 Found 335 hits with Last Name = 'won' and Initial = 'ka'
Found 335 hits with Last Name = 'won' and Initial = 'ka' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356075
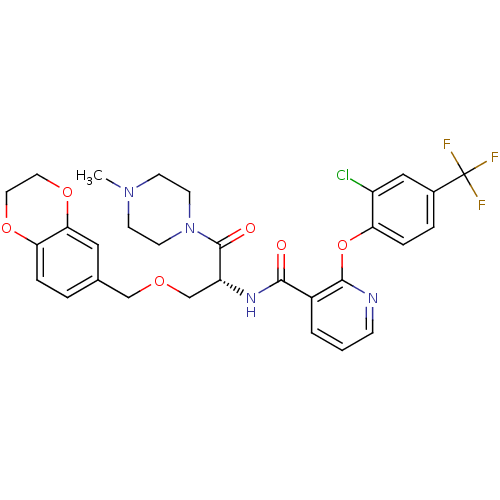
(CHEMBL1911818)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccc2OCCOc2c1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C30H30ClF3N4O6/c1-37-9-11-38(12-10-37)29(40)23(18-41-17-19-4-6-25-26(15-19)43-14-13-42-25)36-27(39)21-3-2-8-35-28(21)44-24-7-5-20(16-22(24)31)30(32,33)34/h2-8,15-16,23H,9-14,17-18H2,1H3,(H,36,39)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50420996
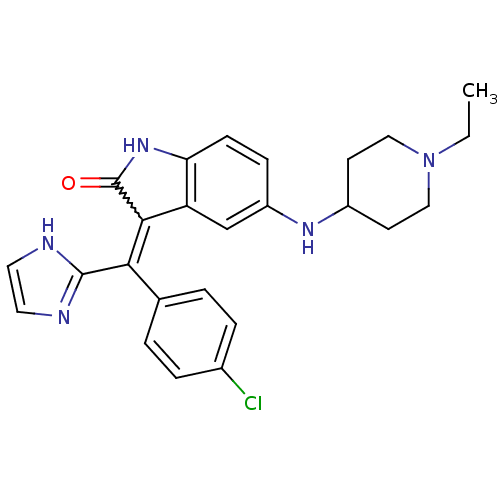
(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356076
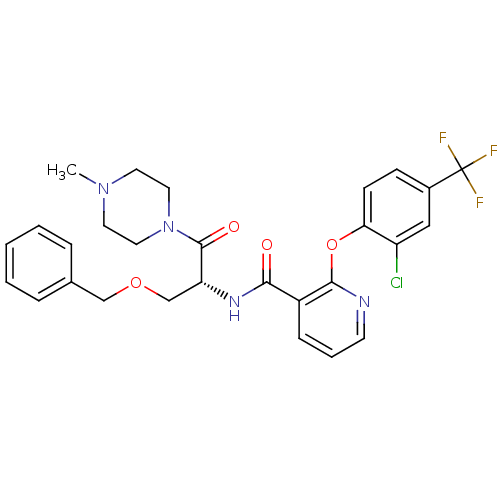
(CHEMBL1911817)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C28H28ClF3N4O4/c1-35-12-14-36(15-13-35)27(38)23(18-39-17-19-6-3-2-4-7-19)34-25(37)21-8-5-11-33-26(21)40-24-10-9-20(16-22(24)29)28(30,31)32/h2-11,16,23H,12-15,17-18H2,1H3,(H,34,37)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged Flt1 using poly(Glu,Tyr) as substrate after 60 mins by alphascreen assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356075

(CHEMBL1911818)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccc2OCCOc2c1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C30H30ClF3N4O6/c1-37-9-11-38(12-10-37)29(40)23(18-41-17-19-4-6-25-26(15-19)43-14-13-42-25)36-27(39)21-3-2-8-35-28(21)44-24-7-5-20(16-22(24)31)30(32,33)34/h2-8,15-16,23H,9-14,17-18H2,1H3,(H,36,39)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394804
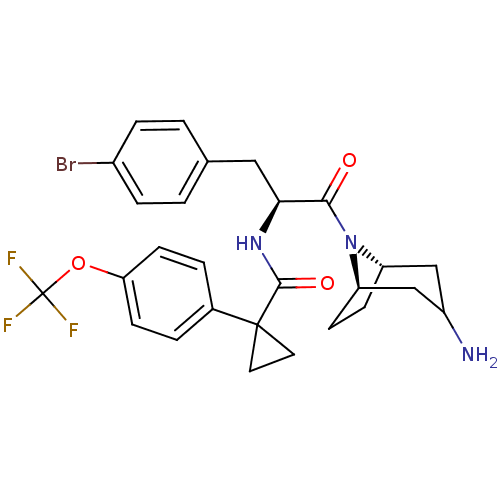
(CHEMBL2163828)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H29BrF3N3O3/c28-18-5-1-16(2-6-18)13-23(24(35)34-20-7-8-21(34)15-19(32)14-20)33-25(36)26(11-12-26)17-3-9-22(10-4-17)37-27(29,30)31/h1-6,9-10,19-21,23H,7-8,11-15,32H2,(H,33,36)/t19?,20-,21+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394807
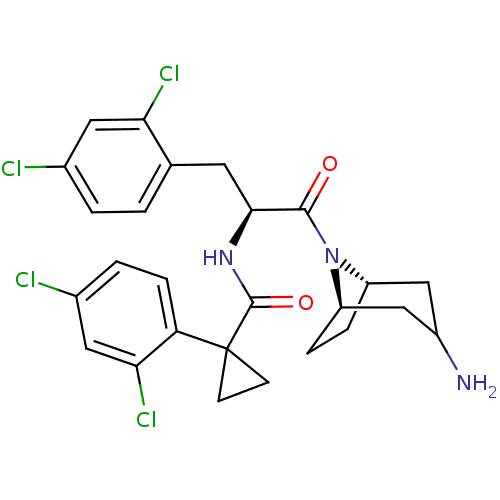
(CHEMBL2163825)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H27Cl4N3O2/c27-15-2-1-14(21(29)10-15)9-23(24(34)33-18-4-5-19(33)13-17(31)12-18)32-25(35)26(7-8-26)20-6-3-16(28)11-22(20)30/h1-3,6,10-11,17-19,23H,4-5,7-9,12-13,31H2,(H,32,35)/t17?,18-,19+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356076

(CHEMBL1911817)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C28H28ClF3N4O4/c1-35-12-14-36(15-13-35)27(38)23(18-39-17-19-6-3-2-4-7-19)34-25(37)21-8-5-11-33-26(21)40-24-10-9-20(16-22(24)29)28(30,31)32/h2-11,16,23H,12-15,17-18H2,1H3,(H,34,37)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356091
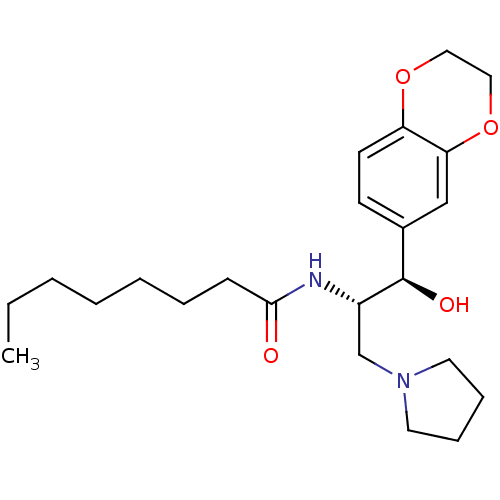
(CHEMBL1911679)Show SMILES CCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C23H36N2O4/c1-2-3-4-5-6-9-22(26)24-19(17-25-12-7-8-13-25)23(27)18-10-11-20-21(16-18)29-15-14-28-20/h10-11,16,19,23,27H,2-9,12-15,17H2,1H3,(H,24,26)/t19-,23+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356090
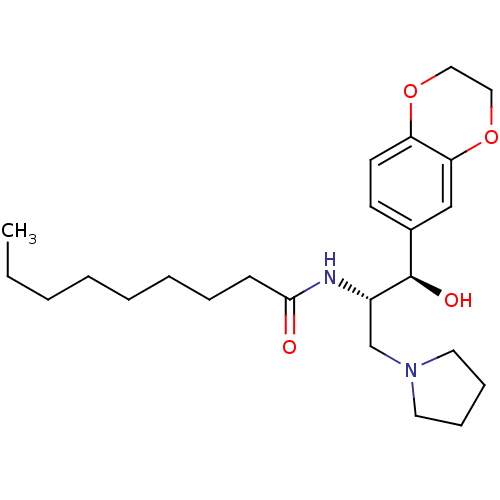
(CHEMBL1911678)Show SMILES CCCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C24H38N2O4/c1-2-3-4-5-6-7-10-23(27)25-20(18-26-13-8-9-14-26)24(28)19-11-12-21-22(17-19)30-16-15-29-21/h11-12,17,20,24,28H,2-10,13-16,18H2,1H3,(H,25,27)/t20-,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356078
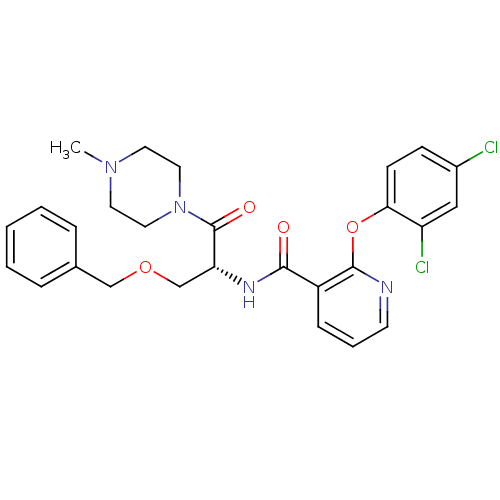
(CHEMBL1911815)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C27H28Cl2N4O4/c1-32-12-14-33(15-13-32)27(35)23(18-36-17-19-6-3-2-4-7-19)31-25(34)21-8-5-11-30-26(21)37-24-10-9-20(28)16-22(24)29/h2-11,16,23H,12-15,17-18H2,1H3,(H,31,34)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356077
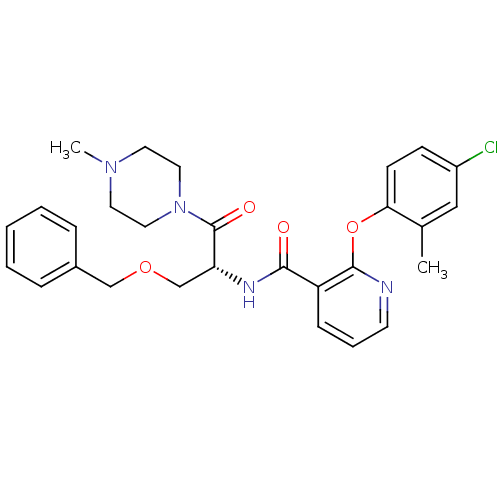
(CHEMBL1911816)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(Cl)cc1C |r| Show InChI InChI=1S/C28H31ClN4O4/c1-20-17-22(29)10-11-25(20)37-27-23(9-6-12-30-27)26(34)31-24(19-36-18-21-7-4-3-5-8-21)28(35)33-15-13-32(2)14-16-33/h3-12,17,24H,13-16,18-19H2,1-2H3,(H,31,34)/t24-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394812
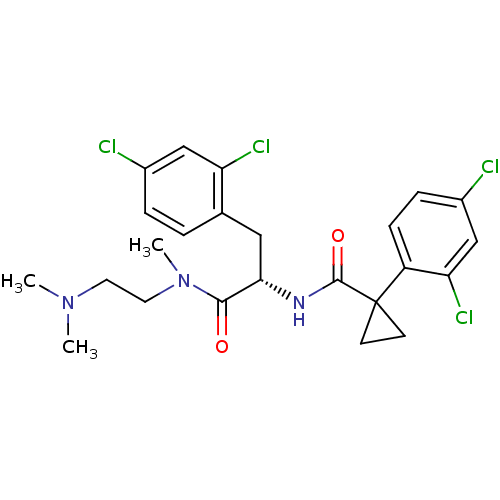
(CHEMBL2163838)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H27Cl4N3O2/c1-30(2)10-11-31(3)22(32)21(12-15-4-5-16(25)13-19(15)27)29-23(33)24(8-9-24)18-7-6-17(26)14-20(18)28/h4-7,13-14,21H,8-12H2,1-3H3,(H,29,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50421033
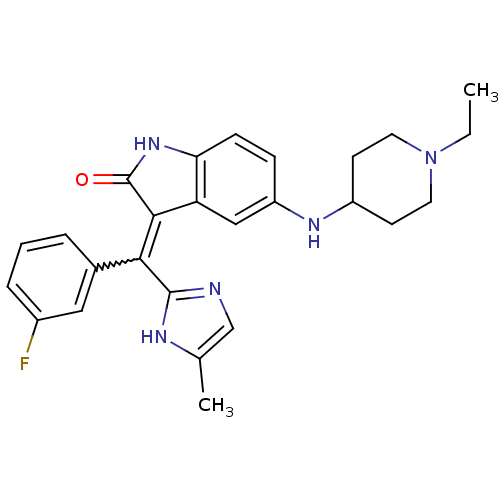
(CHEMBL2087167)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3cccc(F)c3)c2c1 |w:17.25| Show InChI InChI=1S/C26H28FN5O/c1-3-32-11-9-19(10-12-32)30-20-7-8-22-21(14-20)24(26(33)31-22)23(25-28-15-16(2)29-25)17-5-4-6-18(27)13-17/h4-8,13-15,19,30H,3,9-12H2,1-2H3,(H,28,29)(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356090

(CHEMBL1911678)Show SMILES CCCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C24H38N2O4/c1-2-3-4-5-6-7-10-23(27)25-20(18-26-13-8-9-14-26)24(28)19-11-12-21-22(17-19)30-16-15-29-21/h11-12,17,20,24,28H,2-10,13-16,18H2,1H3,(H,25,27)/t20-,24+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356091

(CHEMBL1911679)Show SMILES CCCCCCCC(=O)N[C@@H](CN1CCCC1)[C@H](O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C23H36N2O4/c1-2-3-4-5-6-9-22(26)24-19(17-25-12-7-8-13-25)23(27)18-10-11-20-21(16-18)29-15-14-28-20/h10-11,16,19,23,27H,2-9,12-15,17H2,1H3,(H,24,26)/t19-,23+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394820
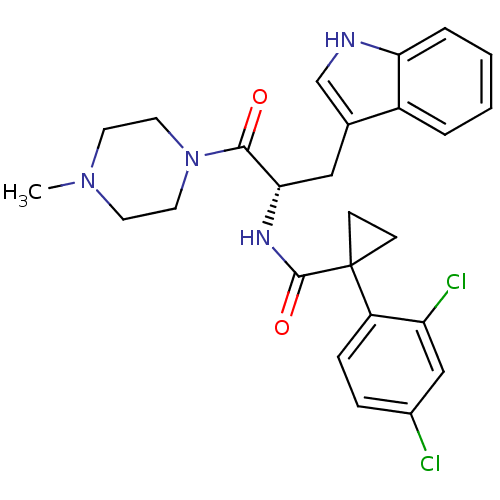
(CHEMBL2163829)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O2/c1-31-10-12-32(13-11-31)24(33)23(14-17-16-29-22-5-3-2-4-19(17)22)30-25(34)26(8-9-26)20-7-6-18(27)15-21(20)28/h2-7,15-16,23,29H,8-14H2,1H3,(H,30,34)/t23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421028
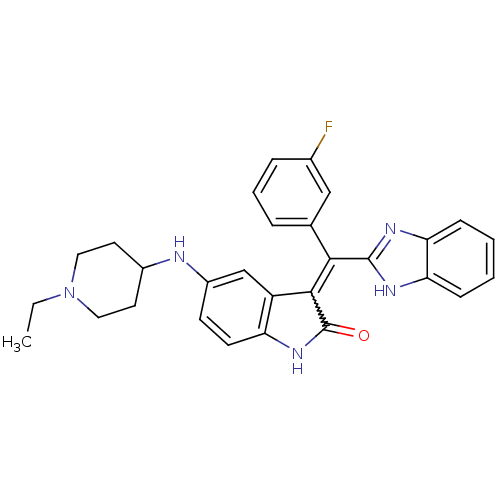
(CHEMBL2086756)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3cccc(F)c3)c2c1 Show InChI InChI=1S/C29H28FN5O/c1-2-35-14-12-20(13-15-35)31-21-10-11-23-22(17-21)27(29(36)34-23)26(18-6-5-7-19(30)16-18)28-32-24-8-3-4-9-25(24)33-28/h3-11,16-17,20,31H,2,12-15H2,1H3,(H,32,33)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394805
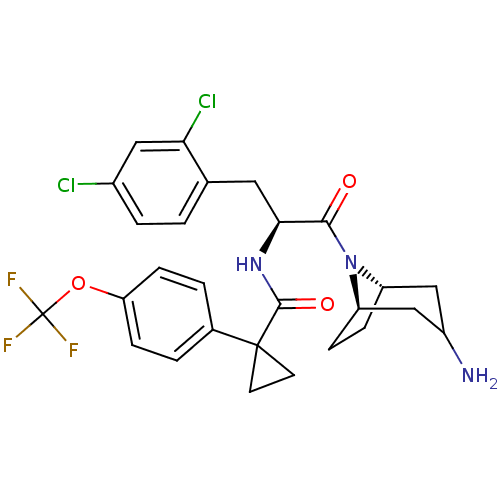
(CHEMBL2163827)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(OC(F)(F)F)cc1 |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C27H28Cl2F3N3O3/c28-17-4-1-15(22(29)12-17)11-23(24(36)35-19-5-6-20(35)14-18(33)13-19)34-25(37)26(9-10-26)16-2-7-21(8-3-16)38-27(30,31)32/h1-4,7-8,12,18-20,23H,5-6,9-11,13-14,33H2,(H,34,37)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394816
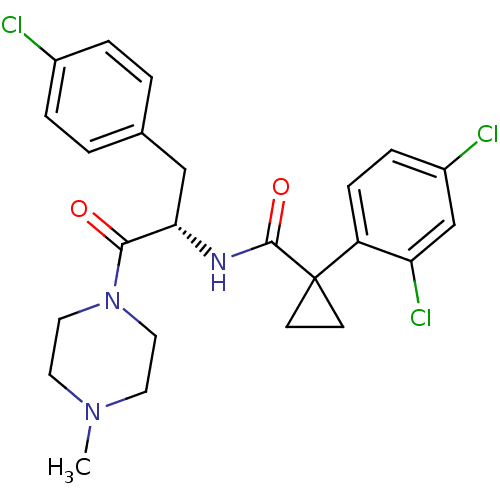
(CHEMBL2163833)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26Cl3N3O2/c1-29-10-12-30(13-11-29)22(31)21(14-16-2-4-17(25)5-3-16)28-23(32)24(8-9-24)19-7-6-18(26)15-20(19)27/h2-7,15,21H,8-14H2,1H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421016
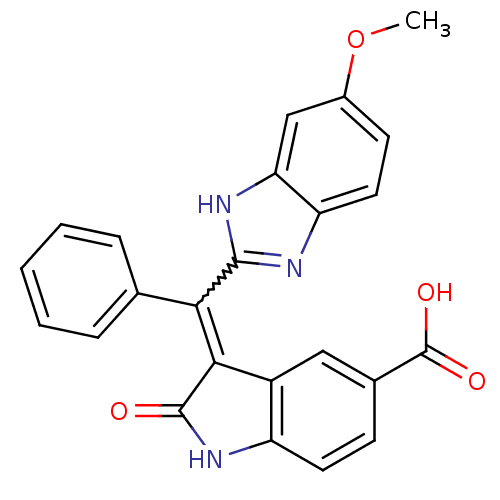
(CHEMBL2086742)Show SMILES COc1ccc2nc([nH]c2c1)C(=C1C(=O)Nc2ccc(cc12)C(O)=O)c1ccccc1 |w:11.12| Show InChI InChI=1S/C24H17N3O4/c1-31-15-8-10-18-19(12-15)26-22(25-18)20(13-5-3-2-4-6-13)21-16-11-14(24(29)30)7-9-17(16)27-23(21)28/h2-12H,1H3,(H,25,26)(H,27,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394806

(CHEMBL2163826)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H28BrCl2N3O2/c27-16-3-1-15(2-4-16)11-23(24(33)32-19-6-7-20(32)14-18(30)13-19)31-25(34)26(9-10-26)21-8-5-17(28)12-22(21)29/h1-5,8,12,18-20,23H,6-7,9-11,13-14,30H2,(H,31,34)/t18?,19-,20+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356079
(CHEMBL1911814)Show SMILES CN1CCN(CC1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cc(F)cnc1Oc1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C27H27Cl2FN4O4/c1-33-9-11-34(12-10-33)27(36)23(17-37-16-18-5-3-2-4-6-18)32-25(35)21-14-20(30)15-31-26(21)38-24-8-7-19(28)13-22(24)29/h2-8,13-15,23H,9-12,16-17H2,1H3,(H,32,35)/t23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421034
(CHEMBL2087168)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3cc(F)cc(F)c3)c2c1 |w:17.25| Show InChI InChI=1S/C26H27F2N5O/c1-3-33-8-6-19(7-9-33)31-20-4-5-22-21(13-20)24(26(34)32-22)23(25-29-14-15(2)30-25)16-10-17(27)12-18(28)11-16/h4-5,10-14,19,31H,3,6-9H2,1-2H3,(H,29,30)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50421026
(CHEMBL2086753)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3ccccc3)c2c1 Show InChI InChI=1S/C29H29N5O/c1-2-34-16-14-20(15-17-34)30-21-12-13-23-22(18-21)27(29(35)33-23)26(19-8-4-3-5-9-19)28-31-24-10-6-7-11-25(24)32-28/h3-13,18,20,30H,2,14-17H2,1H3,(H,31,32)(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421035
(CHEMBL2087169)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3ccccc3F)c2c1 |w:17.25| Show InChI InChI=1S/C26H28FN5O/c1-3-32-12-10-17(11-13-32)30-18-8-9-22-20(14-18)24(26(33)31-22)23(25-28-15-16(2)29-25)19-6-4-5-7-21(19)27/h4-9,14-15,17,30H,3,10-13H2,1-2H3,(H,28,29)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50421034

(CHEMBL2087168)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3cc(F)cc(F)c3)c2c1 |w:17.25| Show InChI InChI=1S/C26H27F2N5O/c1-3-33-8-6-19(7-9-33)31-20-4-5-22-21(13-20)24(26(34)32-22)23(25-29-14-15(2)30-25)16-10-17(27)12-18(28)11-16/h4-5,10-14,19,31H,3,6-9H2,1-2H3,(H,29,30)(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394809
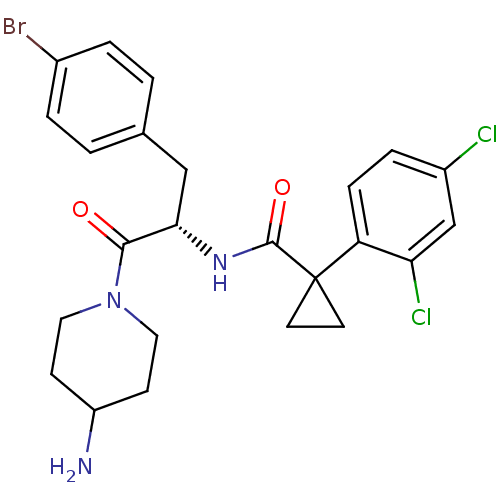
(CHEMBL2163823)Show SMILES NC1CCN(CC1)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H26BrCl2N3O2/c25-16-3-1-15(2-4-16)13-21(22(31)30-11-7-18(28)8-12-30)29-23(32)24(9-10-24)19-6-5-17(26)14-20(19)27/h1-6,14,18,21H,7-13,28H2,(H,29,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of Flt4 |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356094

(CHEMBL1911829)Show SMILES FC(F)(F)c1ccc(Oc2ncccc2C(=O)N[C@H](COCc2ccccc2)C(=O)N[C@@H]2CN3CCC2CC3)c(Cl)c1 |r,wD:18.18,31.32,(8.76,-41.16,;8.77,-39.62,;10.11,-38.85,;10.1,-40.39,;7.44,-38.85,;6.1,-39.61,;4.77,-38.83,;4.79,-37.3,;3.46,-36.52,;3.47,-34.98,;4.8,-34.21,;4.8,-32.67,;3.46,-31.9,;2.13,-32.67,;2.14,-34.2,;.8,-34.97,;.8,-36.51,;-.53,-34.2,;-1.86,-34.96,;-1.87,-36.5,;-3.2,-37.27,;-3.2,-38.81,;-1.87,-39.59,;-1.87,-41.13,;-.54,-41.91,;.8,-41.14,;.79,-39.59,;-.54,-38.83,;-3.2,-34.19,;-3.19,-32.65,;-4.53,-34.96,;-5.86,-34.19,;-7.2,-34.96,;-8.53,-34.18,;-8.53,-32.64,;-7.19,-31.88,;-5.86,-32.65,;-7.4,-32.68,;-7,-34.18,;6.11,-36.53,;6.11,-34.99,;7.44,-37.3,)| Show InChI InChI=1S/C30H30ClF3N4O4/c31-23-15-21(30(32,33)34)8-9-26(23)42-29-22(7-4-12-35-29)27(39)37-25(18-41-17-19-5-2-1-3-6-19)28(40)36-24-16-38-13-10-20(24)11-14-38/h1-9,12,15,20,24-25H,10-11,13-14,16-18H2,(H,36,40)(H,37,39)/t24-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394817
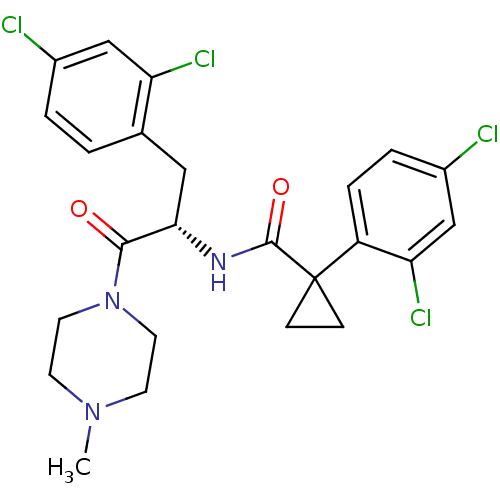
(CHEMBL2163832)Show SMILES CN1CCN(CC1)C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H25Cl4N3O2/c1-30-8-10-31(11-9-30)22(32)21(12-15-2-3-16(25)13-19(15)27)29-23(33)24(6-7-24)18-5-4-17(26)14-20(18)28/h2-5,13-14,21H,6-12H2,1H3,(H,29,33)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394807

(CHEMBL2163825)Show SMILES NC1C[C@@H]2CC[C@H](C1)N2C(=O)[C@H](Cc1ccc(Cl)cc1Cl)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r,TLB:9:8:1.2.7:5.4| Show InChI InChI=1S/C26H27Cl4N3O2/c27-15-2-1-14(21(29)10-15)9-23(24(34)33-18-4-5-19(33)13-17(31)12-18)32-25(35)26(7-8-26)20-6-3-16(28)11-22(20)30/h1-3,6,10-11,17-19,23H,4-5,7-9,12-13,31H2,(H,32,35)/t17?,18-,19+,23-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50394811
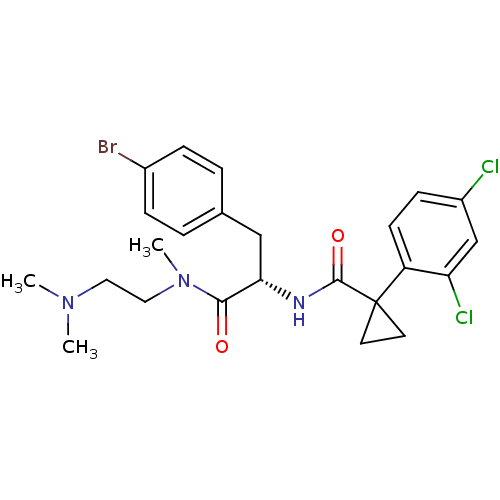
(CHEMBL2163839)Show SMILES CN(C)CCN(C)C(=O)[C@H](Cc1ccc(Br)cc1)NC(=O)C1(CC1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C24H28BrCl2N3O2/c1-29(2)12-13-30(3)22(31)21(14-16-4-6-17(25)7-5-16)28-23(32)24(10-11-24)19-9-8-18(26)15-20(19)27/h4-9,15,21H,10-14H2,1-3H3,(H,28,32)/t21-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis |
J Med Chem 55: 4322-35 (2012)
Article DOI: 10.1021/jm300122u
BindingDB Entry DOI: 10.7270/Q2MG7QMF |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421029
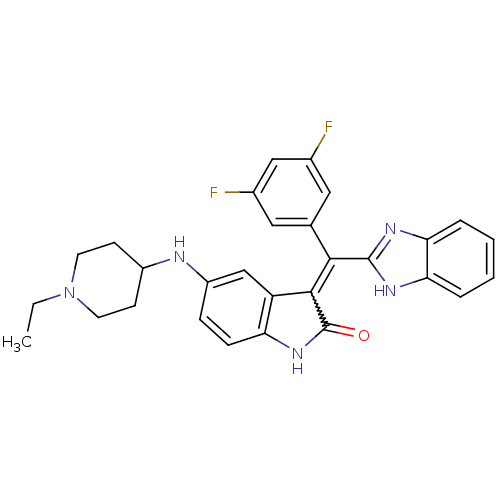
(CHEMBL2086757)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3cc(F)cc(F)c3)c2c1 Show InChI InChI=1S/C29H27F2N5O/c1-2-36-11-9-20(10-12-36)32-21-7-8-23-22(16-21)27(29(37)35-23)26(17-13-18(30)15-19(31)14-17)28-33-24-5-3-4-6-25(24)34-28/h3-8,13-16,20,32H,2,9-12H2,1H3,(H,33,34)(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421033

(CHEMBL2087167)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3cccc(F)c3)c2c1 |w:17.25| Show InChI InChI=1S/C26H28FN5O/c1-3-32-11-9-19(10-12-32)30-20-7-8-22-21(14-20)24(26(33)31-22)23(25-28-15-16(2)29-25)17-5-4-6-18(27)13-17/h4-8,13-15,19,30H,3,9-12H2,1-2H3,(H,28,29)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50421033

(CHEMBL2087167)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3cccc(F)c3)c2c1 |w:17.25| Show InChI InChI=1S/C26H28FN5O/c1-3-32-11-9-19(10-12-32)30-20-7-8-22-21(14-20)24(26(33)31-22)23(25-28-15-16(2)29-25)17-5-4-6-18(27)13-17/h4-8,13-15,19,30H,3,9-12H2,1-2H3,(H,28,29)(H,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged FGFR1 using poly(Glu,Tyr) as substrate after 30 mins by alphascreen assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421036
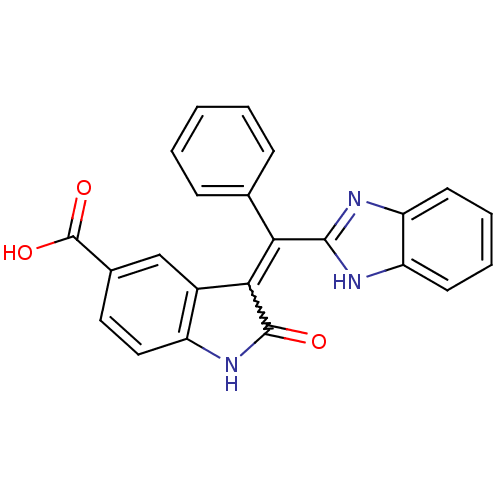
(CHEMBL2086736)Show SMILES OC(=O)c1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3ccccc3)c2c1 Show InChI InChI=1S/C23H15N3O3/c27-22-20(15-12-14(23(28)29)10-11-16(15)26-22)19(13-6-2-1-3-7-13)21-24-17-8-4-5-9-18(17)25-21/h1-12H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50421026

(CHEMBL2086753)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3ccccc3)c2c1 Show InChI InChI=1S/C29H29N5O/c1-2-34-16-14-20(15-17-34)30-21-12-13-23-22(18-21)27(29(35)33-23)26(19-8-4-3-5-9-19)28-31-24-10-6-7-11-25(24)32-28/h3-13,18,20,30H,2,14-17H2,1H3,(H,31,32)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged FGFR1 using poly(Glu,Tyr) as substrate after 30 mins by alphascreen assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50421028

(CHEMBL2086756)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3cccc(F)c3)c2c1 Show InChI InChI=1S/C29H28FN5O/c1-2-35-14-12-20(13-15-35)31-21-10-11-23-22(17-21)27(29(36)34-23)26(18-6-5-7-19(30)16-18)28-32-24-8-3-4-9-25(24)33-28/h3-11,16-17,20,31H,2,12-15H2,1H3,(H,32,33)(H,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged FGFR1 using poly(Glu,Tyr) as substrate after 30 mins by alphascreen assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50421032
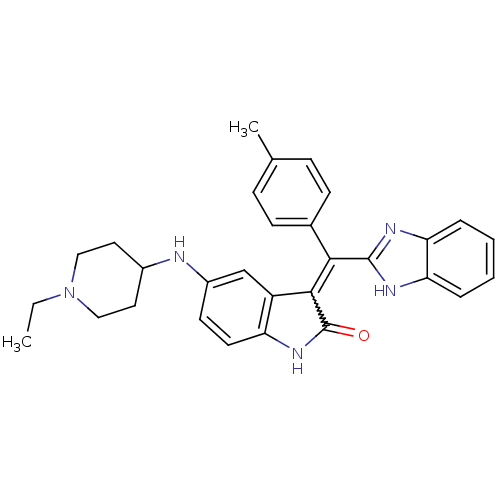
(CHEMBL2086754)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccccc4[nH]3)c3ccc(C)cc3)c2c1 Show InChI InChI=1S/C30H31N5O/c1-3-35-16-14-21(15-17-35)31-22-12-13-24-23(18-22)28(30(36)34-24)27(20-10-8-19(2)9-11-20)29-32-25-6-4-5-7-26(25)33-29/h4-13,18,21,31H,3,14-17H2,1-2H3,(H,32,33)(H,34,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50421014
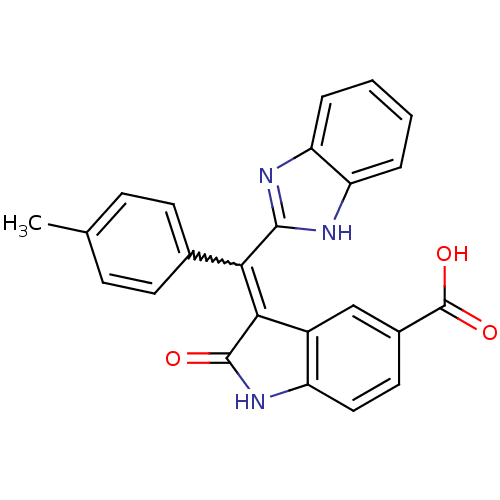
(CHEMBL2086740)Show SMILES Cc1ccc(cc1)C(=C1C(=O)Nc2ccc(cc12)C(O)=O)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C24H17N3O3/c1-13-6-8-14(9-7-13)20(22-25-18-4-2-3-5-19(18)26-22)21-16-12-15(24(29)30)10-11-17(16)27-23(21)28/h2-12H,1H3,(H,25,26)(H,27,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged KDR after 4 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356066
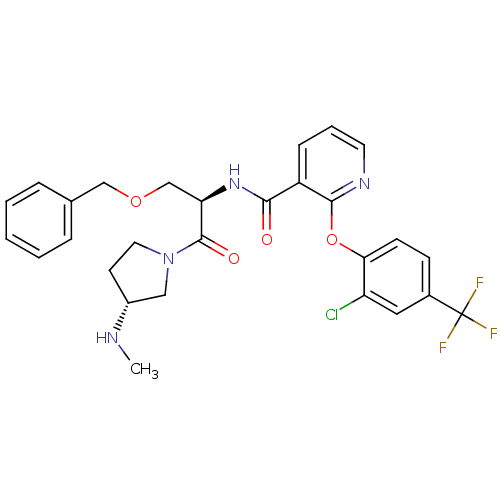
(CHEMBL1911827)Show SMILES CN[C@@H]1CCN(C1)C(=O)[C@@H](COCc1ccccc1)NC(=O)c1cccnc1Oc1ccc(cc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C28H28ClF3N4O4/c1-33-20-11-13-36(15-20)27(38)23(17-39-16-18-6-3-2-4-7-18)35-25(37)21-8-5-12-34-26(21)40-24-10-9-19(14-22(24)29)28(30,31)32/h2-10,12,14,20,23,33H,11,13,15-17H2,1H3,(H,35,37)/t20-,23-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Ceramide glucosyltransferase
(Homo sapiens (Human)) | BDBM50356094

(CHEMBL1911829)Show SMILES FC(F)(F)c1ccc(Oc2ncccc2C(=O)N[C@H](COCc2ccccc2)C(=O)N[C@@H]2CN3CCC2CC3)c(Cl)c1 |r,wD:18.18,31.32,(8.76,-41.16,;8.77,-39.62,;10.11,-38.85,;10.1,-40.39,;7.44,-38.85,;6.1,-39.61,;4.77,-38.83,;4.79,-37.3,;3.46,-36.52,;3.47,-34.98,;4.8,-34.21,;4.8,-32.67,;3.46,-31.9,;2.13,-32.67,;2.14,-34.2,;.8,-34.97,;.8,-36.51,;-.53,-34.2,;-1.86,-34.96,;-1.87,-36.5,;-3.2,-37.27,;-3.2,-38.81,;-1.87,-39.59,;-1.87,-41.13,;-.54,-41.91,;.8,-41.14,;.79,-39.59,;-.54,-38.83,;-3.2,-34.19,;-3.19,-32.65,;-4.53,-34.96,;-5.86,-34.19,;-7.2,-34.96,;-8.53,-34.18,;-8.53,-32.64,;-7.19,-31.88,;-5.86,-32.65,;-7.4,-32.68,;-7,-34.18,;6.11,-36.53,;6.11,-34.99,;7.44,-37.3,)| Show InChI InChI=1S/C30H30ClF3N4O4/c31-23-15-21(30(32,33)34)8-9-26(23)42-29-22(7-4-12-35-29)27(39)37-25(18-41-17-19-5-2-1-3-6-19)28(40)36-24-16-38-13-10-20(24)11-14-38/h1-9,12,15,20,24-25H,10-11,13-14,16-18H2,(H,36,40)(H,37,39)/t24-,25-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction |
Bioorg Med Chem Lett 21: 6773-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.037
BindingDB Entry DOI: 10.7270/Q2833SG2 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50421035

(CHEMBL2087169)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc(C)[nH]3)c3ccccc3F)c2c1 |w:17.25| Show InChI InChI=1S/C26H28FN5O/c1-3-32-12-10-17(11-13-32)30-18-8-9-22-20(14-18)24(26(33)31-22)23(25-28-15-16(2)29-25)19-6-4-5-7-21(19)27/h4-9,14-15,17,30H,3,10-13H2,1-2H3,(H,28,29)(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged FGFR1 using poly(Glu,Tyr) as substrate after 30 mins by alphascreen assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50420997
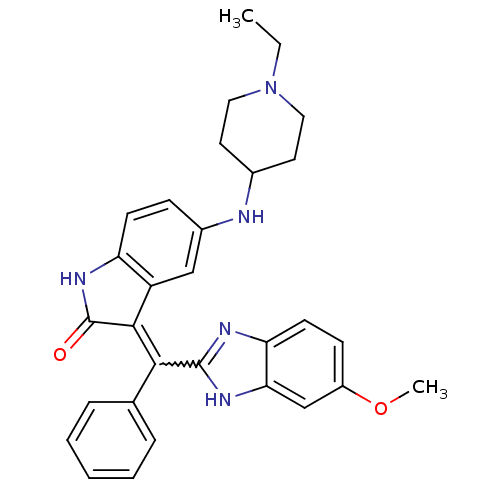
(CHEMBL2086751)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3nc4ccc(OC)cc4[nH]3)c3ccccc3)c2c1 |w:17.18| Show InChI InChI=1S/C30H31N5O2/c1-3-35-15-13-20(14-16-35)31-21-9-11-24-23(17-21)28(30(36)34-24)27(19-7-5-4-6-8-19)29-32-25-12-10-22(37-2)18-26(25)33-29/h4-12,17-18,20,31H,3,13-16H2,1-2H3,(H,32,33)(H,34,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged PDGFRalpha after 2 hrs by luciferase-luciferin coupled chemiluminescence assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data