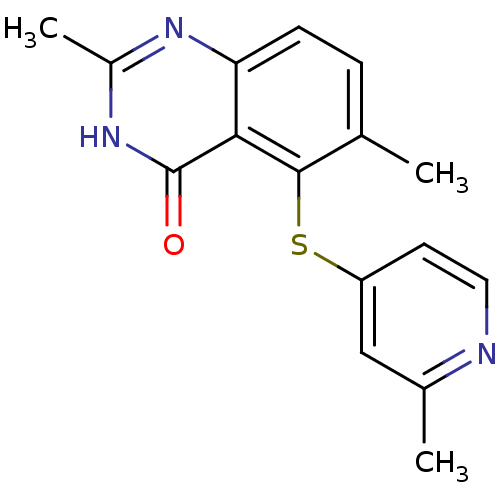
(2,6-Dimethyl-5-(2-methyl-pyridin-4-ylsulfanyl)-3H-...)Show InChI InChI=1S/C16H15N3OS/c1-9-4-5-13-14(16(20)19-11(3)18-13)15(9)21-12-6-7-17-10(2)8-12/h4-8H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Thymidylate synthase |
J Med Chem 36: 733-46 (1993)
BindingDB Entry DOI: 10.7270/Q2HM57JF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005322

(CHEMBL353813 | [6-(2-Amino-1,6,7,8-tetrahydro-imid...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc5cc(CO)ccc5c4)c3cc2[nH]1 Show InChI InChI=1S/C22H22N4O/c23-22-24-19-10-18-2-1-7-26(21(18)11-20(19)25-22)12-14-3-5-17-9-15(13-27)4-6-16(17)8-14/h3-6,8-11,27H,1-2,7,12-13H2,(H3,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005332

(5-[4-(4-Methoxy-benzenesulfonyl)-benzyl]-5,6,7,8-t...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(CN2CCCc3cc4nc(N)[nH]c4cc23)cc1 Show InChI InChI=1S/C24H24N4O3S/c1-31-18-6-10-20(11-7-18)32(29,30)19-8-4-16(5-9-19)15-28-12-2-3-17-13-21-22(14-23(17)28)27-24(25)26-21/h4-11,13-14H,2-3,12,15H2,1H3,(H3,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50005330

(5-(4-Benzenesulfonyl-benzyl)-5,6,7,8-tetrahydro-1H...)Show SMILES Nc1nc2cc3CCCN(Cc4ccc(cc4)S(=O)(=O)c4ccccc4)c3cc2[nH]1 Show InChI InChI=1S/C23H22N4O2S/c24-23-25-20-13-17-5-4-12-27(22(17)14-21(20)26-23)15-16-8-10-19(11-9-16)30(28,29)18-6-2-1-3-7-18/h1-3,6-11,13-14H,4-5,12,15H2,(H3,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Thymidylate synthase (TS) |
J Med Chem 35: 847-58 (1992)
BindingDB Entry DOI: 10.7270/Q2X0660R |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056521

(6-{[(4-Benzenesulfonyl-3-fluoro-phenyl)-prop-2-yny...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(c(F)c3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20FN3O3S/c1-3-13-29(16-18-9-11-23-21(14-18)25(30)28-17(2)27-23)19-10-12-24(22(26)15-19)33(31,32)20-7-5-4-6-8-20/h1,4-12,14-15H,13,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant thymidylate synthase in E. coli |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056521

(6-{[(4-Benzenesulfonyl-3-fluoro-phenyl)-prop-2-yny...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(c(F)c3)S(=O)(=O)c3ccccc3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20FN3O3S/c1-3-13-29(16-18-9-11-23-21(14-18)25(30)28-17(2)27-23)19-10-12-24(22(26)15-19)33(31,32)20-7-5-4-6-8-20/h1,4-12,14-15H,13,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant thymidylate synthase in E. coli |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056523
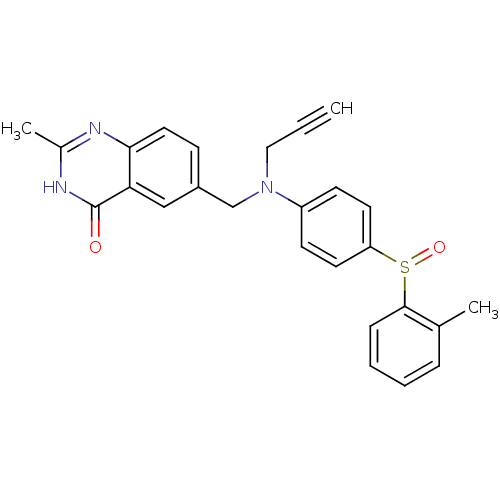
(2-Methyl-6-({prop-2-ynyl-[4-(toluene-2-sulfinyl)-p...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)c3ccccc3C)cc2c(=O)[nH]1 Show InChI InChI=1S/C26H23N3O2S/c1-4-15-29(17-20-9-14-24-23(16-20)26(30)28-19(3)27-24)21-10-12-22(13-11-21)32(31)25-8-6-5-7-18(25)2/h1,5-14,16H,15,17H2,2-3H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50035011
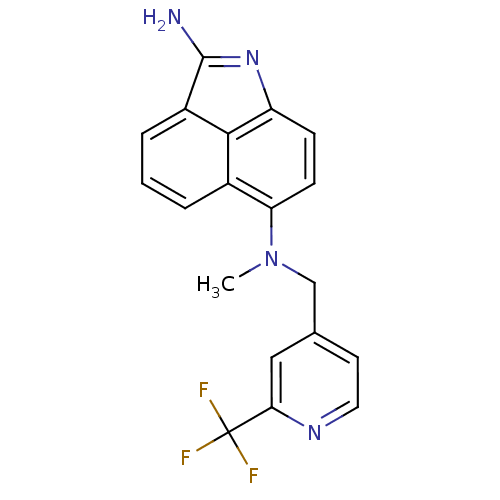
(CHEMBL301218 | N*6*-Methyl-N*6*-(2-trifluoromethyl...)Show SMILES CN(Cc1ccnc(c1)C(F)(F)F)c1ccc2N=C(N)c3cccc1c23 |t:18| Show InChI InChI=1S/C19H15F3N4/c1-26(10-11-7-8-24-16(9-11)19(20,21)22)15-6-5-14-17-12(15)3-2-4-13(17)18(23)25-14/h2-9H,10H2,1H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human thymidylate synthase |
J Med Chem 38: 1892-903 (1995)
BindingDB Entry DOI: 10.7270/Q2X929B2 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056523

(2-Methyl-6-({prop-2-ynyl-[4-(toluene-2-sulfinyl)-p...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)c3ccccc3C)cc2c(=O)[nH]1 Show InChI InChI=1S/C26H23N3O2S/c1-4-15-29(17-20-9-14-24-23(16-20)26(30)28-19(3)27-24)21-10-12-22(13-11-21)32(31)25-8-6-5-7-18(25)2/h1,5-14,16H,15,17H2,2-3H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50056515

(6-({[4-(2-Chloro-benzenesulfinyl)-phenyl]-prop-2-y...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)S(=O)c3ccccc3Cl)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H20ClN3O2S/c1-3-14-29(16-18-8-13-23-21(15-18)25(30)28-17(2)27-23)19-9-11-20(12-10-19)32(31)24-7-5-4-6-22(24)26/h1,4-13,15H,14,16H2,2H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human thymidylate synthase |
J Med Chem 40: 677-83 (1997)
Article DOI: 10.1021/jm960613f
BindingDB Entry DOI: 10.7270/Q25X2810 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data