

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132988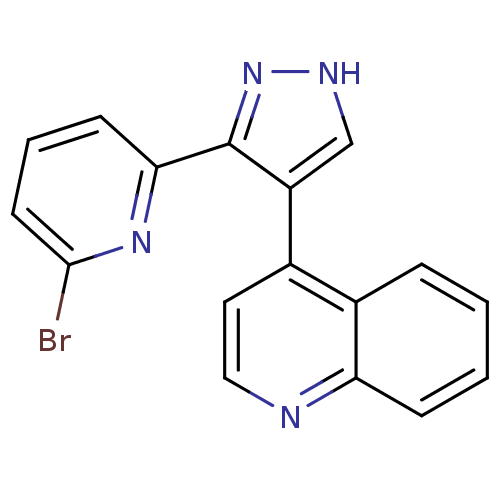 (4-[3-(6-Bromo-pyridin-2-yl)-1H-pyrazol-4-yl]-quino...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase growth in mouse fibroblasts (NIH 3T3) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132989 (4-[3-(6-Methyl-pyridin-2-yl)-1H-pyrazol-4-yl]-quin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase production in mink lung cells (p3TP Lux) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132988 (4-[3-(6-Bromo-pyridin-2-yl)-1H-pyrazol-4-yl]-quino...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase production in mink lung cells (p3TP Lux) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132986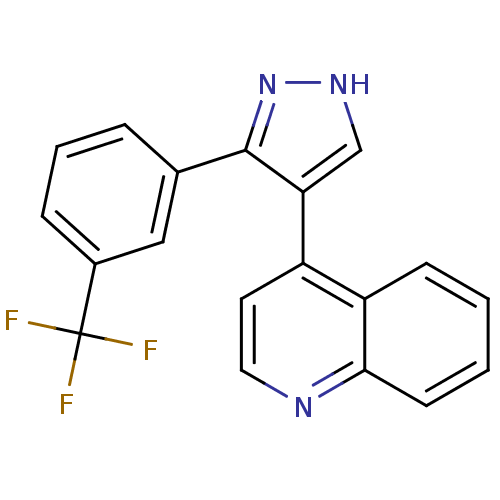 (4-(3-(3-(trifluoromethyl)phenyl)-1H-pyrazol-4-yl)q...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Mitogen-activated protein kinase p38 | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132989 (4-[3-(6-Methyl-pyridin-2-yl)-1H-pyrazol-4-yl]-quin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase growth in mouse fibroblasts (NIH 3T3) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184486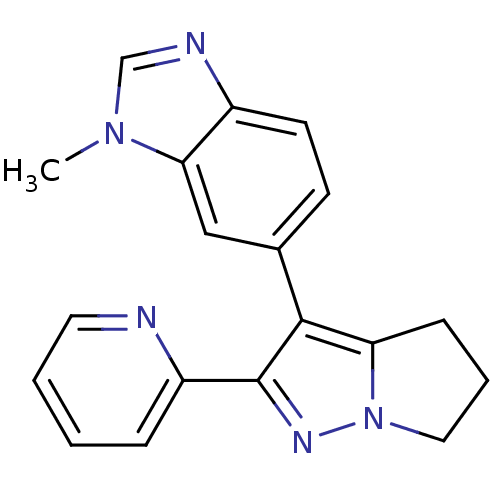 (1-methyl-6-(2-pyridin-2-yl-5,6-dihydro-4H-pyrrolo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184488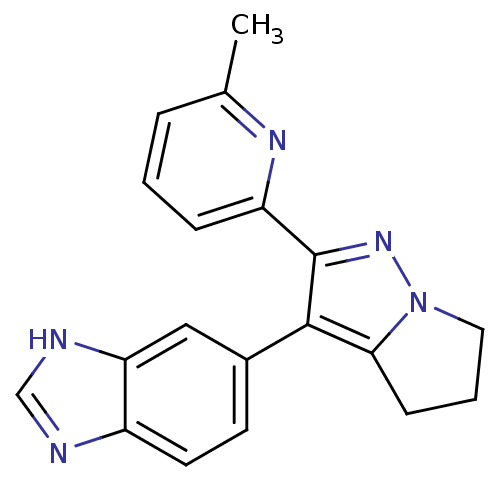 (6-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrol...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184483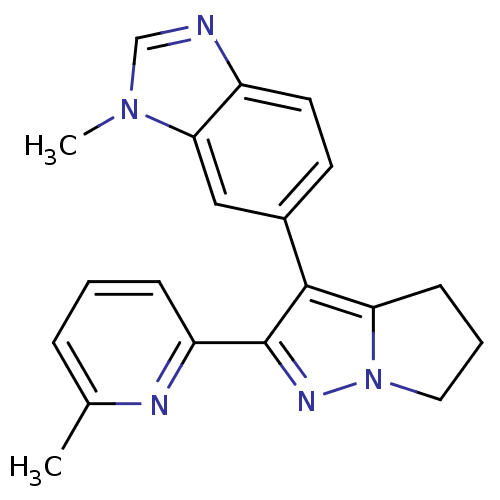 (1-methyl-6-[2-(6-methylpyridin-2-yl)-5,6-dihydro-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21506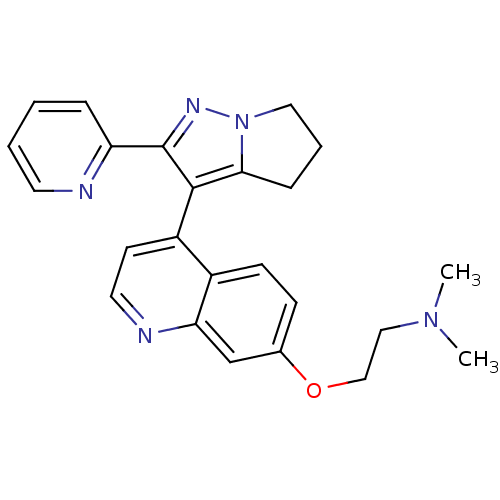 (Dihydropyrrolopyrazole, 15a | dimethyl[2-({4-[2-(p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | 29 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132981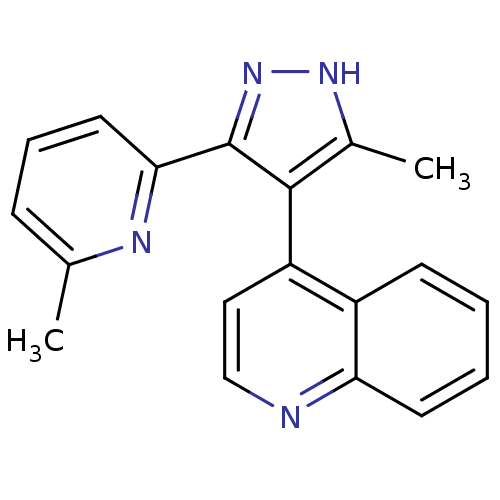 (4-[5-Methyl-3-(6-methyl-pyridin-2-yl)-1H-pyrazol-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase production in mink lung cells (p3TP Lux) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132993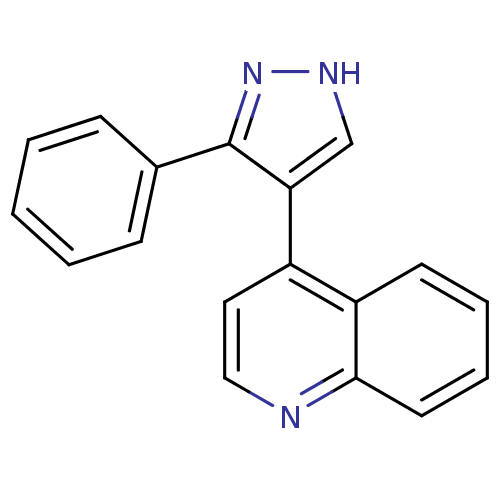 (4-(3-Phenyl-1H-pyrazol-4-yl)-quinoline | 4-(3-phen...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Mitogen-activated protein kinase p38 | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184483 (1-methyl-6-[2-(6-methylpyridin-2-yl)-5,6-dihydro-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132988 (4-[3-(6-Bromo-pyridin-2-yl)-1H-pyrazol-4-yl]-quino...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Transforming growth factor beta-1 receptor kinase (TGF-beta RIK) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132992 (4-(3-(6-methylpyridin-2-yl)-1H-pyrazol-4-yl)phenol...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Transforming growth factor beta-1 receptor kinase (TGF-beta RIK) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132992 (4-(3-(6-methylpyridin-2-yl)-1H-pyrazol-4-yl)phenol...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase growth in mouse fibroblasts (NIH 3T3) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21505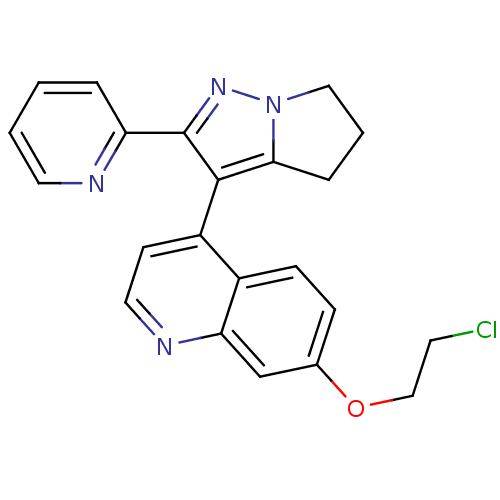 (7-(2-chloroethoxy)-4-[2-(pyridin-2-yl)-4H,5H,6H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | 66 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132989 (4-[3-(6-Methyl-pyridin-2-yl)-1H-pyrazol-4-yl]-quin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Transforming growth factor beta-1 receptor kinase (TGF-beta RIK) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM21492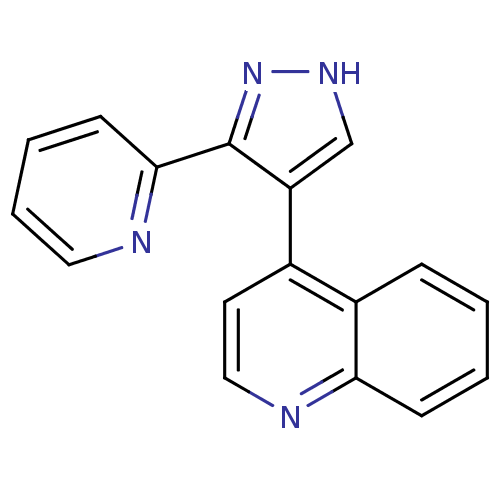 (4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]quinoline | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184489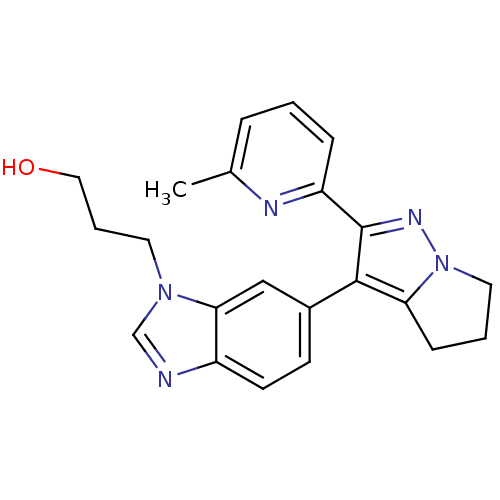 (3-(6-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrr...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132976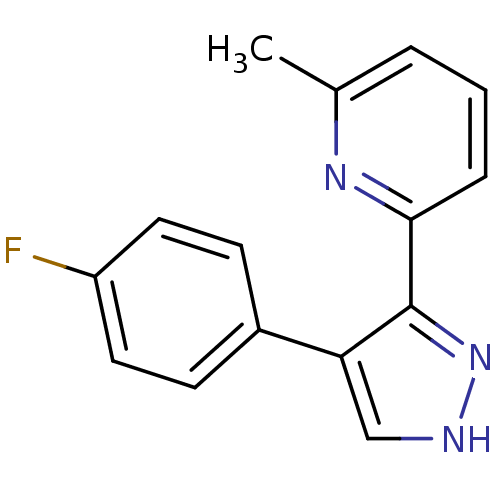 (2-[4-(4-Fluoro-phenyl)-1H-pyrazol-3-yl]-6-methyl-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase growth in mouse fibroblasts (NIH 3T3) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132987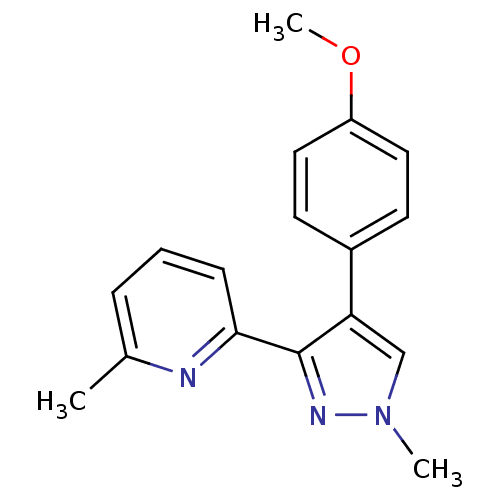 (2-[4-(4-Methoxy-phenyl)-1-methyl-1H-pyrazol-3-yl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Transforming growth factor beta-1 receptor kinase (TGF-beta RIK) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132992 (4-(3-(6-methylpyridin-2-yl)-1H-pyrazol-4-yl)phenol...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase production in mink lung cells (p3TP Lux) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM21492 (4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]quinoline | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase production in mink lung cells (p3TP Lux) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50148668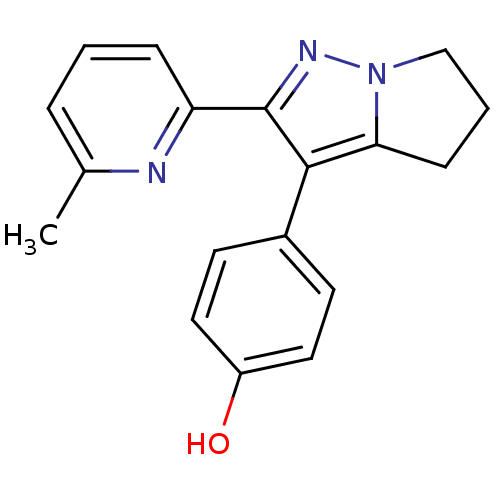 (4-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against TGF-beta type I receptor | Bioorg Med Chem Lett 14: 3585-8 (2004) Article DOI: 10.1016/j.bmcl.2004.04.065 BindingDB Entry DOI: 10.7270/Q23R0SCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21510 (Dihydropyrrolopyrazole, 16a | dimethyl[5-({4-[2-(p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | 24 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184488 (6-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrol...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132987 (2-[4-(4-Methoxy-phenyl)-1-methyl-1H-pyrazol-3-yl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase production in mink lung cells (p3TP Lux) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50148666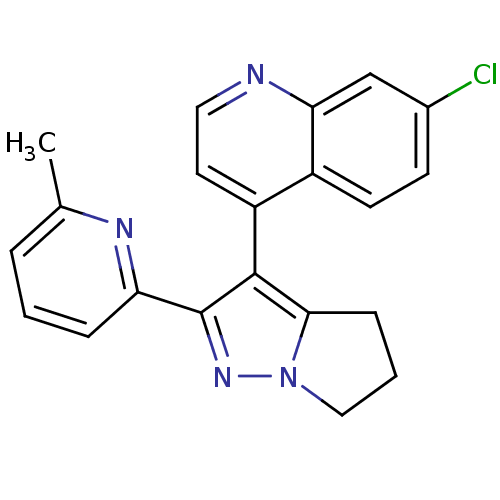 (7-Chloro-4-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against TGF-beta type I receptor | Bioorg Med Chem Lett 14: 3585-8 (2004) Article DOI: 10.1016/j.bmcl.2004.04.065 BindingDB Entry DOI: 10.7270/Q23R0SCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21511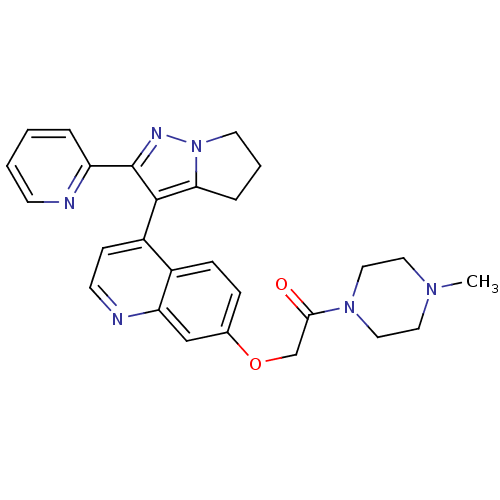 (1-(4-methylpiperazin-1-yl)-2-({4-[2-(pyridin-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | 260 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184494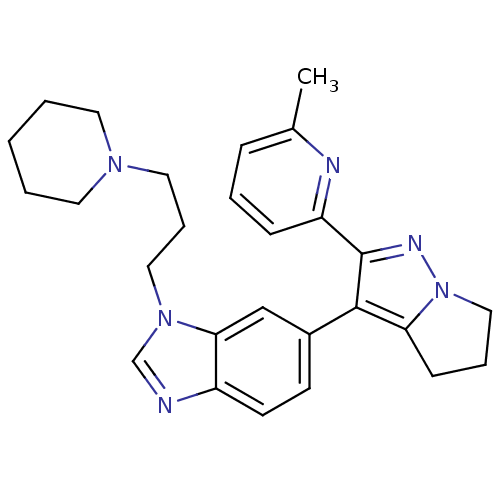 (6-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrol...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM21492 (4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]quinoline | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Transforming growth factor (TGF) beta-1 receptor (T204D mutation) autophosphorylation in Sf9 cells | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184496 (CHEMBL441176 | dimethyl-(3-{6-[2-(6-methyl-pyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50148655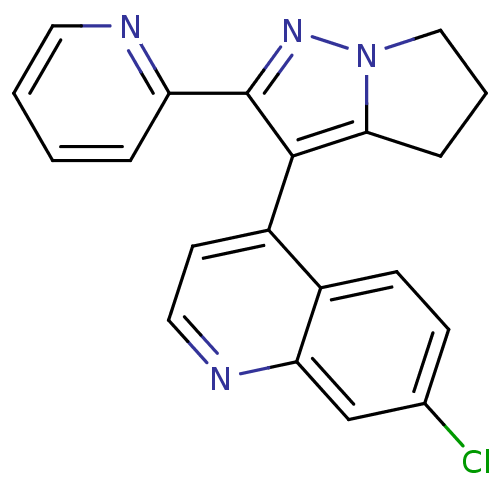 (7-Chloro-4-(2-pyridin-2-yl-5,6-dihydro-4H-pyrrolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against TGF-beta type I receptor | Bioorg Med Chem Lett 14: 3585-8 (2004) Article DOI: 10.1016/j.bmcl.2004.04.065 BindingDB Entry DOI: 10.7270/Q23R0SCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132994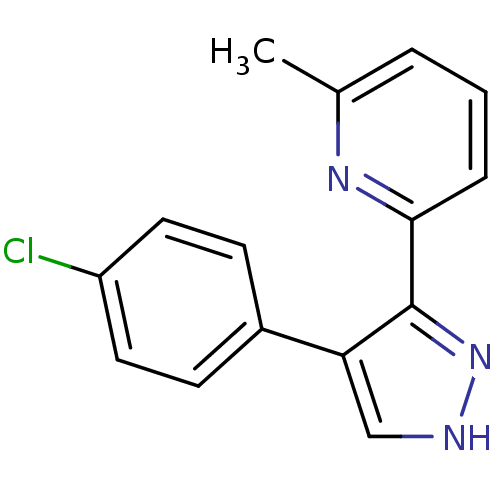 (2-[4-(4-Chloro-phenyl)-1H-pyrazol-3-yl]-6-methyl-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Transforming growth factor beta-1 receptor kinase (TGF-beta RIK) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21492 (4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]quinoline | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 59 | n/a | 40 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM21492 (4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]quinoline | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50148657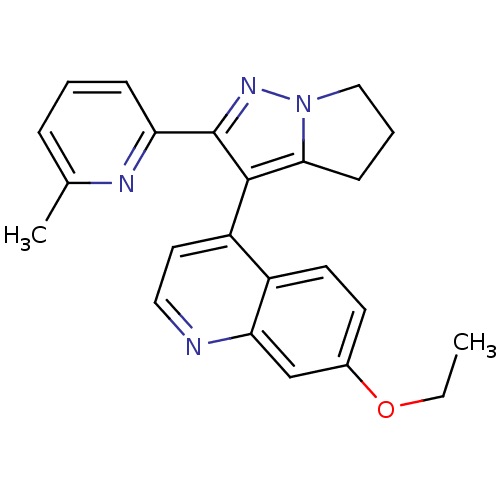 (7-Ethoxy-4-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against Mitogen-activated protein kinase p38 | Bioorg Med Chem Lett 14: 3585-8 (2004) Article DOI: 10.1016/j.bmcl.2004.04.065 BindingDB Entry DOI: 10.7270/Q23R0SCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132987 (2-[4-(4-Methoxy-phenyl)-1-methyl-1H-pyrazol-3-yl]-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase growth in mouse fibroblasts (NIH 3T3) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21509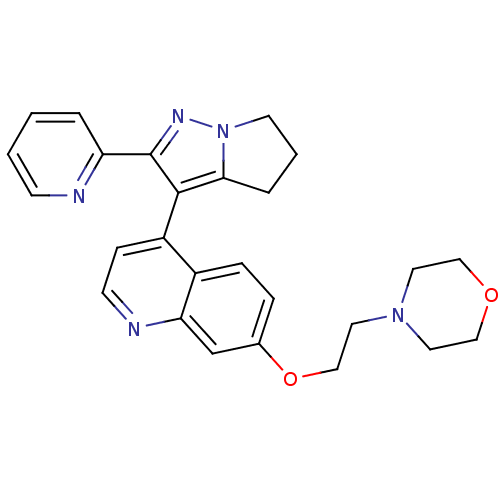 (4-[2-({4-[2-(pyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-a]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | 180 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184487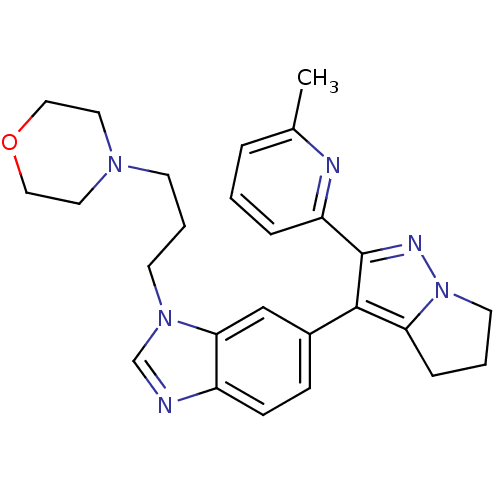 (4-(3-(6-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132976 (2-[4-(4-Fluoro-phenyl)-1H-pyrazol-3-yl]-6-methyl-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Transforming growth factor beta-1 receptor kinase (TGF-beta RIK) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50148656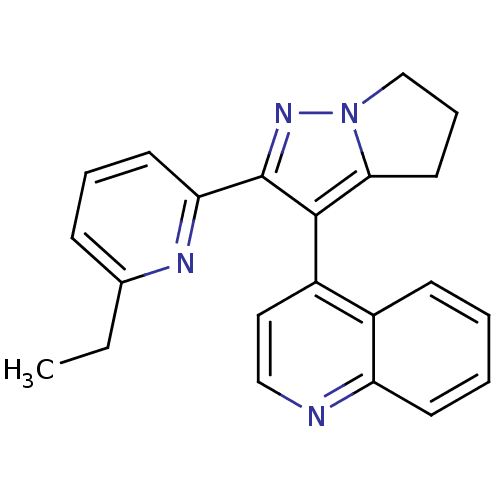 (4-(2-(6-ethylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against TGF-beta type I receptor | Bioorg Med Chem Lett 14: 3585-8 (2004) Article DOI: 10.1016/j.bmcl.2004.04.065 BindingDB Entry DOI: 10.7270/Q23R0SCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21513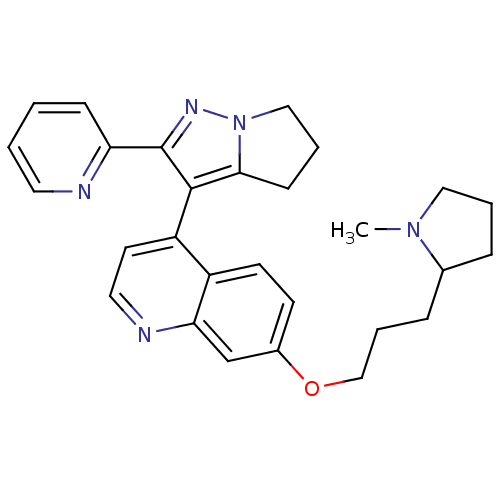 (7-[3-(1-methylpyrrolidin-2-yl)propoxy]-4-[2-(pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | 17 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184497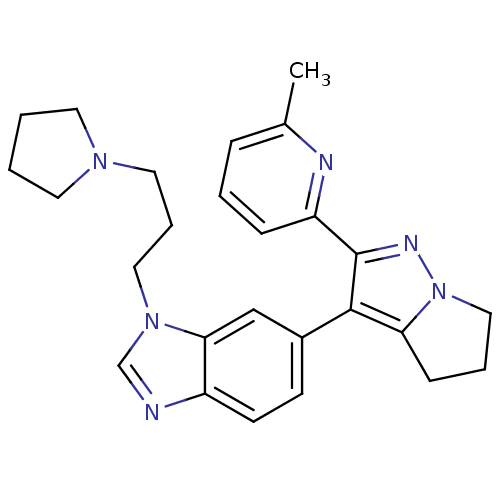 (6-[2-(6-methyl-pyridin-2-yl)-5,6-dihydro-4H-pyrrol...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21504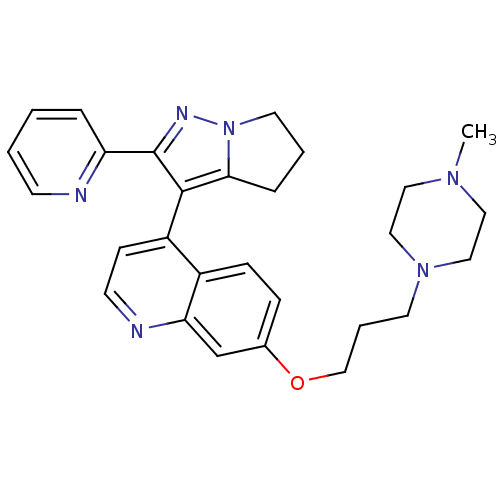 (7-[3-(4-methylpiperazin-1-yl)propoxy]-4-[2-(pyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | 44 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184486 (1-methyl-6-(2-pyridin-2-yl-5,6-dihydro-4H-pyrrolo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of recombinant human TGFbeta R1 expressed in Sf9 cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132980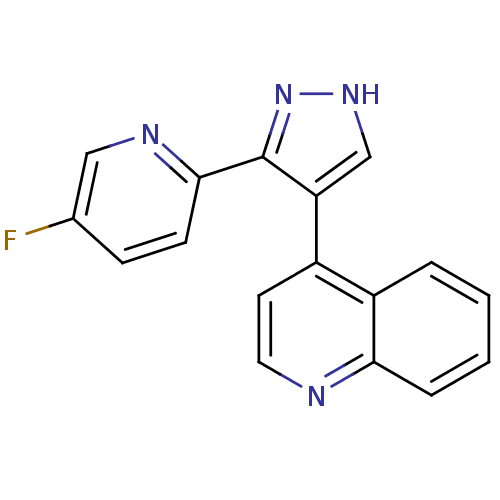 (4-(3-(5-fluoropyridin-2-yl)-1H-pyrazol-4-yl)quinol...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase growth in mouse fibroblasts (NIH 3T3) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132989 (4-[3-(6-Methyl-pyridin-2-yl)-1H-pyrazol-4-yl]-quin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Mitogen-activated protein kinase p38 | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50184489 (3-(6-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrr...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibition of TGFbeta R1 induced transcriptional activation of p3TP-Lux in mink Mv1Lu lung cells | J Med Chem 49: 2138-42 (2006) Article DOI: 10.1021/jm058209g BindingDB Entry DOI: 10.7270/Q2V40TS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM50148661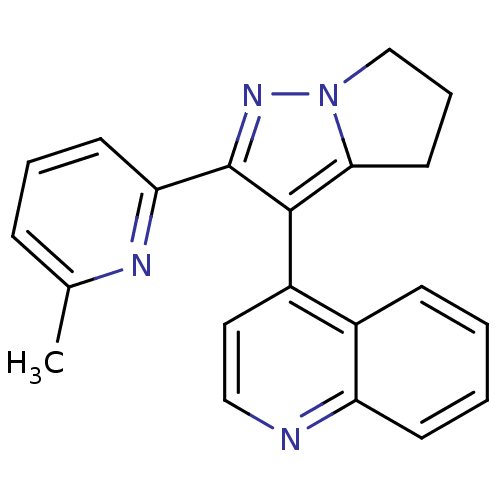 (4-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory Curated by ChEMBL | Assay Description Inhibitory activity against TGF-beta type I receptor | Bioorg Med Chem Lett 14: 3585-8 (2004) Article DOI: 10.1016/j.bmcl.2004.04.065 BindingDB Entry DOI: 10.7270/Q23R0SCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 227 total ) | Next | Last >> |