 Found 5936 hits with Last Name = 'you' and Initial = 'j'
Found 5936 hits with Last Name = 'you' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Bcl2 (unknown origin) |
J Med Chem 61: 6421-6467 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00180
BindingDB Entry DOI: 10.7270/Q2DJ5J62 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50051007
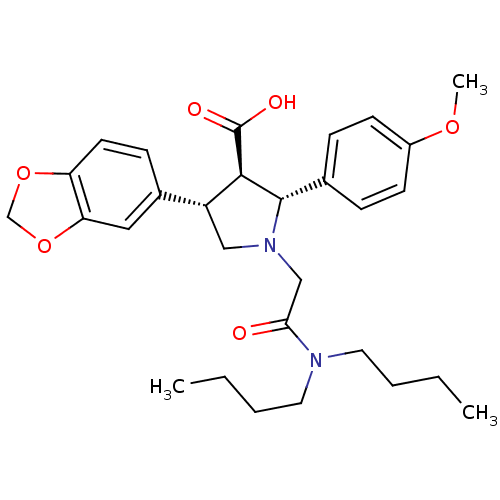
((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...)Show SMILES CCCCN(CCCC)C(=O)CN1C[C@@H]([C@H]([C@@H]1c1ccc(OC)cc1)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O6/c1-4-6-14-30(15-7-5-2)26(32)18-31-17-23(21-10-13-24-25(16-21)37-19-36-24)27(29(33)34)28(31)20-8-11-22(35-3)12-9-20/h8-13,16,23,27-28H,4-7,14-15,17-19H2,1-3H3,(H,33,34)/t23-,27-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Endothelin A receptor |
J Med Chem 47: 1969-86 (2004)
Article DOI: 10.1021/jm030528p
BindingDB Entry DOI: 10.7270/Q2HD7WD6 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50143784
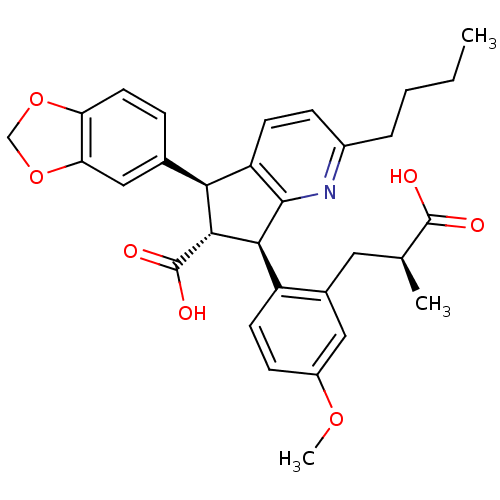
((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...)Show SMILES CCCCc1ccc2[C@@H]([C@H]([C@@H](c2n1)c1ccc(OC)cc1C[C@H](C)C(O)=O)C(O)=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C31H33NO7/c1-4-5-6-20-8-10-23-26(18-7-12-24-25(15-18)39-16-38-24)28(31(35)36)27(29(23)32-20)22-11-9-21(37-3)14-19(22)13-17(2)30(33)34/h7-12,14-15,17,26-28H,4-6,13,16H2,1-3H3,(H,33,34)(H,35,36)/t17-,26-,27-,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Endothelin A receptor |
J Med Chem 47: 1969-86 (2004)
Article DOI: 10.1021/jm030528p
BindingDB Entry DOI: 10.7270/Q2HD7WD6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228930
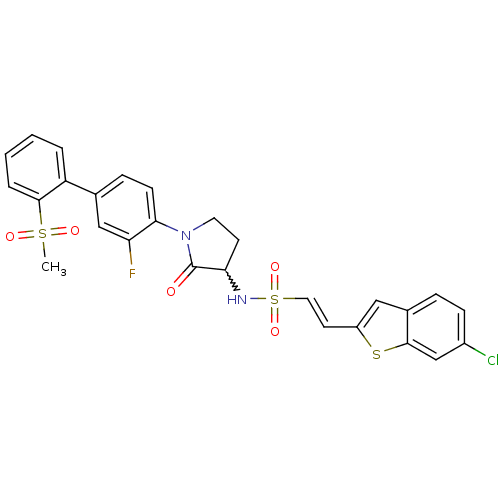
((E)-2-(6-chloro-benzo[b]thiophen-2-yl)-ethenesulfo...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3cc4ccc(Cl)cc4s3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C27H22ClFN2O5S3/c1-38(33,34)26-5-3-2-4-21(26)17-7-9-24(22(29)15-17)31-12-10-23(27(31)32)30-39(35,36)13-11-20-14-18-6-8-19(28)16-25(18)37-20/h2-9,11,13-16,23,30H,10,12H2,1H3/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (828-1132) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374259
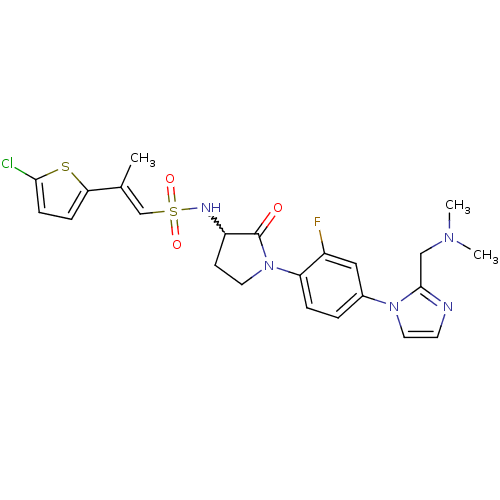
(CHEMBL257741)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CCC(NS(=O)(=O)\C=C(/C)c3ccc(Cl)s3)C2=O)c(F)c1 |w:16.17| Show InChI InChI=1S/C23H25ClFN5O3S2/c1-15(20-6-7-21(24)34-20)14-35(32,33)27-18-8-10-30(23(18)31)19-5-4-16(12-17(19)25)29-11-9-26-22(29)13-28(2)3/h4-7,9,11-12,14,18,27H,8,10,13H2,1-3H3/b15-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228676
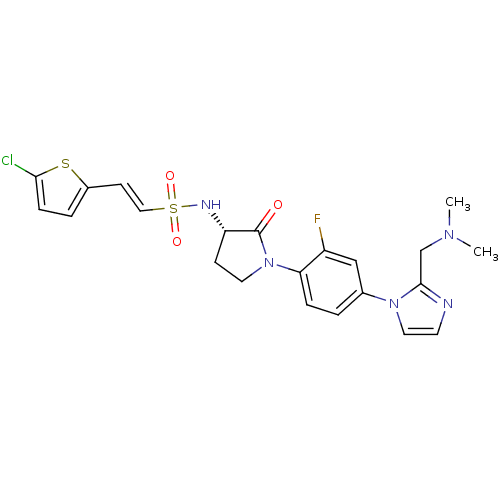
((S)-2-(5-chlorothiophen-2-yl)-N-(1-(4-(2-((dimethy...)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CC[C@H](NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 Show InChI InChI=1S/C22H23ClFN5O3S2/c1-27(2)14-21-25-9-11-28(21)15-3-5-19(17(24)13-15)29-10-7-18(22(29)30)26-34(31,32)12-8-16-4-6-20(23)33-16/h3-6,8-9,11-13,18,26H,7,10,14H2,1-2H3/b12-8+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228950
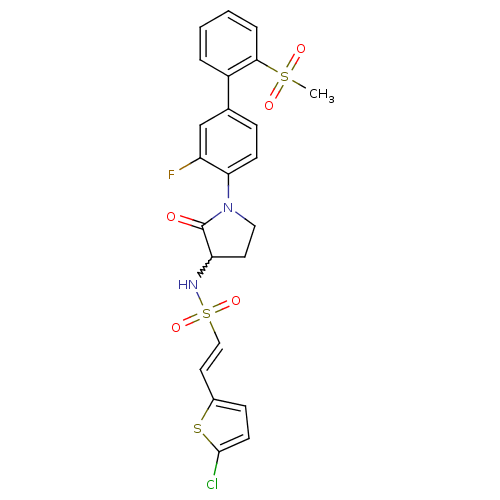
((E)-2-(5-chloro-thiophen-2-yl)-ethenesulfonic acid...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C23H20ClFN2O5S3/c1-34(29,30)21-5-3-2-4-17(21)15-6-8-20(18(25)14-15)27-12-10-19(23(27)28)26-35(31,32)13-11-16-7-9-22(24)33-16/h2-9,11,13-14,19,26H,10,12H2,1H3/b13-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306153
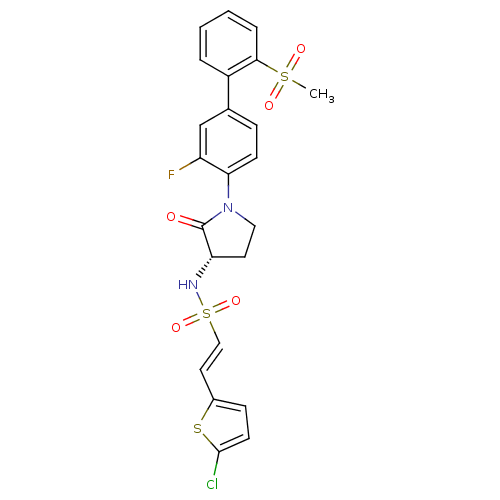
((S)-2-(5-chlorothiophen-2-yl)-N-(1-(3-fluoro-2'-(m...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(N2CC[C@H](NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C23H20ClFN2O5S3/c1-34(29,30)21-5-3-2-4-17(21)15-6-8-20(18(25)14-15)27-12-10-19(23(27)28)26-35(31,32)13-11-16-7-9-22(24)33-16/h2-9,11,13-14,19,26H,10,12H2,1H3/b13-11+/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human JAK1 (837-1142) expressed in baculovirus-infected Sf9 cells using EQEDEPEGDYFEWLE as substrate after 1 hr by HTRF assay |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228676

((S)-2-(5-chlorothiophen-2-yl)-N-(1-(4-(2-((dimethy...)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CC[C@H](NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 Show InChI InChI=1S/C22H23ClFN5O3S2/c1-27(2)14-21-25-9-11-28(21)15-3-5-19(17(24)13-15)29-10-7-18(22(29)30)26-34(31,32)12-8-16-4-6-20(23)33-16/h3-6,8-9,11-13,18,26H,7,10,14H2,1-2H3/b12-8+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17759
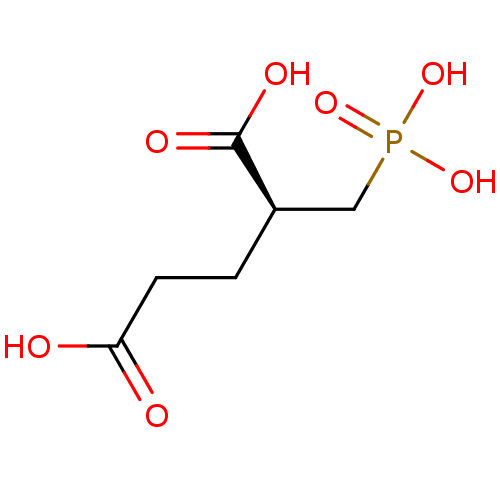
((2S)-2-(phosphonomethyl)pentanedioic acid | (S)-2-...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13)/t4-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GCPII (unknown origin) NAALADase activity |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128044
BindingDB Entry DOI: 10.7270/Q2SQ9459 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338686

((R/S)-3-chloro-N-((3S)-1-(1-(methylamino)-2,3-dihy...)Show SMILES CNC1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C22H23ClN4O3S/c1-24-19-7-2-13-10-14(3-5-16(13)19)27-9-8-20(22(27)28)26-31(29,30)15-4-6-17-18(23)12-25-21(17)11-15/h3-6,10-12,19-20,24-26H,2,7-9H2,1H3/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228954
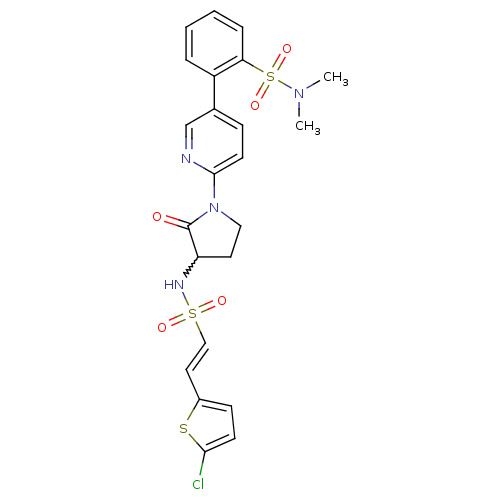
((E)-2-(6-(3-(2-(5-chlorothiophen-2-yl)vinylsulfona...)Show SMILES CN(C)S(=O)(=O)c1ccccc1-c1ccc(nc1)N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |w:21.23| Show InChI InChI=1S/C23H23ClN4O5S3/c1-27(2)36(32,33)20-6-4-3-5-18(20)16-7-10-22(25-15-16)28-13-11-19(23(28)29)26-35(30,31)14-12-17-8-9-21(24)34-17/h3-10,12,14-15,19,26H,11,13H2,1-2H3/b14-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374262
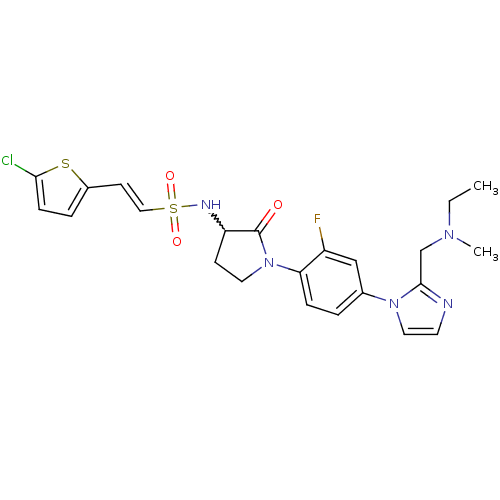
(CHEMBL272779)Show SMILES CCN(C)Cc1nccn1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:17.18| Show InChI InChI=1S/C23H25ClFN5O3S2/c1-3-28(2)15-22-26-10-12-29(22)16-4-6-20(18(25)14-16)30-11-8-19(23(30)31)27-35(32,33)13-9-17-5-7-21(24)34-17/h4-7,9-10,12-14,19,27H,3,8,11,15H2,1-2H3/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused JAK3 catalytic domain expressed in baculovirus-infected Sf9 cells using polyglutamic acid-tyrosine as substrate after 3... |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374264

(CHEMBL257863)Show SMILES Fc1cc(ccc1N1CCC(NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O)-n1ccnc1CN1CCCC1 |w:10.11| Show InChI InChI=1S/C24H25ClFN5O3S2/c25-22-6-4-18(35-22)8-14-36(33,34)28-20-7-12-31(24(20)32)21-5-3-17(15-19(21)26)30-13-9-27-23(30)16-29-10-1-2-11-29/h3-6,8-9,13-15,20,28H,1-2,7,10-12,16H2/b14-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50058126
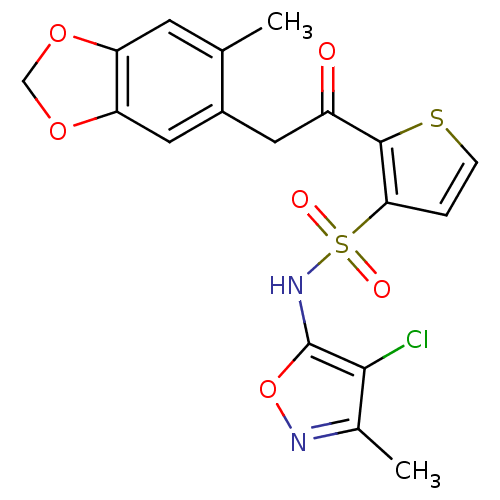
(2-[2-(6-Methyl-benzo[1,3]dioxol-5-yl)-acetyl]-thio...)Show SMILES Cc1noc(NS(=O)(=O)c2ccsc2C(=O)Cc2cc3OCOc3cc2C)c1Cl Show InChI InChI=1S/C18H15ClN2O6S2/c1-9-5-13-14(26-8-25-13)7-11(9)6-12(22)17-15(3-4-28-17)29(23,24)21-18-16(19)10(2)20-27-18/h3-5,7,21H,6,8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Encysive Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards Endothelin A receptor |
J Med Chem 47: 1969-86 (2004)
Article DOI: 10.1021/jm030528p
BindingDB Entry DOI: 10.7270/Q2HD7WD6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374270
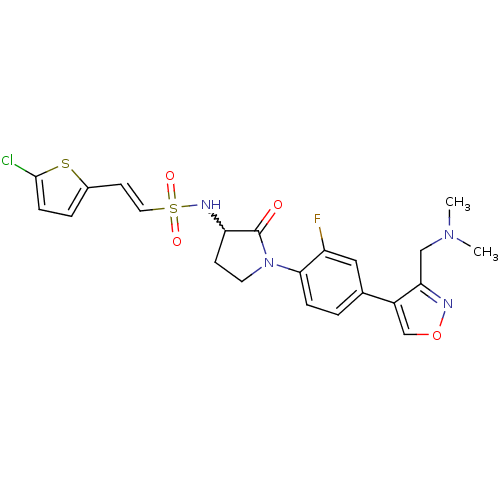
(CHEMBL271015)Show SMILES CN(C)Cc1nocc1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:16.17| Show InChI InChI=1S/C22H22ClFN4O4S2/c1-27(2)12-19-16(13-32-25-19)14-3-5-20(17(24)11-14)28-9-7-18(22(28)29)26-34(30,31)10-8-15-4-6-21(23)33-15/h3-6,8,10-11,13,18,26H,7,9,12H2,1-2H3/b10-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374263
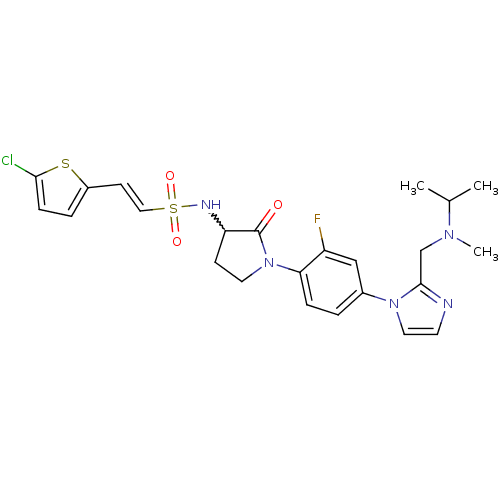
(CHEMBL437097)Show SMILES CC(C)N(C)Cc1nccn1-c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |w:18.19| Show InChI InChI=1S/C24H27ClFN5O3S2/c1-16(2)29(3)15-23-27-10-12-30(23)17-4-6-21(19(26)14-17)31-11-8-20(24(31)32)28-36(33,34)13-9-18-5-7-22(25)35-18/h4-7,9-10,12-14,16,20,28H,8,11,15H2,1-3H3/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339708

((S)-2-(5-chlorothiophen-2-yl)-N-(1-(5-fluoro-1,2,3...)Show SMILES Fc1c2CCNCc2ccc1N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C19H19ClFN3O3S2/c20-17-4-2-13(28-17)7-10-29(26,27)23-15-6-9-24(19(15)25)16-3-1-12-11-22-8-5-14(12)18(16)21/h1-4,7,10,15,22-23H,5-6,8-9,11H2/b10-7+/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339718
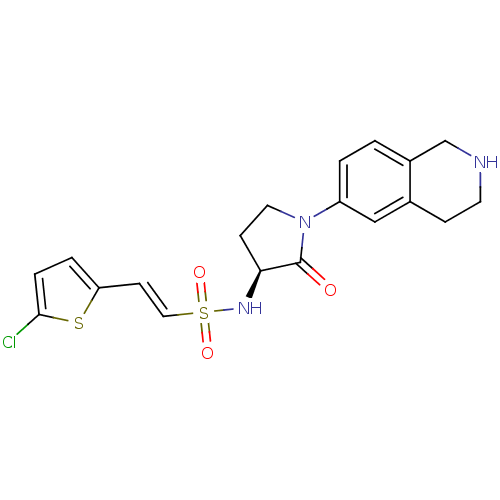
((S)-2-(5-chlorothiophen-2-yl)-N-(2-oxo-1-(1,2,3,4-...)Show SMILES Clc1ccc(\C=C\S(=O)(=O)N[C@H]2CCN(C2=O)c2ccc3CNCCc3c2)s1 |r| Show InChI InChI=1S/C19H20ClN3O3S2/c20-18-4-3-16(27-18)7-10-28(25,26)22-17-6-9-23(19(17)24)15-2-1-14-12-21-8-5-13(14)11-15/h1-4,7,10-11,17,21-22H,5-6,8-9,12H2/b10-7+/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328712
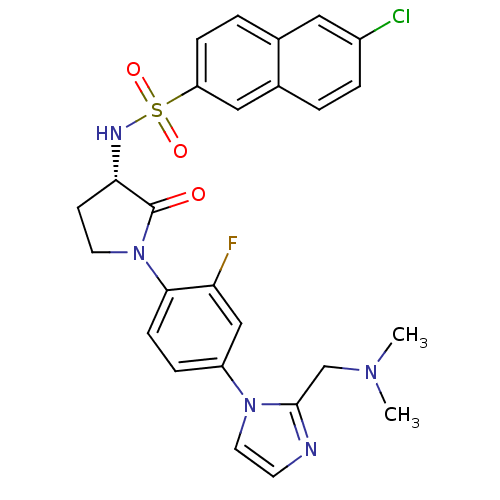
((S)-6-chloro-N-(1-(4-(2-((dimethylamino)methyl)-1H...)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C26H25ClFN5O3S/c1-31(2)16-25-29-10-12-32(25)20-6-8-24(22(28)15-20)33-11-9-23(26(33)34)30-37(35,36)21-7-4-17-13-19(27)5-3-18(17)14-21/h3-8,10,12-15,23,30H,9,11,16H2,1-2H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused JAK1 catalytic domain expressed in baculovirus-infected Sf9 cells using polyglutamic acid-tyrosine as substrate after 3... |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human GST-fused JAK2 catalytic domain expressed in baculovirus-infected Sf9 cells using polyglutamic acid-tyrosine as substrate after 3... |
J Med Chem 56: 4521-36 (2013)
Article DOI: 10.1021/jm400266t
BindingDB Entry DOI: 10.7270/Q2VX0HX0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228940
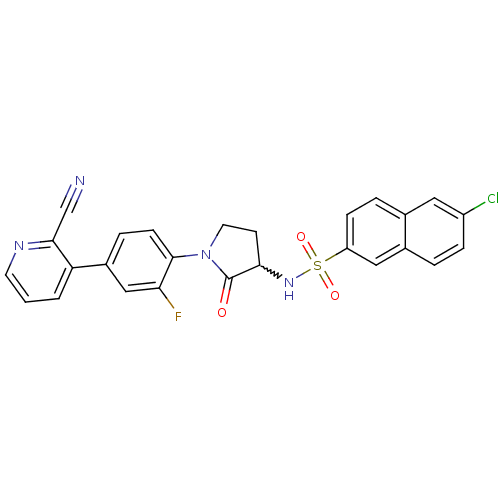
(6-chloro-N-(1-(4-(2-cyanopyridin-3-yl)-2-fluorophe...)Show SMILES Fc1cc(ccc1N1CCC(NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)-c1cccnc1C#N |w:10.11| Show InChI InChI=1S/C26H18ClFN4O3S/c27-19-6-3-17-13-20(7-4-16(17)12-19)36(34,35)31-23-9-11-32(26(23)33)25-8-5-18(14-22(25)28)21-2-1-10-30-24(21)15-29/h1-8,10,12-14,23,31H,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human F10a by fluorescence assay |
Bioorg Med Chem Lett 18: 23-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.023
BindingDB Entry DOI: 10.7270/Q2SX6CZF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50053947
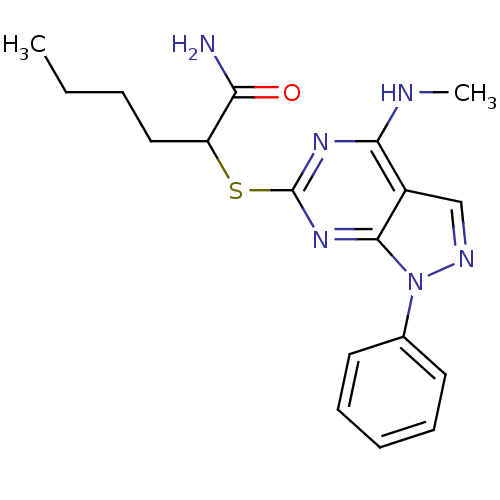
(2-(4-Methylamino-1-phenyl-1H-pyrazolo[3,4-d]pyrimi...)Show InChI InChI=1S/C18H22N6OS/c1-3-4-10-14(15(19)25)26-18-22-16(20-2)13-11-21-24(17(13)23-18)12-8-6-5-7-9-12/h5-9,11,14H,3-4,10H2,1-2H3,(H2,19,25)(H,20,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.745 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Griffith University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N6-PIA binding from A1 receptor in whole rat brain membranes |
Bioorg Med Chem Lett 11: 191-3 (2001)
BindingDB Entry DOI: 10.7270/Q23F4NW2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306146
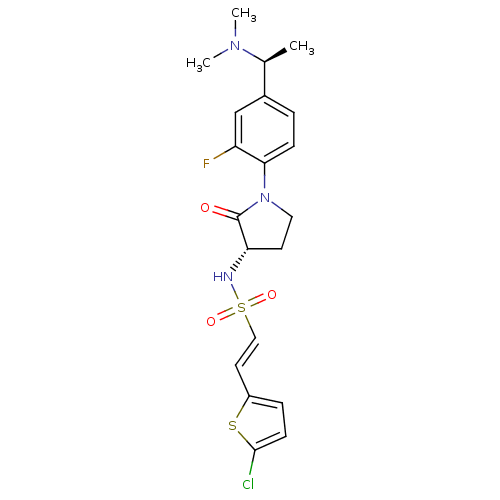
((E)-2-(5-CHLOROTHIOPHEN-2-YL)-N-[(3S)-1-{4-[(1S)-1...)Show SMILES C[C@H](N(C)C)c1ccc(N2CC[C@H](NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C20H23ClFN3O3S2/c1-13(24(2)3)14-4-6-18(16(22)12-14)25-10-8-17(20(25)26)23-30(27,28)11-9-15-5-7-19(21)29-15/h4-7,9,11-13,17,23H,8,10H2,1-3H3/b11-9+/t13-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339716

((S)-6-chloro-N-(1-(6-fluoro-2,3,4,5-tetrahydro-1H-...)Show SMILES Fc1c2CCCNCc2ccc1N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C24H23ClFN3O3S/c25-18-6-3-16-13-19(7-4-15(16)12-18)33(31,32)28-21-9-11-29(24(21)30)22-8-5-17-14-27-10-1-2-20(17)23(22)26/h3-8,12-13,21,27-28H,1-2,9-11,14H2/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339713
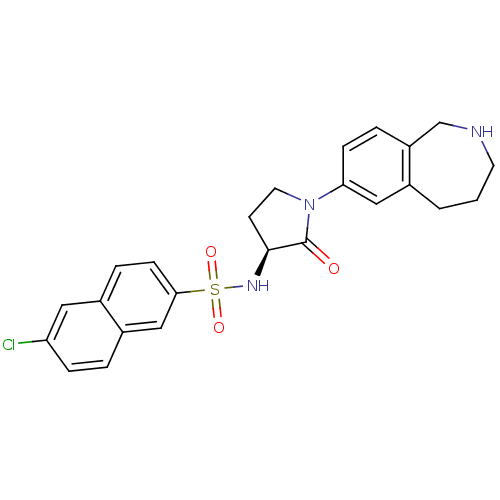
((S)-6-chloro-N-(2-oxo-1-(2,3,4,5-tetrahydro-1H-ben...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCCc2c1 |r| Show InChI InChI=1S/C24H24ClN3O3S/c25-20-6-3-18-14-22(8-5-17(18)12-20)32(30,31)27-23-9-11-28(24(23)29)21-7-4-19-15-26-10-1-2-16(19)13-21/h3-8,12-14,23,26-27H,1-2,9-11,15H2/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306143
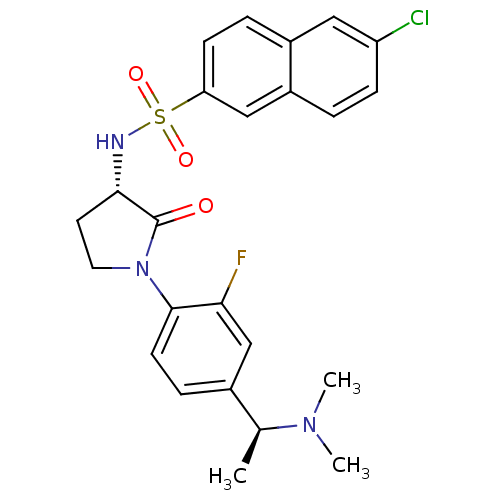
(6-chloro-N-((S)-1-(4-((S)-1-(dimethylamino)ethyl)-...)Show SMILES C[C@H](N(C)C)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C24H25ClFN3O3S/c1-15(28(2)3)16-6-9-23(21(26)14-16)29-11-10-22(24(29)30)27-33(31,32)20-8-5-17-12-19(25)7-4-18(17)13-20/h4-9,12-15,22,27H,10-11H2,1-3H3/t15-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374274

(CHEMBL403111)Show SMILES CN(C)Cc1nccn1-c1ccc(N2CCC(NS(=O)(=O)c3cc4ccc(Cl)cc4s3)C2=O)c(F)c1 |w:16.17| Show InChI InChI=1S/C24H23ClFN5O3S2/c1-29(2)14-22-27-8-10-30(22)17-5-6-20(18(26)13-17)31-9-7-19(24(31)32)28-36(33,34)23-11-15-3-4-16(25)12-21(15)35-23/h3-6,8,10-13,19,28H,7,9,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a |
Bioorg Med Chem Lett 18: 28-33 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.019
BindingDB Entry DOI: 10.7270/Q29024PS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306138
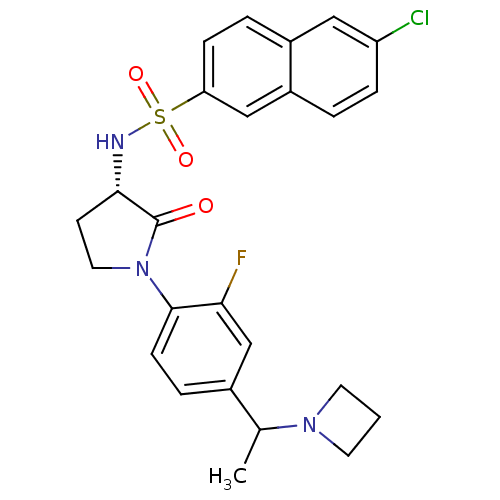
(CHEMBL604887 | N-((3S)-1-(4-(1-(azetidin-1-yl)ethy...)Show SMILES CC(N1CCC1)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C25H25ClFN3O3S/c1-16(29-10-2-11-29)17-5-8-24(22(27)15-17)30-12-9-23(25(30)31)28-34(32,33)21-7-4-18-13-20(26)6-3-19(18)14-21/h3-8,13-16,23,28H,2,9-12H2,1H3/t16?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339714
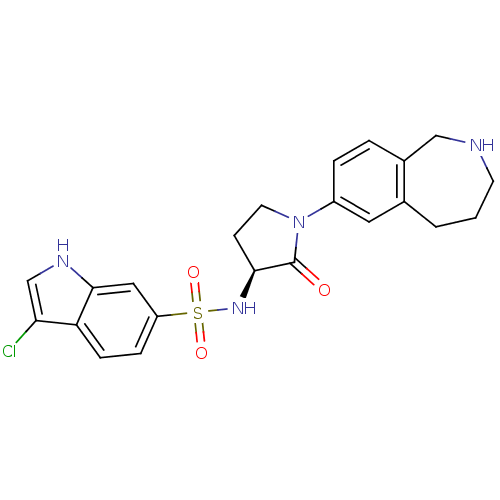
((S)-3-chloro-N-(2-oxo-1-(2,3,4,5-tetrahydro-1H-ben...)Show SMILES Clc1c[nH]c2cc(ccc12)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCCc2c1 |r| Show InChI InChI=1S/C22H23ClN4O3S/c23-19-13-25-21-11-17(5-6-18(19)21)31(29,30)26-20-7-9-27(22(20)28)16-4-3-15-12-24-8-1-2-14(15)10-16/h3-6,10-11,13,20,24-26H,1-2,7-9,12H2/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306142
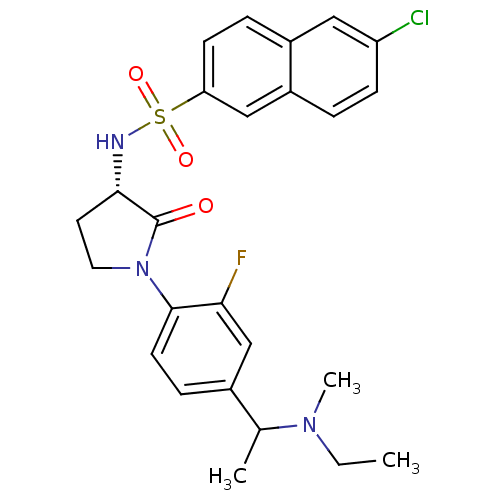
(6-chloro-N-((3S)-1-(4-(1-(ethyl(methyl)amino)ethyl...)Show SMILES CCN(C)C(C)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C25H27ClFN3O3S/c1-4-29(3)16(2)17-7-10-24(22(27)15-17)30-12-11-23(25(30)31)28-34(32,33)21-9-6-18-13-20(26)8-5-19(18)14-21/h5-10,13-16,23,28H,4,11-12H2,1-3H3/t16?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339711

((S)-3-chloro-N-(1-(7-fluoro-1,2,3,4-tetrahydroisoq...)Show SMILES Fc1cc2CNCCc2cc1N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C21H20ClFN4O3S/c22-16-11-25-19-9-14(1-2-15(16)19)31(29,30)26-18-4-6-27(21(18)28)20-8-12-3-5-24-10-13(12)7-17(20)23/h1-2,7-9,11,18,24-26H,3-6,10H2/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50338689

((R/S)-3-chloro-N-((3S)-1-(1-(dimethylamino)-2,3-di...)Show SMILES CN(C)C1CCc2cc(ccc12)N1CC[C@H](NS(=O)(=O)c2ccc3c(Cl)c[nH]c3c2)C1=O |r| Show InChI InChI=1S/C23H25ClN4O3S/c1-27(2)22-8-3-14-11-15(4-6-17(14)22)28-10-9-20(23(28)29)26-32(30,31)16-5-7-18-19(24)13-25-21(18)12-16/h4-7,11-13,20,22,25-26H,3,8-10H2,1-2H3/t20-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a activity measured using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1582-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.131
BindingDB Entry DOI: 10.7270/Q28052WG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12539
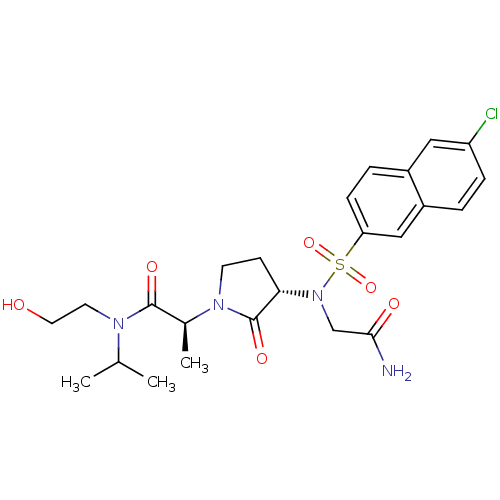
((2S)-2-[(3S)-3-{2-[(6-chloronaphthalene-2-)sulfona...)Show SMILES CC(C)N(CCO)C(=O)[C@H](C)N1CC[C@H](N(CC(N)=O)S(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C24H31ClN4O6S/c1-15(2)27(10-11-30)23(32)16(3)28-9-8-21(24(28)33)29(14-22(26)31)36(34,35)20-7-5-17-12-19(25)6-4-18(17)13-20/h4-7,12-13,15-16,21,30H,8-11,14H2,1-3H3,(H2,26,31)/t16-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 5953-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.001
BindingDB Entry DOI: 10.7270/Q2CF9NB8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12540

((2S)-N-(2-aminoethyl)-2-[(3S)-3-{2-[(6-chloronapht...)Show SMILES CC(C)N(CCN)C(=O)[C@H](C)N1CC[C@H](N(CC(N)=O)S(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C24H32ClN5O5S/c1-15(2)28(11-9-26)23(32)16(3)29-10-8-21(24(29)33)30(14-22(27)31)36(34,35)20-7-5-17-12-19(25)6-4-18(17)13-20/h4-7,12-13,15-16,21H,8-11,14,26H2,1-3H3,(H2,27,31)/t16-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 5953-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.001
BindingDB Entry DOI: 10.7270/Q2CF9NB8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12541

((2S)-N-[2-(carbamoylamino)ethyl]-2-[(3S)-3-{2-[(6-...)Show SMILES CC(C)N(CCNC(N)=O)C(=O)[C@H](C)N1CC[C@H](N(CC(N)=O)S(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C25H33ClN6O6S/c1-15(2)30(11-9-29-25(28)36)23(34)16(3)31-10-8-21(24(31)35)32(14-22(27)33)39(37,38)20-7-5-17-12-19(26)6-4-18(17)13-20/h4-7,12-13,15-16,21H,8-11,14H2,1-3H3,(H2,27,33)(H3,28,29,36)/t16-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 5953-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.001
BindingDB Entry DOI: 10.7270/Q2CF9NB8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12542
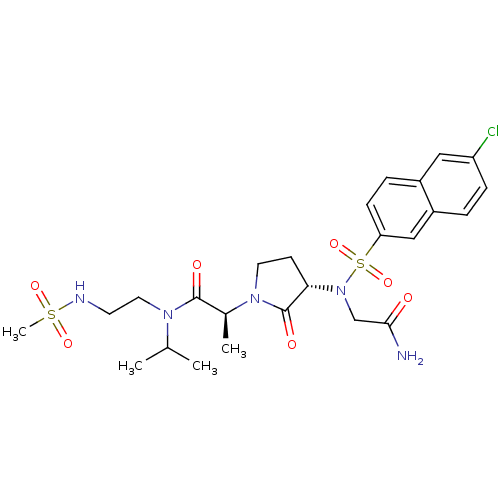
((2S)-2-[(3S)-3-{2-[(6-chloronaphthalene-2-)sulfona...)Show SMILES CC(C)N(CCNS(C)(=O)=O)C(=O)[C@H](C)N1CC[C@H](N(CC(N)=O)S(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C25H34ClN5O7S2/c1-16(2)29(12-10-28-39(4,35)36)24(33)17(3)30-11-9-22(25(30)34)31(15-23(27)32)40(37,38)21-8-6-18-13-20(26)7-5-19(18)14-21/h5-8,13-14,16-17,22,28H,9-12,15H2,1-4H3,(H2,27,32)/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 5953-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.001
BindingDB Entry DOI: 10.7270/Q2CF9NB8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12543

((2S)-2-[(3S)-3-{2-[(6-chloronaphthalene-2-)sulfona...)Show SMILES CC(C)N(CCN1CCCCC1)C(=O)[C@H](C)N1CC[C@H](N(CC(N)=O)S(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C29H40ClN5O5S/c1-20(2)33(16-15-32-12-5-4-6-13-32)28(37)21(3)34-14-11-26(29(34)38)35(19-27(31)36)41(39,40)25-10-8-22-17-24(30)9-7-23(22)18-25/h7-10,17-18,20-21,26H,4-6,11-16,19H2,1-3H3,(H2,31,36)/t21-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 5953-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.001
BindingDB Entry DOI: 10.7270/Q2CF9NB8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12544
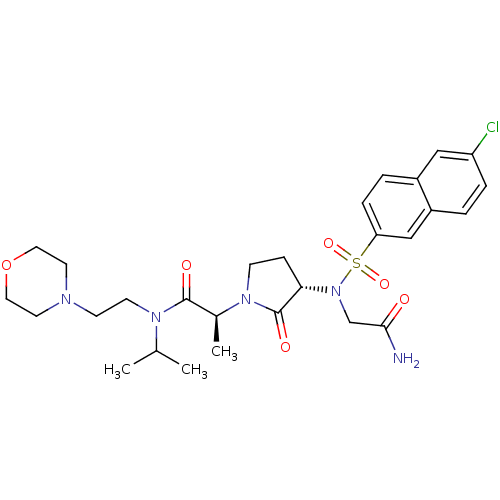
((2S)-2-[(3S)-3-{2-[(6-chloronaphthalene-2-)sulfona...)Show SMILES CC(C)N(CCN1CCOCC1)C(=O)[C@H](C)N1CC[C@H](N(CC(N)=O)S(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r| Show InChI InChI=1S/C28H38ClN5O6S/c1-19(2)32(11-10-31-12-14-40-15-13-31)27(36)20(3)33-9-8-25(28(33)37)34(18-26(30)35)41(38,39)24-7-5-21-16-23(29)6-4-22(21)17-24/h4-7,16-17,19-20,25H,8-15,18H2,1-3H3,(H2,30,35)/t20-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 5953-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.001
BindingDB Entry DOI: 10.7270/Q2CF9NB8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12545
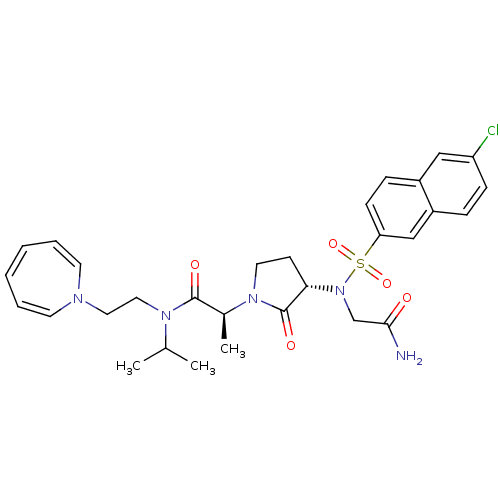
((2S)-N-[2-(1H-azepin-1-yl)ethyl]-2-[(3S)-3-{2-[(6-...)Show SMILES CC(C)N(CCN1C=CC=CC=C1)C(=O)[C@H](C)N1CC[C@H](N(CC(N)=O)S(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O |r,c:7,9,11| Show InChI InChI=1S/C30H36ClN5O5S/c1-21(2)34(17-16-33-13-6-4-5-7-14-33)29(38)22(3)35-15-12-27(30(35)39)36(20-28(32)37)42(40,41)26-11-9-23-18-25(31)10-8-24(23)19-26/h4-11,13-14,18-19,21-22,27H,12,15-17,20H2,1-3H3,(H2,32,37)/t22-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 5953-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.001
BindingDB Entry DOI: 10.7270/Q2CF9NB8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12557
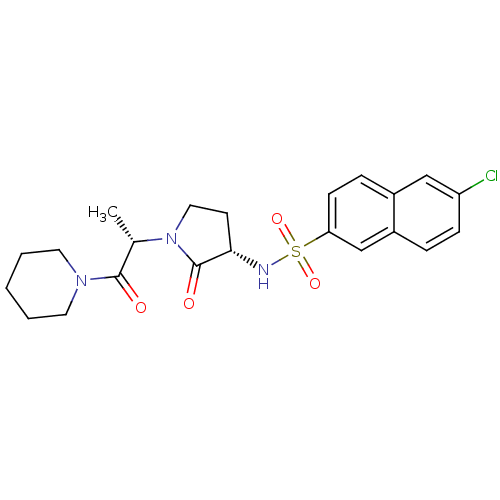
(6-chloro-N-[(3S)-2-oxo-1-[(2S)-1-oxo-1-(piperidin-...)Show SMILES C[C@H](N1CC[C@H](NS(=O)(=O)c2ccc3cc(Cl)ccc3c2)C1=O)C(=O)N1CCCCC1 |r| Show InChI InChI=1S/C22H26ClN3O4S/c1-15(21(27)25-10-3-2-4-11-25)26-12-9-20(22(26)28)24-31(29,30)19-8-6-16-13-18(23)7-5-17(16)14-19/h5-8,13-15,20,24H,2-4,9-12H2,1H3/t15-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339720
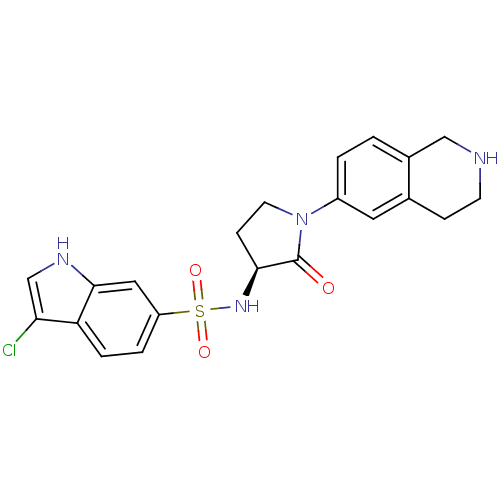
((S)-3-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...)Show SMILES Clc1c[nH]c2cc(ccc12)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCc2c1 |r| Show InChI InChI=1S/C21H21ClN4O3S/c22-18-12-24-20-10-16(3-4-17(18)20)30(28,29)25-19-6-8-26(21(19)27)15-2-1-14-11-23-7-5-13(14)9-15/h1-4,9-10,12,19,23-25H,5-8,11H2/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12569
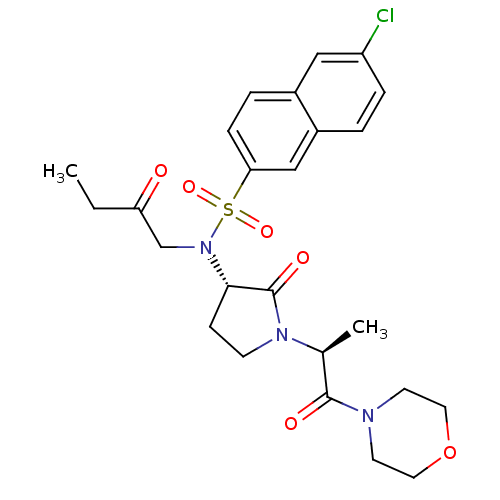
(GTC000006A | N-(6-chloronaphthalen-2-yl)-N'-[(3S)-...)Show SMILES CCC(=O)CN([C@H]1CCN([C@@H](C)C(=O)N2CCOCC2)C1=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 |r| Show InChI InChI=1S/C25H30ClN3O6S/c1-3-21(30)16-29(36(33,34)22-7-5-18-14-20(26)6-4-19(18)15-22)23-8-9-28(25(23)32)17(2)24(31)27-10-12-35-13-11-27/h4-7,14-15,17,23H,3,8-13,16H2,1-2H3/t17-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... |
Bioorg Med Chem Lett 16: 3784-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.053
BindingDB Entry DOI: 10.7270/Q2416V9V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339719
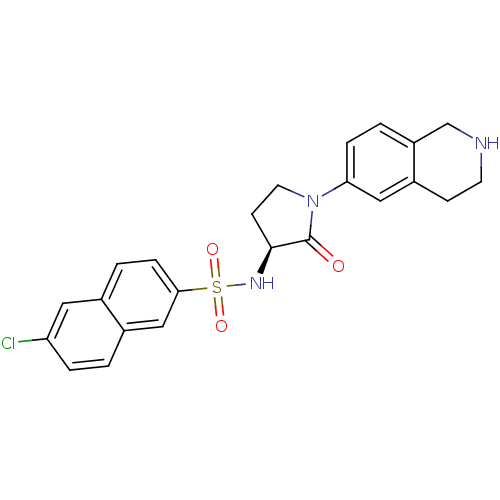
((S)-6-chloro-N-(2-oxo-1-(1,2,3,4-tetrahydroisoquin...)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCN(C1=O)c1ccc2CNCCc2c1 |r| Show InChI InChI=1S/C23H22ClN3O3S/c24-19-4-1-16-13-21(6-3-15(16)11-19)31(29,30)26-22-8-10-27(23(22)28)20-5-2-18-14-25-9-7-17(18)12-20/h1-6,11-13,22,25-26H,7-10,14H2/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50339717

((S)-2-(5-chlorothiophen-2-yl)-N-(1-(2-methyl-1,2,3...)Show SMILES CN1CCc2cc(ccc2C1)N1CC[C@H](NS(=O)(=O)\C=C\c2ccc(Cl)s2)C1=O |r| Show InChI InChI=1S/C20H22ClN3O3S2/c1-23-9-6-14-12-16(3-2-15(14)13-23)24-10-7-18(20(24)25)22-29(26,27)11-8-17-4-5-19(21)28-17/h2-5,8,11-12,18,22H,6-7,9-10,13H2,1H3/b11-8+/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a using bis-(CBZ-glycylglycly)-L-arginine amide fluorogenic substrate |
Bioorg Med Chem Lett 21: 1588-92 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.129
BindingDB Entry DOI: 10.7270/Q2RN385P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50306140
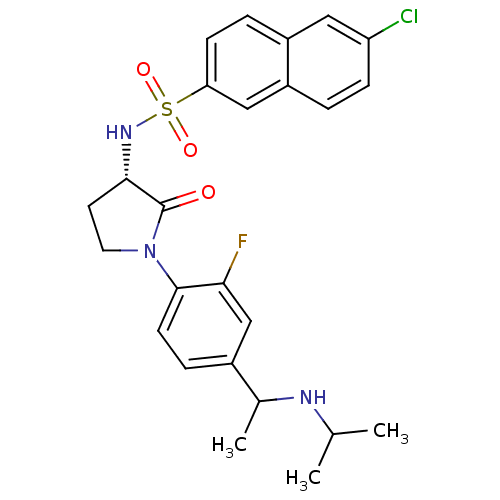
(6-chloro-N-((3S)-1-(2-fluoro-4-(1-(isopropylamino)...)Show SMILES CC(C)NC(C)c1ccc(N2CC[C@H](NS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C2=O)c(F)c1 |r| Show InChI InChI=1S/C25H27ClFN3O3S/c1-15(2)28-16(3)17-6-9-24(22(27)14-17)30-11-10-23(25(30)31)29-34(32,33)21-8-5-18-12-20(26)7-4-19(18)13-21/h4-9,12-16,23,28-29H,10-11H2,1-3H3/t16?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by fluorescence assay |
Bioorg Med Chem Lett 20: 618-22 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.077
BindingDB Entry DOI: 10.7270/Q24M94NN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data