 Found 521 hits with Last Name = 'wilkinson' and Initial = 'bl'
Found 521 hits with Last Name = 'wilkinson' and Initial = 'bl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10860
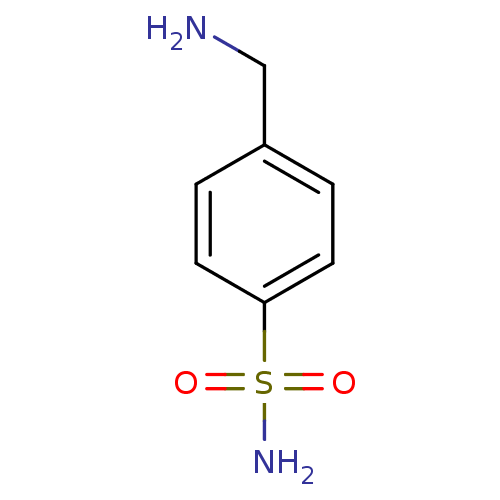
(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM12941
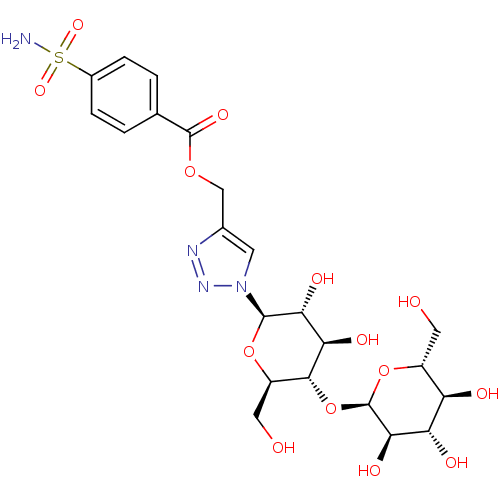
(4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)OCc1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O[C@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O Show InChI InChI=1S/C22H30N4O14S/c23-41(35,36)11-3-1-9(2-4-11)21(34)37-8-10-5-26(25-24-10)20-17(32)16(31)19(13(7-28)38-20)40-22-18(33)15(30)14(29)12(6-27)39-22/h1-5,12-20,22,27-33H,6-8H2,(H2,23,35,36)/t12-,13-,14-,15+,16-,17-,18-,19-,20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM12916
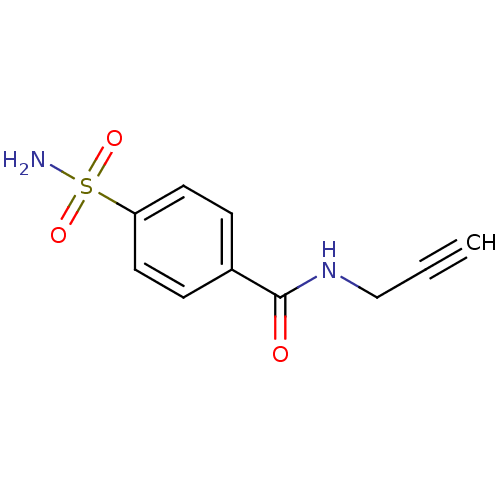
(CHEMBL386542 | N-(Prop-2-ynyl)-4-sulfamoylbenzamid...)Show InChI InChI=1S/C10H10N2O3S/c1-2-7-12-10(13)8-3-5-9(6-4-8)16(11,14)15/h1,3-6H,7H2,(H,12,13)(H2,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50200451
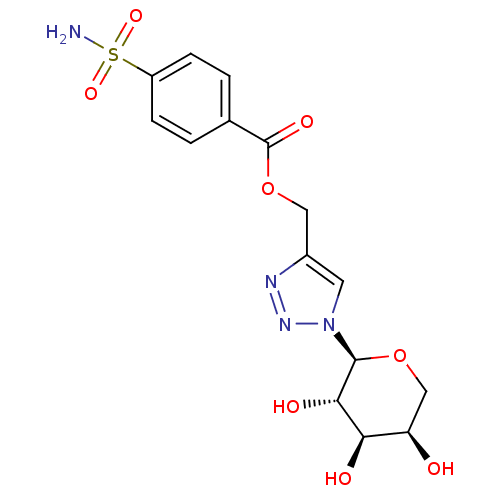
(4-sulfamoyl-benzoic acid 1-((2S,3S,4R,5R)-3,4,5-tr...)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)OCc1cn(nn1)[C@H]1OC[C@@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C15H18N4O8S/c16-28(24,25)10-3-1-8(2-4-10)15(23)27-6-9-5-19(18-17-9)14-13(22)12(21)11(20)7-26-14/h1-5,11-14,20-22H,6-7H2,(H2,16,24,25)/t11-,12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372664
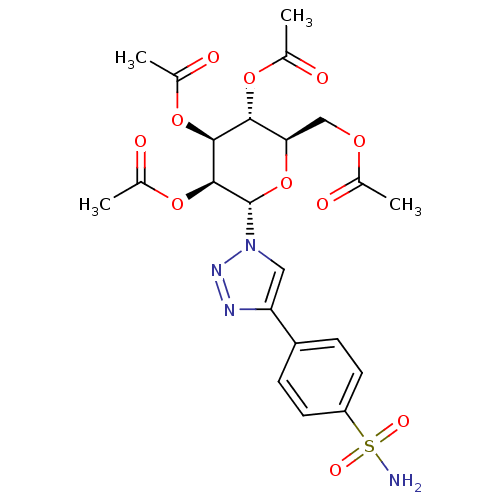
(CHEMBL273046)Show SMILES CC(=O)OC[C@H]1O[C@@H]([C@@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19-,20+,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372664

(CHEMBL273046)Show SMILES CC(=O)OC[C@H]1O[C@@H]([C@@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19-,20+,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50366063

(CHEMBL1956769)Show SMILES NS(=O)(=O)c1ccc(CCNC(=S)N[C@@H]2O[C@H](CO)[C@@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C21H33N3O12S2/c22-38(32,33)10-3-1-9(2-4-10)5-6-23-21(37)24-19-16(30)15(29)18(12(8-26)34-19)36-20-17(31)14(28)13(27)11(7-25)35-20/h1-4,11-20,25-31H,5-8H2,(H2,22,32,33)(H2,23,24,37)/t11-,12-,13-,14+,15-,16-,17-,18-,19-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50372658
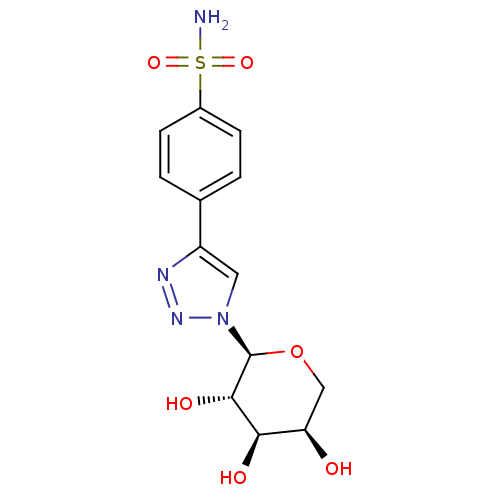
(CHEMBL409306)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@H]1OC[C@@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C13H16N4O6S/c14-24(21,22)8-3-1-7(2-4-8)9-5-17(16-15-9)13-12(20)11(19)10(18)6-23-13/h1-5,10-13,18-20H,6H2,(H2,14,21,22)/t10-,11-,12+,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372658

(CHEMBL409306)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@H]1OC[C@@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C13H16N4O6S/c14-24(21,22)8-3-1-7(2-4-8)9-5-17(16-15-9)13-12(20)11(19)10(18)6-23-13/h1-5,10-13,18-20H,6H2,(H2,14,21,22)/t10-,11-,12+,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50366059
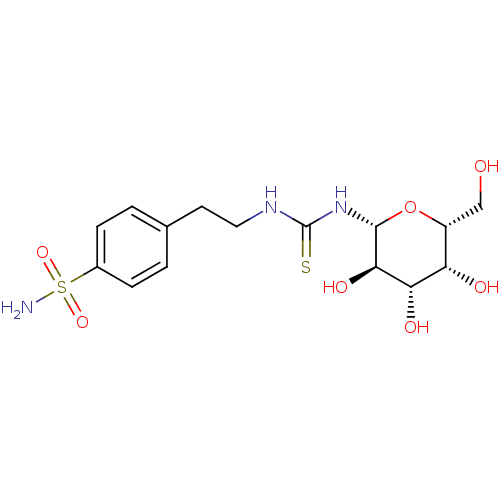
(CHEMBL1956765)Show SMILES NS(=O)(=O)c1ccc(CCNC(=S)N[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C15H23N3O7S2/c16-27(23,24)9-3-1-8(2-4-9)5-6-17-15(26)18-14-13(22)12(21)11(20)10(7-19)25-14/h1-4,10-14,19-22H,5-7H2,(H2,16,23,24)(H2,17,18,26)/t10-,11+,12+,13-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50372659
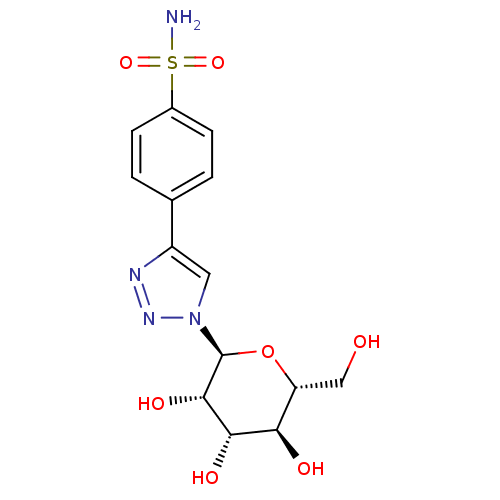
(CHEMBL259682)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O Show InChI InChI=1S/C14H18N4O7S/c15-26(23,24)8-3-1-7(2-4-8)9-5-18(17-16-9)14-13(22)12(21)11(20)10(6-19)25-14/h1-5,10-14,19-22H,6H2,(H2,15,23,24)/t10-,11-,12+,13+,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA1 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM12939

(4-({[4-(Aminosulfonyl)benzoyl]amino}methyl-1-(beta...)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)NCc1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O[C@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O Show InChI InChI=1S/C22H31N5O13S/c23-41(36,37)11-3-1-9(2-4-11)20(35)24-5-10-6-27(26-25-10)21-17(33)16(32)19(13(8-29)38-21)40-22-18(34)15(31)14(30)12(7-28)39-22/h1-4,6,12-19,21-22,28-34H,5,7-8H2,(H,24,35)(H2,23,36,37)/t12-,13-,14-,15+,16-,17-,18-,19-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10861
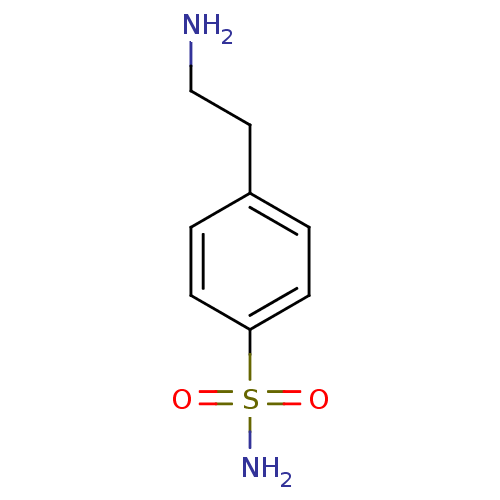
(4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...)Show InChI InChI=1S/C8H12N2O2S/c9-6-5-7-1-3-8(4-2-7)13(10,11)12/h1-4H,5-6,9H2,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50366076
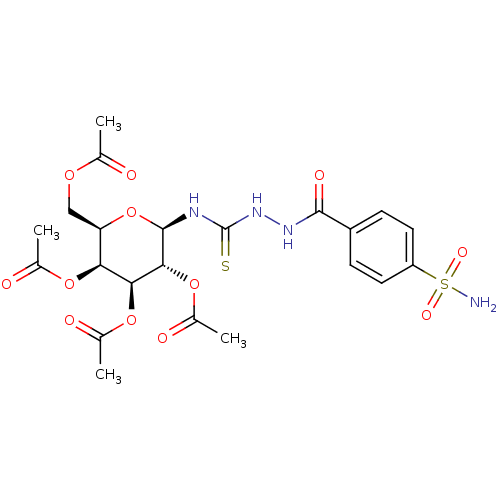
(CHEMBL1956750)Show SMILES CC(=O)OC[C@H]1O[C@@H](NC(=S)NNC(=O)c2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O |r| Show InChI InChI=1S/C22H28N4O12S2/c1-10(27)34-9-16-17(35-11(2)28)18(36-12(3)29)19(37-13(4)30)21(38-16)24-22(39)26-25-20(31)14-5-7-15(8-6-14)40(23,32)33/h5-8,16-19,21H,9H2,1-4H3,(H,25,31)(H2,23,32,33)(H2,24,26,39)/t16-,17+,18+,19-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM12943

(4-sulfamoyl-N-[(1-{[(2R,3S,4S,5R,6S)-3,4,5-trihydr...)Show SMILES CO[C@H]1O[C@H](Cn2cc(CNC(=O)c3ccc(cc3)S(N)(=O)=O)nn2)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C17H23N5O8S/c1-29-17-15(25)14(24)13(23)12(30-17)8-22-7-10(20-21-22)6-19-16(26)9-2-4-11(5-3-9)31(18,27)28/h2-5,7,12-15,17,23-25H,6,8H2,1H3,(H,19,26)(H2,18,27,28)/t12-,13-,14+,15-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM12932

(4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2-acet...)Show SMILES CC(=O)N[C@@H]1[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@@H](COC(C)=O)O[C@H]1n1cc(COC(=O)c2ccc(cc2)S(N)(=O)=O)nn1 Show InChI InChI=1S/C24H29N5O12S/c1-12(30)26-20-22(40-15(4)33)21(39-14(3)32)19(11-37-13(2)31)41-23(20)29-9-17(27-28-29)10-38-24(34)16-5-7-18(8-6-16)42(25,35)36/h5-9,19-23H,10-11H2,1-4H3,(H,26,30)(H2,25,35,36)/t19-,20-,21-,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM15231
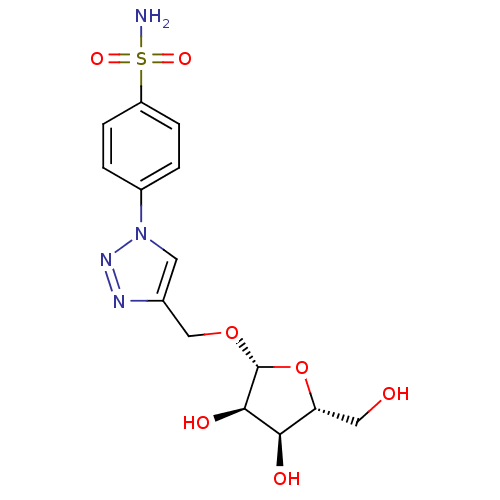
(4-[4-({[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymeth...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1cc(CO[C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C14H18N4O7S/c15-26(22,23)10-3-1-9(2-4-10)18-5-8(16-17-18)7-24-14-13(21)12(20)11(6-19)25-14/h1-5,11-14,19-21H,6-7H2,(H2,15,22,23)/t11-,12-,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 50: 1651-7 (2007)
Article DOI: 10.1021/jm061320h
BindingDB Entry DOI: 10.7270/Q2668BFJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM12918
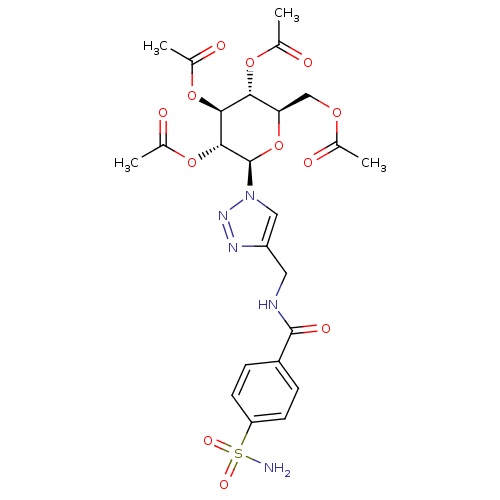
(4-({[4-(Aminosulfonyl)benzoyl]amino}methyl-1-(2,3,...)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(CNC(=O)c2ccc(cc2)S(N)(=O)=O)nn1 Show InChI InChI=1S/C24H29N5O12S/c1-12(30)37-11-19-20(38-13(2)31)21(39-14(3)32)22(40-15(4)33)24(41-19)29-10-17(27-28-29)9-26-23(34)16-5-7-18(8-6-16)42(25,35)36/h5-8,10,19-22,24H,9,11H2,1-4H3,(H,26,34)(H2,25,35,36)/t19-,20-,21+,22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372664

(CHEMBL273046)Show SMILES CC(=O)OC[C@H]1O[C@@H]([C@@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(nn1)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C22H26N4O11S/c1-11(27)33-10-18-19(34-12(2)28)20(35-13(3)29)21(36-14(4)30)22(37-18)26-9-17(24-25-26)15-5-7-16(8-6-15)38(23,31)32/h5-9,18-22H,10H2,1-4H3,(H2,23,31,32)/t18-,19-,20+,21+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50236140
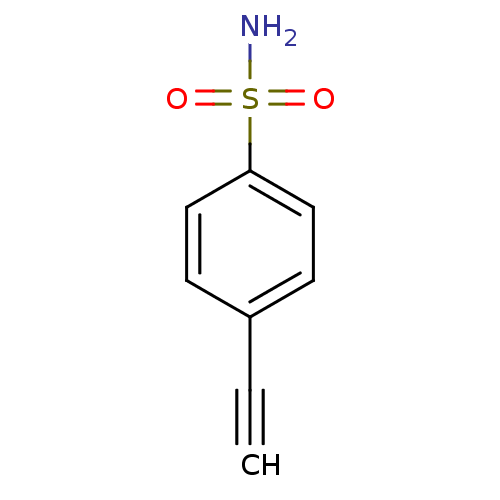
(4-ethynyl benzene sulfonamide | 4-ethynylbenzenesu...)Show InChI InChI=1S/C8H7NO2S/c1-2-7-3-5-8(6-4-7)12(9,10)11/h1,3-6H,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned CA2 by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50372659

(CHEMBL259682)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cn(nn1)[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O Show InChI InChI=1S/C14H18N4O7S/c15-26(23,24)8-3-1-7(2-4-8)9-5-18(17-16-9)14-13(22)12(21)11(20)10(6-19)25-14/h1-5,10-14,19-22H,6H2,(H2,15,23,24)/t10-,11-,12+,13+,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human CA9 catalytic domain by CO2 hydration method |
J Med Chem 51: 1945-53 (2008)
Article DOI: 10.1021/jm701426t
BindingDB Entry DOI: 10.7270/Q28P61CM |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50366074

(CHEMBL1956748)Show SMILES CC(=O)OC[C@H]1O[C@@H](NC(=S)NCc2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O |r| Show InChI InChI=1S/C22H29N3O11S2/c1-11(26)32-10-17-18(33-12(2)27)19(34-13(3)28)20(35-14(4)29)21(36-17)25-22(37)24-9-15-5-7-16(8-6-15)38(23,30)31/h5-8,17-21H,9-10H2,1-4H3,(H2,23,30,31)(H2,24,25,37)/t17-,18+,19+,20-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM12929

(4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)OCc1cn(nn1)[C@@H]1OC[C@@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C15H18N4O8S/c16-28(24,25)10-3-1-8(2-4-10)15(23)27-6-9-5-19(18-17-9)14-13(22)12(21)11(20)7-26-14/h1-5,11-14,20-22H,6-7H2,(H2,16,24,25)/t11-,12-,13+,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 49: 6539-48 (2006)
Article DOI: 10.1021/jm060967z
BindingDB Entry DOI: 10.7270/Q2X928HW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM12936
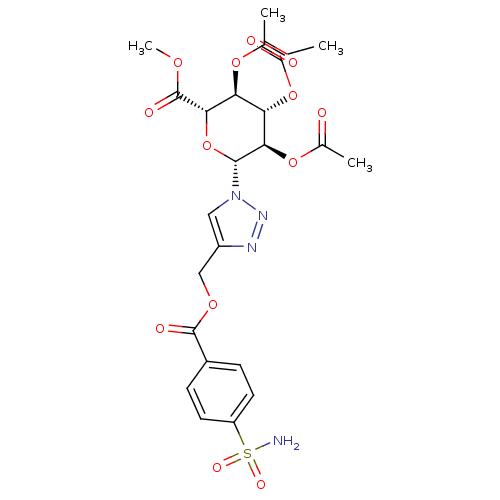
(4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2,3,4-...)Show SMILES COC(=O)[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(COC(=O)c2ccc(cc2)S(N)(=O)=O)nn1 Show InChI InChI=1S/C23H26N4O13S/c1-11(28)37-17-18(38-12(2)29)20(23(32)35-4)40-21(19(17)39-13(3)30)27-9-15(25-26-27)10-36-22(31)14-5-7-16(8-6-14)41(24,33)34/h5-9,17-21H,10H2,1-4H3,(H2,24,33,34)/t17-,18-,19+,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50366052
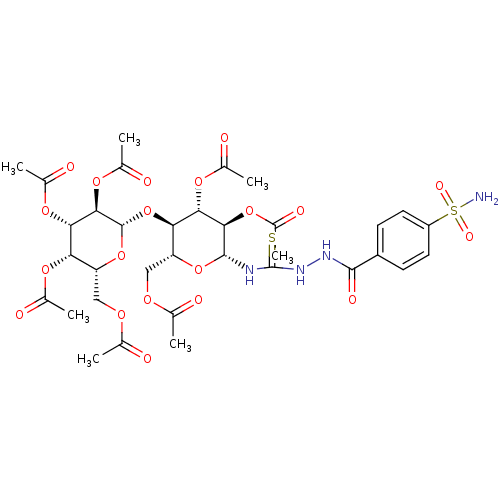
(CHEMBL1956758)Show SMILES CC(=O)OC[C@H]1O[C@@H](NC(=S)NNC(=O)c2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1O[C@@H]1O[C@H](COC(C)=O)[C@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O |r| Show InChI InChI=1S/C34H44N4O20S2/c1-14(39)49-12-23-26(58-33-30(55-20(7)45)28(53-18(5)43)25(51-16(3)41)24(57-33)13-50-15(2)40)27(52-17(4)42)29(54-19(6)44)32(56-23)36-34(59)38-37-31(46)21-8-10-22(11-9-21)60(35,47)48/h8-11,23-30,32-33H,12-13H2,1-7H3,(H,37,46)(H2,35,47,48)(H2,36,38,59)/t23-,24-,25+,26-,27+,28+,29-,30-,32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM12924
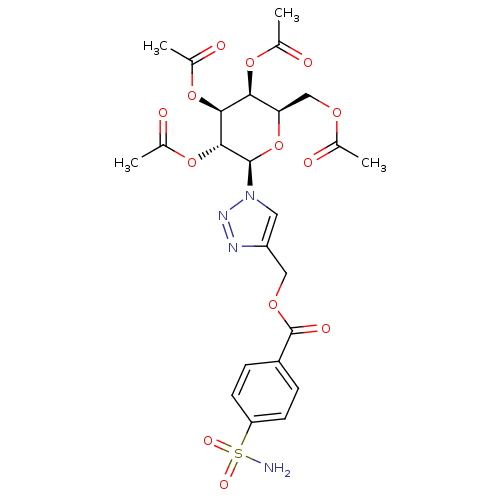
(4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2,3,4,...)Show SMILES CC(=O)OC[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O)n1cc(COC(=O)c2ccc(cc2)S(N)(=O)=O)nn1 Show InChI InChI=1S/C24H28N4O13S/c1-12(29)36-11-19-20(38-13(2)30)21(39-14(3)31)22(40-15(4)32)23(41-19)28-9-17(26-27-28)10-37-24(33)16-5-7-18(8-6-16)42(25,34)35/h5-9,19-23H,10-11H2,1-4H3,(H2,25,34,35)/t19-,20+,21+,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 49: 6539-48 (2006)
Article DOI: 10.1021/jm060967z
BindingDB Entry DOI: 10.7270/Q2X928HW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM15224
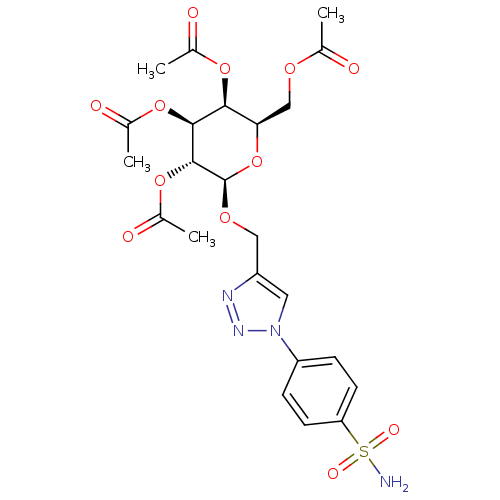
(4-(4-{[(2,3,4,6-tetra-O-acetyl-beta-D-galactopyran...)Show SMILES CC(=O)OC[C@H]1O[C@@H](OCc2cn(nn2)-c2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O |r| Show InChI InChI=1S/C23H28N4O12S/c1-12(28)34-11-19-20(36-13(2)29)21(37-14(3)30)22(38-15(4)31)23(39-19)35-10-16-9-27(26-25-16)17-5-7-18(8-6-17)40(24,32)33/h5-9,19-23H,10-11H2,1-4H3,(H2,24,32,33)/t19-,20+,21+,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 50: 1651-7 (2007)
Article DOI: 10.1021/jm061320h
BindingDB Entry DOI: 10.7270/Q2668BFJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50366065
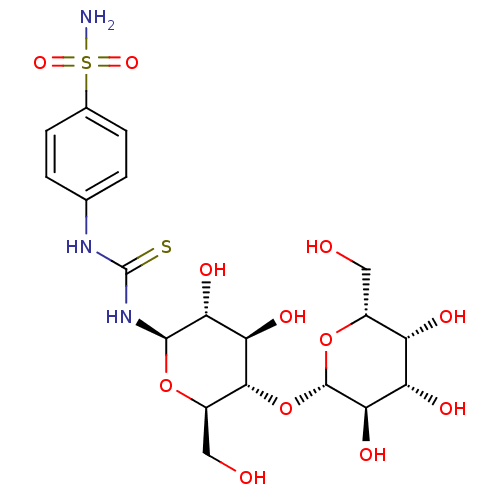
(CHEMBL1956771)Show SMILES NS(=O)(=O)c1ccc(NC(=S)N[C@@H]2O[C@H](CO)[C@@H](O[C@@H]3O[C@H](CO)[C@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C19H29N3O12S2/c20-36(30,31)8-3-1-7(2-4-8)21-19(35)22-17-14(28)13(27)16(10(6-24)32-17)34-18-15(29)12(26)11(25)9(5-23)33-18/h1-4,9-18,23-29H,5-6H2,(H2,20,30,31)(H2,21,22,35)/t9-,10-,11+,12+,13-,14-,15-,16-,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM15224

(4-(4-{[(2,3,4,6-tetra-O-acetyl-beta-D-galactopyran...)Show SMILES CC(=O)OC[C@H]1O[C@@H](OCc2cn(nn2)-c2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O |r| Show InChI InChI=1S/C23H28N4O12S/c1-12(28)34-11-19-20(36-13(2)29)21(37-14(3)30)22(38-15(4)31)23(39-19)35-10-16-9-27(26-25-16)17-5-7-18(8-6-17)40(24,32)33/h5-9,19-23H,10-11H2,1-4H3,(H2,24,32,33)/t19-,20+,21+,22-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 50: 1651-7 (2007)
Article DOI: 10.1021/jm061320h
BindingDB Entry DOI: 10.7270/Q2668BFJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM15230
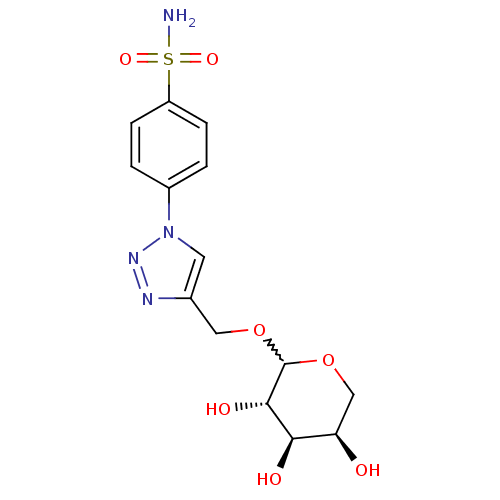
(4-[4-({[(3S,4R,5R)-3,4,5-trihydroxyoxan-2-yl]oxy}m...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1cc(COC2OC[C@@H](O)[C@@H](O)[C@@H]2O)nn1 |r,w:15.15| Show InChI InChI=1S/C14H18N4O7S/c15-26(22,23)10-3-1-9(2-4-10)18-5-8(16-17-18)6-24-14-13(21)12(20)11(19)7-25-14/h1-5,11-14,19-21H,6-7H2,(H2,15,22,23)/t11-,12-,13+,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 50: 1651-7 (2007)
Article DOI: 10.1021/jm061320h
BindingDB Entry DOI: 10.7270/Q2668BFJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50366058

(CHEMBL1956764)Show SMILES NS(=O)(=O)c1ccc(CNC(=S)N[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C14H21N3O7S2/c15-26(22,23)8-3-1-7(2-4-8)5-16-14(25)17-13-12(21)11(20)10(19)9(6-18)24-13/h1-4,9-13,18-21H,5-6H2,(H2,15,22,23)(H2,16,17,25)/t9-,10+,11+,12-,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50366069
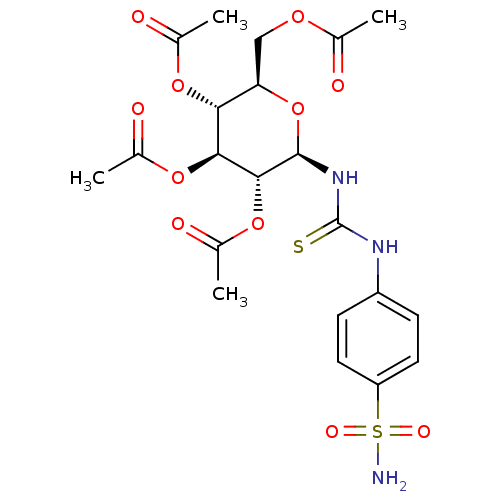
(CHEMBL1956743)Show SMILES CC(=O)OC[C@H]1O[C@@H](NC(=S)Nc2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C21H27N3O11S2/c1-10(25)31-9-16-17(32-11(2)26)18(33-12(3)27)19(34-13(4)28)20(35-16)24-21(36)23-14-5-7-15(8-6-14)37(22,29)30/h5-8,16-20H,9H2,1-4H3,(H2,22,29,30)(H2,23,24,36)/t16-,17-,18+,19-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM15228
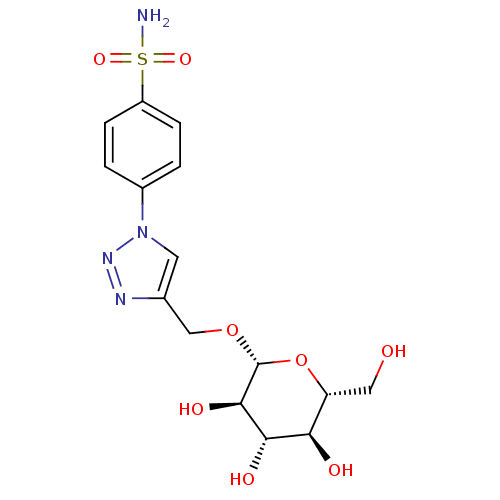
(4-[4-({[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydro...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1cc(CO[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C15H20N4O8S/c16-28(24,25)10-3-1-9(2-4-10)19-5-8(17-18-19)7-26-15-14(23)13(22)12(21)11(6-20)27-15/h1-5,11-15,20-23H,6-7H2,(H2,16,24,25)/t11-,12-,13+,14-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 50: 1651-7 (2007)
Article DOI: 10.1021/jm061320h
BindingDB Entry DOI: 10.7270/Q2668BFJ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50366053
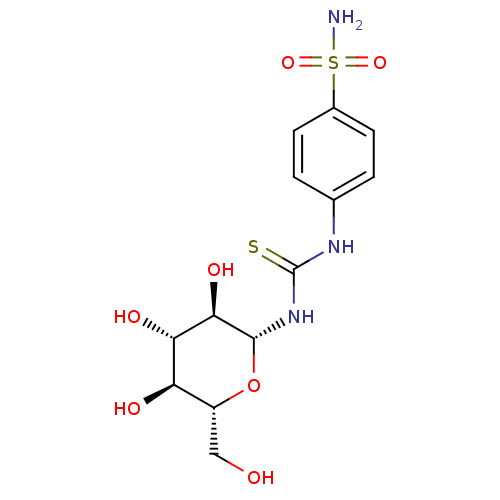
(CHEMBL1956759)Show SMILES NS(=O)(=O)c1ccc(NC(=S)N[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C13H19N3O7S2/c14-25(21,22)7-3-1-6(2-4-7)15-13(24)16-12-11(20)10(19)9(18)8(5-17)23-12/h1-4,8-12,17-20H,5H2,(H2,14,21,22)(H2,15,16,24)/t8-,9-,10+,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50366069

(CHEMBL1956743)Show SMILES CC(=O)OC[C@H]1O[C@@H](NC(=S)Nc2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C21H27N3O11S2/c1-10(25)31-9-16-17(32-11(2)26)18(33-12(3)27)19(34-13(4)28)20(35-16)24-21(36)23-14-5-7-15(8-6-14)37(22,29)30/h5-8,16-20H,9H2,1-4H3,(H2,22,29,30)(H2,23,24,36)/t16-,17-,18+,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM12936

(4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(2,3,4-...)Show SMILES COC(=O)[C@H]1O[C@H]([C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O)n1cc(COC(=O)c2ccc(cc2)S(N)(=O)=O)nn1 Show InChI InChI=1S/C23H26N4O13S/c1-11(28)37-17-18(38-12(2)29)20(23(32)35-4)40-21(19(17)39-13(3)30)27-9-15(25-26-27)10-36-22(31)14-5-7-16(8-6-14)41(24,33)34/h5-9,17-21H,10H2,1-4H3,(H2,24,33,34)/t17-,18-,19+,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 49: 6539-48 (2006)
Article DOI: 10.1021/jm060967z
BindingDB Entry DOI: 10.7270/Q2X928HW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM12921
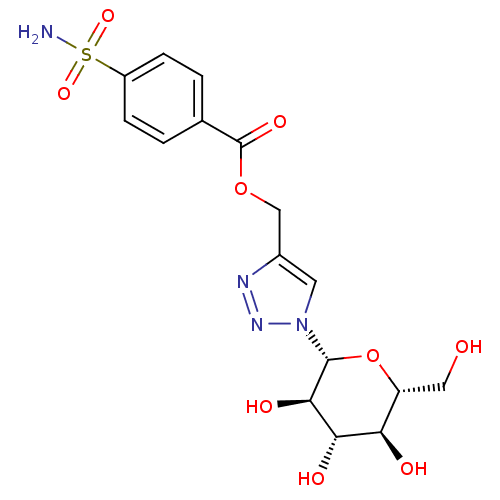
(4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)OCc1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C16H20N4O9S/c17-30(26,27)10-3-1-8(2-4-10)16(25)28-7-9-5-20(19-18-9)15-14(24)13(23)12(22)11(6-21)29-15/h1-5,11-15,21-24H,6-7H2,(H2,17,26,27)/t11-,12-,13+,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 49: 6539-48 (2006)
Article DOI: 10.1021/jm060967z
BindingDB Entry DOI: 10.7270/Q2X928HW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50366057
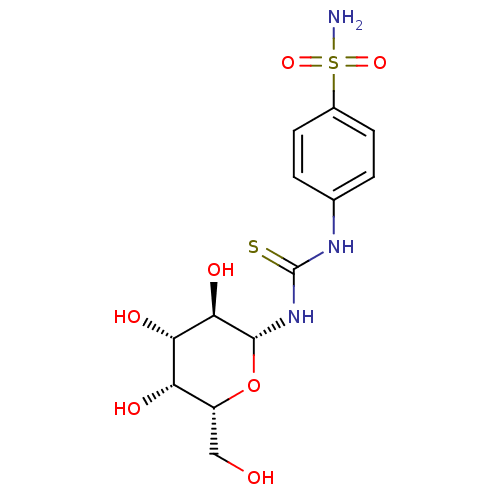
(CHEMBL1956763)Show SMILES NS(=O)(=O)c1ccc(NC(=S)N[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C13H19N3O7S2/c14-25(21,22)7-3-1-6(2-4-7)15-13(24)16-12-11(20)10(19)9(18)8(5-17)23-12/h1-4,8-12,17-20H,5H2,(H2,14,21,22)(H2,15,16,24)/t8-,9+,10+,11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM12921

(4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...)Show SMILES NS(=O)(=O)c1ccc(cc1)C(=O)OCc1cn(nn1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C16H20N4O9S/c17-30(26,27)10-3-1-8(2-4-10)16(25)28-7-9-5-20(19-18-9)15-14(24)13(23)12(22)11(6-21)29-15/h1-5,11-15,21-24H,6-7H2,(H2,17,26,27)/t11-,12-,13+,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50366069

(CHEMBL1956743)Show SMILES CC(=O)OC[C@H]1O[C@@H](NC(=S)Nc2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C21H27N3O11S2/c1-10(25)31-9-16-17(32-11(2)26)18(33-12(3)27)19(34-13(4)28)20(35-16)24-21(36)23-14-5-7-15(8-6-14)37(22,29)30/h5-8,16-20H,9H2,1-4H3,(H2,22,29,30)(H2,23,24,36)/t16-,17-,18+,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 12 catalytic domain preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM12937
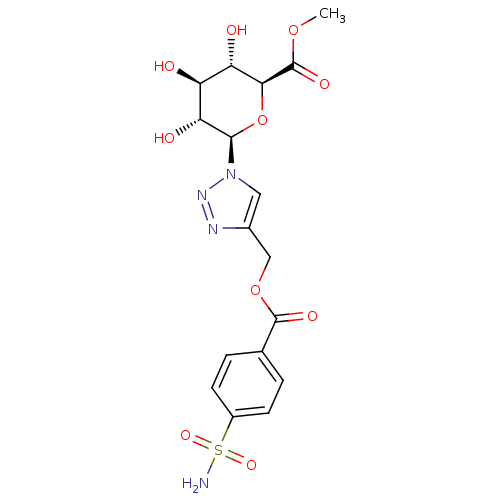
(4-({[4-(Aminosulfonyl)benzoyl]oxy}methyl-1-(beta-D...)Show SMILES COC(=O)[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1cc(COC(=O)c2ccc(cc2)S(N)(=O)=O)nn1 Show InChI InChI=1S/C17H20N4O10S/c1-29-17(26)14-12(23)11(22)13(24)15(31-14)21-6-9(19-20-21)7-30-16(25)8-2-4-10(5-3-8)32(18,27)28/h2-6,11-15,22-24H,7H2,1H3,(H2,18,27,28)/t11-,12-,13+,14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 49: 6539-48 (2006)
Article DOI: 10.1021/jm060967z
BindingDB Entry DOI: 10.7270/Q2X928HW |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM12945
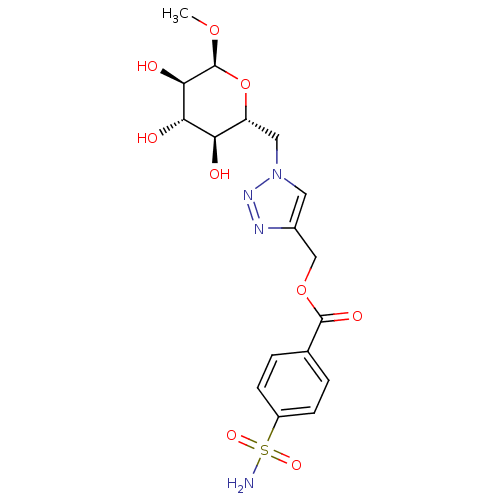
((1-{[(2R,3S,4S,5R,6S)-3,4,5-trihydroxy-6-methoxyox...)Show SMILES CO[C@H]1O[C@H](Cn2cc(COC(=O)c3ccc(cc3)S(N)(=O)=O)nn2)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C17H22N4O9S/c1-28-17-15(24)14(23)13(22)12(30-17)7-21-6-10(19-20-21)8-29-16(25)9-2-4-11(5-3-9)31(18,26)27/h2-6,12-15,17,22-24H,7-8H2,1H3,(H2,18,26,27)/t12-,13-,14+,15-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA12 by CO2 hydration assay |
Bioorg Med Chem Lett 17: 987-92 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.046
BindingDB Entry DOI: 10.7270/Q2JD4XMG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50366046
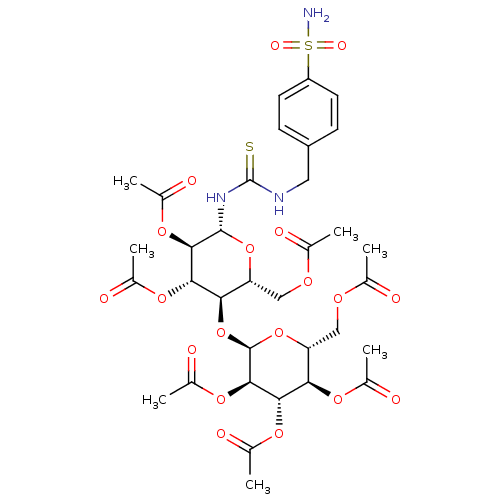
(CHEMBL1956752)Show SMILES CC(=O)OC[C@H]1O[C@@H](NC(=S)NCc2ccc(cc2)S(N)(=O)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1O[C@H]1O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]1OC(C)=O |r| Show InChI InChI=1S/C34H45N3O19S2/c1-15(38)47-13-24-27(56-33-31(53-21(7)44)29(51-19(5)42)26(49-17(3)40)25(55-33)14-48-16(2)39)28(50-18(4)41)30(52-20(6)43)32(54-24)37-34(57)36-12-22-8-10-23(11-9-22)58(35,45)46/h8-11,24-33H,12-14H2,1-7H3,(H2,35,45,46)(H2,36,37,57)/t24-,25-,26-,27-,28+,29+,30-,31-,32-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of human cloned carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration assay |
Bioorg Med Chem 20: 2392-404 (2012)
Article DOI: 10.1016/j.bmc.2012.01.052
BindingDB Entry DOI: 10.7270/Q22B8ZGK |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM15232
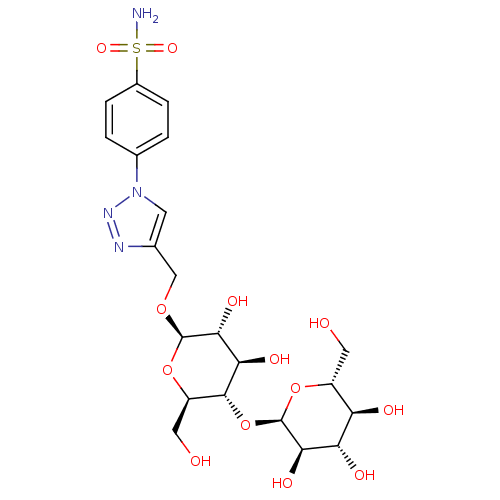
(4-(4-{[(4-O-alpha-D-glucopyranosyl-beta-D-glucopyr...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1cc(CO[C@@H]2O[C@H](CO)[C@@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)nn1 |r| Show InChI InChI=1S/C21H30N4O13S/c22-39(33,34)11-3-1-10(2-4-11)25-5-9(23-24-25)8-35-20-18(32)16(30)19(13(7-27)37-20)38-21-17(31)15(29)14(28)12(6-26)36-21/h1-5,12-21,26-32H,6-8H2,(H2,22,33,34)/t12-,13-,14-,15+,16-,17-,18-,19-,20-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30 | -46.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Griffith University
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 50: 1651-7 (2007)
Article DOI: 10.1021/jm061320h
BindingDB Entry DOI: 10.7270/Q2668BFJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data