 Found 940 hits with Last Name = 'you' and Initial = 'l'
Found 940 hits with Last Name = 'you' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50059510

(8-Methoxy-3-phenyl-1,2,3,4-tetrahydro-chromeno[3,4...)Show InChI InChI=1S/C19H17NO3/c1-22-14-7-8-16-15-9-10-20(13-5-3-2-4-6-13)12-17(15)19(21)23-18(16)11-14/h2-8,11H,9-10,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Inositol phosphorylceramide synthase
(Candida albicans) | BDBM50408926
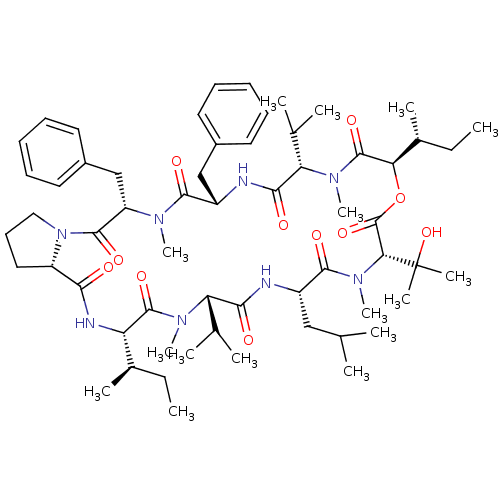
(Aureobasidin A | CHEMBL1793802)Show SMILES CC[C@@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@H](C)CC |r| Show InChI InChI=1S/C60H92N8O11/c1-17-38(9)46-57(75)65(14)47(36(5)6)52(70)61-42(32-35(3)4)55(73)67(16)50(60(11,12)78)59(77)79-49(39(10)18-2)58(76)66(15)48(37(7)8)53(71)62-43(33-40-26-21-19-22-27-40)54(72)64(13)45(34-41-28-23-20-24-29-41)56(74)68-31-25-30-44(68)51(69)63-46/h19-24,26-29,35-39,42-50,78H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t38-,39-,42+,43+,44+,45+,46+,47+,48+,49-,50-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp.
Curated by ChEMBL
| Assay Description
Inhibition of Candida albicans ATCC 38247 inositol phosphorylceramide synthase preincubated for 30 mins |
Antimicrob Agents Chemother 53: 496-504 (2009)
Article DOI: 10.1128/AAC.00633-08
BindingDB Entry DOI: 10.7270/Q27H1JV5 |
More data for this
Ligand-Target Pair | |
Inositol phosphorylceramide synthase catalytic subunit AUR1
(Saccharomyces cerevisiae S288c) | BDBM50408926

(Aureobasidin A | CHEMBL1793802)Show SMILES CC[C@@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@H](C)CC |r| Show InChI InChI=1S/C60H92N8O11/c1-17-38(9)46-57(75)65(14)47(36(5)6)52(70)61-42(32-35(3)4)55(73)67(16)50(60(11,12)78)59(77)79-49(39(10)18-2)58(76)66(15)48(37(7)8)53(71)62-43(33-40-26-21-19-22-27-40)54(72)64(13)45(34-41-28-23-20-24-29-41)56(74)68-31-25-30-44(68)51(69)63-46/h19-24,26-29,35-39,42-50,78H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t38-,39-,42+,43+,44+,45+,46+,47+,48+,49-,50-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp.
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae SJ21R inositol phosphorylceramide synthase preincubated for 30 mins |
Antimicrob Agents Chemother 53: 496-504 (2009)
Article DOI: 10.1128/AAC.00633-08
BindingDB Entry DOI: 10.7270/Q27H1JV5 |
More data for this
Ligand-Target Pair | |
Inositol phosphorylceramide synthase
(Candida albicans) | BDBM50333121
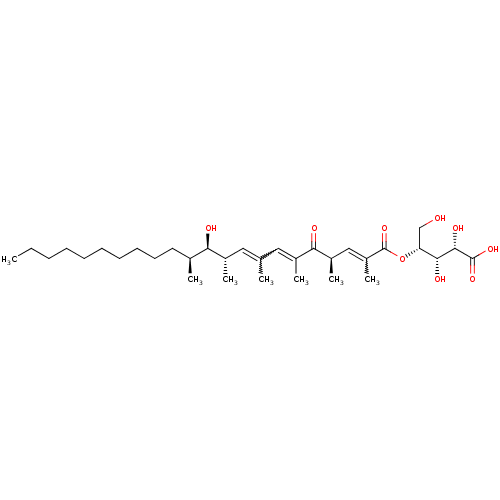
(CHEMBL1631582 | Khafrefungin)Show SMILES CCCCCCCCCC[C@H](C)[C@@H](O)[C@@H](C)C=C(C)C=C(C)C(=O)[C@H](C)C=C(C)C(=O)O[C@H](CO)[C@@H](O)[C@H](O)C(O)=O |r,w:19.18,17.17,27.27| Show InChI InChI=1S/C33H56O9/c1-8-9-10-11-12-13-14-15-16-22(3)28(35)23(4)17-21(2)18-24(5)29(36)25(6)19-26(7)33(41)42-27(20-34)30(37)31(38)32(39)40/h17-19,22-23,25,27-28,30-31,34-35,37-38H,8-16,20H2,1-7H3,(H,39,40)/t22-,23-,25+,27+,28+,30+,31-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp.
Curated by ChEMBL
| Assay Description
Inhibition of Candida albicans ATCC 38247 inositol phosphorylceramide synthase preincubated for 30 mins |
Antimicrob Agents Chemother 53: 496-504 (2009)
Article DOI: 10.1128/AAC.00633-08
BindingDB Entry DOI: 10.7270/Q27H1JV5 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50028059
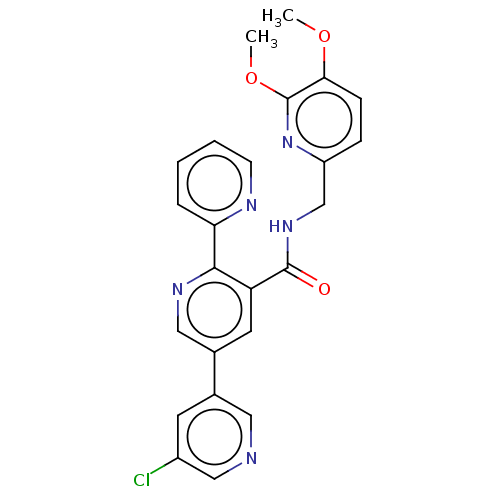
(CHEMBL3338866)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2ccccn2)-c2cncc(Cl)c2)nc1OC Show InChI InChI=1S/C24H20ClN5O3/c1-32-21-7-6-18(30-24(21)33-2)14-29-23(31)19-10-16(15-9-17(25)13-26-11-15)12-28-22(19)20-5-3-4-8-27-20/h3-13H,14H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec... |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Inositol phosphorylceramide synthase
(Candida albicans) | BDBM50408926

(Aureobasidin A | CHEMBL1793802)Show SMILES CC[C@@H](C)[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](C(C)C)N(C)C(=O)[C@H](OC(=O)[C@@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N(C)C1=O)C(C)(C)O)[C@H](C)CC |r| Show InChI InChI=1S/C60H92N8O11/c1-17-38(9)46-57(75)65(14)47(36(5)6)52(70)61-42(32-35(3)4)55(73)67(16)50(60(11,12)78)59(77)79-49(39(10)18-2)58(76)66(15)48(37(7)8)53(71)62-43(33-40-26-21-19-22-27-40)54(72)64(13)45(34-41-28-23-20-24-29-41)56(74)68-31-25-30-44(68)51(69)63-46/h19-24,26-29,35-39,42-50,78H,17-18,25,30-34H2,1-16H3,(H,61,70)(H,62,71)(H,63,69)/t38-,39-,42+,43+,44+,45+,46+,47+,48+,49-,50-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp.
Curated by ChEMBL
| Assay Description
Inhibition of Candida albicans inositol phosphorylceramide synthase |
Antimicrob Agents Chemother 53: 496-504 (2009)
Article DOI: 10.1128/AAC.00633-08
BindingDB Entry DOI: 10.7270/Q27H1JV5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059503
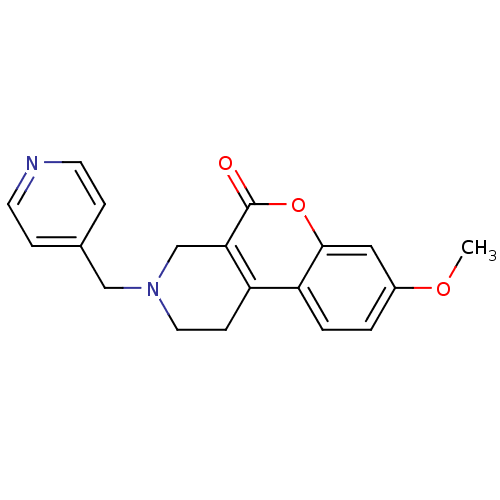
(8-Methoxy-3-pyridin-4-ylmethyl-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C19H18N2O3/c1-23-14-2-3-16-15-6-9-21(11-13-4-7-20-8-5-13)12-17(15)19(22)24-18(16)10-14/h2-5,7-8,10H,6,9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059507

(3-Benzyl-8-methoxy-1,2,3,4-tetrahydro-chromeno[3,4...)Show InChI InChI=1S/C20H19NO3/c1-23-15-7-8-17-16-9-10-21(12-14-5-3-2-4-6-14)13-18(16)20(22)24-19(17)11-15/h2-8,11H,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Microtus ochrogaster) | BDBM50380758
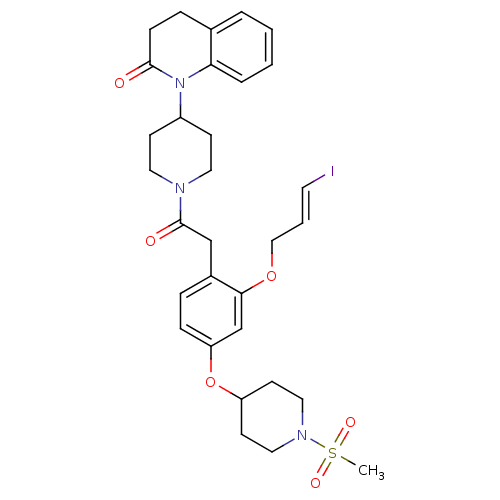
(CHEMBL2017867)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C\I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-LVA from prairie vole vasopressin V1A receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097380
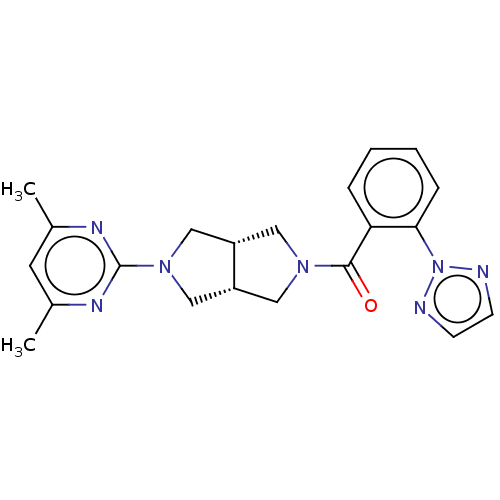
(CHEMBL3586432)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-n1nccn1 |r| Show InChI InChI=1S/C21H23N7O/c1-14-9-15(2)25-21(24-14)27-12-16-10-26(11-17(16)13-27)20(29)18-5-3-4-6-19(18)28-22-7-8-23-28/h3-9,16-17H,10-13H2,1-2H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059505
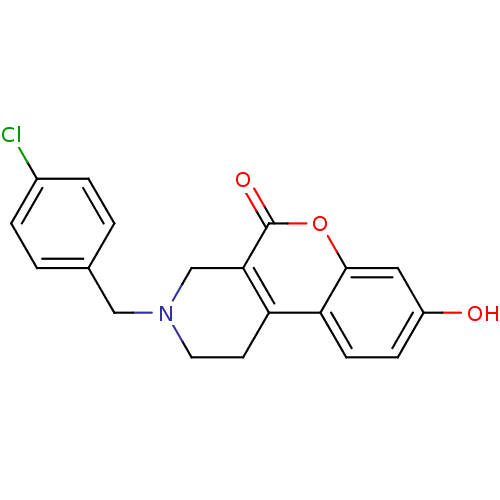
(3-(4-Chloro-benzyl)-8-hydroxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C19H16ClNO3/c20-13-3-1-12(2-4-13)10-21-8-7-15-16-6-5-14(22)9-18(16)24-19(23)17(15)11-21/h1-6,9,22H,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059504

(8-Methoxy-3-(4-methyl-benzyl)-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C21H21NO3/c1-14-3-5-15(6-4-14)12-22-10-9-17-18-8-7-16(24-2)11-20(18)25-21(23)19(17)13-22/h3-8,11H,9-10,12-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Microtus ochrogaster) | BDBM50380759
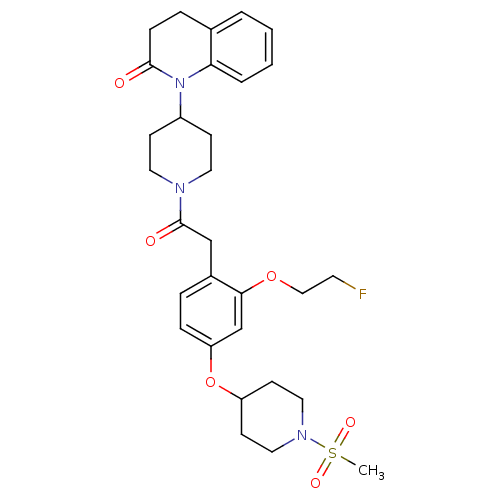
(CHEMBL2017869)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OCCF)c1 Show InChI InChI=1S/C30H38FN3O6S/c1-41(37,38)33-17-12-25(13-18-33)40-26-8-6-23(28(21-26)39-19-14-31)20-30(36)32-15-10-24(11-16-32)34-27-5-3-2-4-22(27)7-9-29(34)35/h2-6,8,21,24-25H,7,9-20H2,1H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-OVTA from prairie vole Oxytocin receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059496
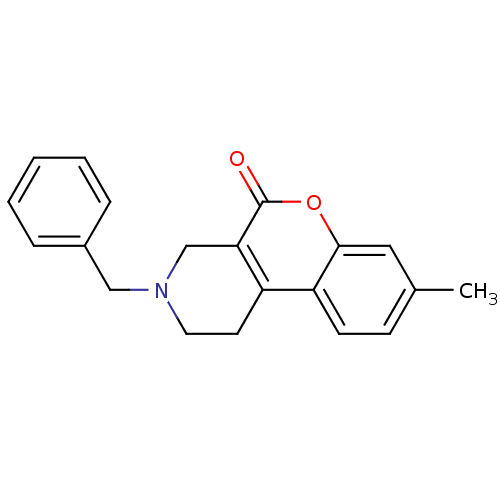
(3-Benzyl-8-methyl-1,2,3,4-tetrahydro-chromeno[3,4-...)Show InChI InChI=1S/C20H19NO2/c1-14-7-8-17-16-9-10-21(12-15-5-3-2-4-6-15)13-18(16)20(22)23-19(17)11-14/h2-8,11H,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Microtus ochrogaster) | BDBM50380758

(CHEMBL2017867)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C\I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-OVTA from prairie vole Oxytocin receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Microtus ochrogaster) | BDBM50380757
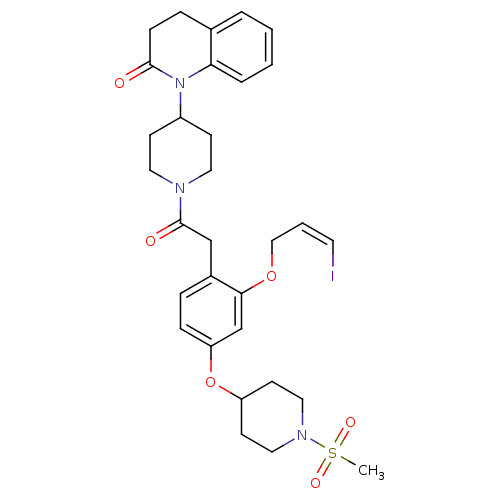
(CHEMBL2017868)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C/I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-OVTA from prairie vole Oxytocin receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059491
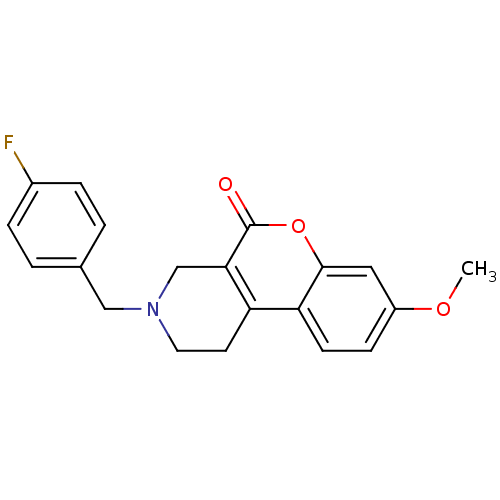
(3-(4-Fluoro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18FNO3/c1-24-15-6-7-17-16-8-9-22(11-13-2-4-14(21)5-3-13)12-18(16)20(23)25-19(17)10-15/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412863
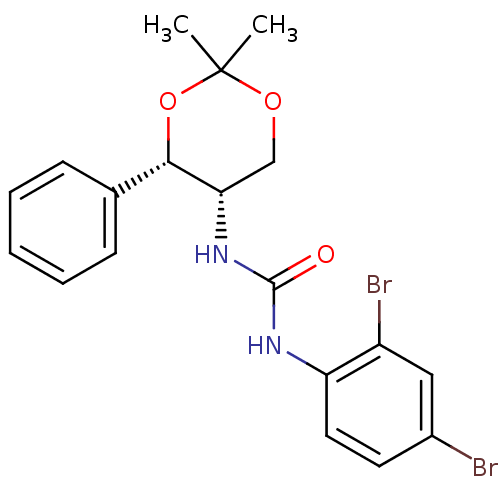
(CHEMBL359632 | JNJ-10397049)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccc(Br)cc2Br)[C@@H](O1)c1ccccc1 |r| Show InChI InChI=1S/C19H20Br2N2O3/c1-19(2)25-11-16(17(26-19)12-6-4-3-5-7-12)23-18(24)22-15-9-8-13(20)10-14(15)21/h3-10,16-17H,11H2,1-2H3,(H2,22,23,24)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50435057

(CHEMBL2391300)Show SMILES CN[C@@H](CCS(C)(=O)=O)C(=O)N[C@H]1C[C@H]2CC[C@]1(CS(=O)(=O)N1CCN(CC1)c1ccccc1C)C2(C)C |r,TLB:11:12:35:16.15| Show InChI InChI=1S/C27H44N4O5S2/c1-20-8-6-7-9-23(20)30-13-15-31(16-14-30)38(35,36)19-27-12-10-21(26(27,2)3)18-24(27)29-25(32)22(28-4)11-17-37(5,33)34/h6-9,21-22,24,28H,10-19H2,1-5H3,(H,29,32)/t21-,22+,24+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yerkes National Primate Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to human OT receptor |
Bioorg Med Chem Lett 23: 902-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.116
BindingDB Entry DOI: 10.7270/Q29Z9690 |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524390
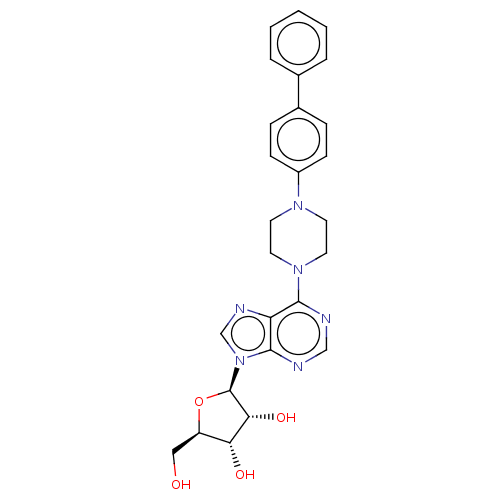
(CHEMBL4475619)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(ncnc12)N1CCN(CC1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C26H28N6O4/c33-14-20-22(34)23(35)26(36-20)32-16-29-21-24(27-15-28-25(21)32)31-12-10-30(11-13-31)19-8-6-18(7-9-19)17-4-2-1-3-5-17/h1-9,15-16,20,22-23,26,33-35H,10-14H2/t20-,22-,23-,26-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... |
J Med Chem 62: 4483-4499 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00020
BindingDB Entry DOI: 10.7270/Q2028VZ7 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380757

(CHEMBL2017868)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C/I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097388
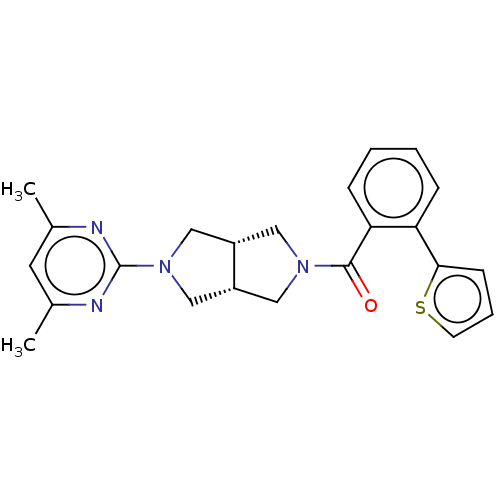
(CHEMBL3586426)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C23H24N4OS/c1-15-10-16(2)25-23(24-15)27-13-17-11-26(12-18(17)14-27)22(28)20-7-4-3-6-19(20)21-8-5-9-29-21/h3-10,17-18H,11-14H2,1-2H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059492
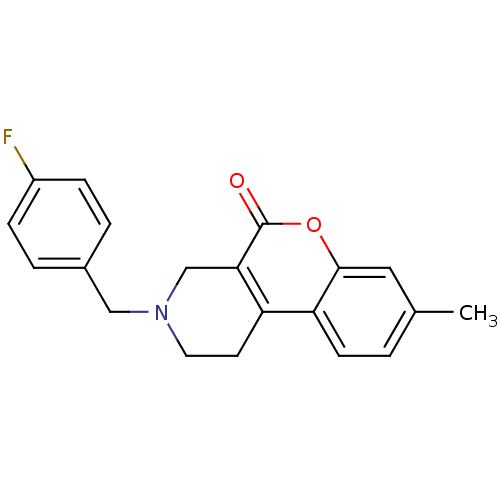
(3-(4-Fluoro-benzyl)-8-methyl-1,2,3,4-tetrahydro-ch...)Show InChI InChI=1S/C20H18FNO2/c1-13-2-7-17-16-8-9-22(11-14-3-5-15(21)6-4-14)12-18(16)20(23)24-19(17)10-13/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059497

(8-Methoxy-3-phenethyl-1,2,3,4-tetrahydro-chromeno[...)Show InChI InChI=1S/C21H21NO3/c1-24-16-7-8-18-17-10-12-22(11-9-15-5-3-2-4-6-15)14-19(17)21(23)25-20(18)13-16/h2-8,13H,9-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097360
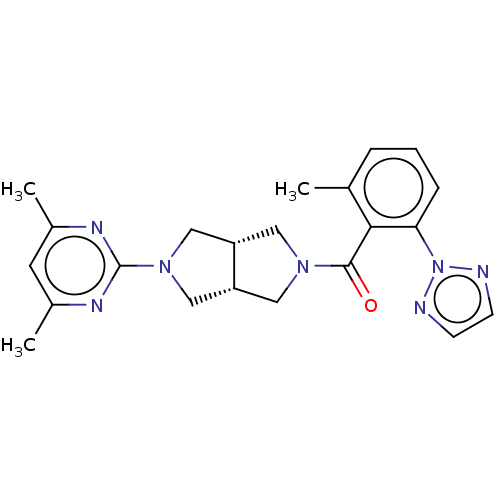
(CHEMBL3586440)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(C)cccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O/c1-14-5-4-6-19(29-23-7-8-24-29)20(14)21(30)27-10-17-12-28(13-18(17)11-27)22-25-15(2)9-16(3)26-22/h4-9,17-18H,10-13H2,1-3H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059501

(8-Methoxy-3-(4-trifluoromethyl-benzyl)-1,2,3,4-tet...)Show SMILES COc1ccc2c3CCN(Cc4ccc(cc4)C(F)(F)F)Cc3c(=O)oc2c1 Show InChI InChI=1S/C21H18F3NO3/c1-27-15-6-7-17-16-8-9-25(12-18(16)20(26)28-19(17)10-15)11-13-2-4-14(5-3-13)21(22,23)24/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059493
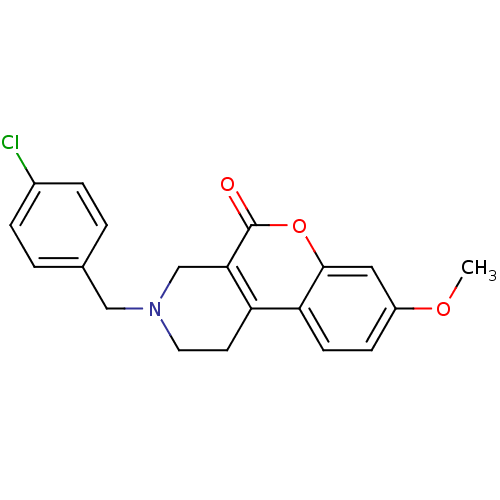
(3-(4-Chloro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18ClNO3/c1-24-15-6-7-17-16-8-9-22(11-13-2-4-14(21)5-3-13)12-18(16)20(23)25-19(17)10-15/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50206172

(1-(benzo[d][1,3]dioxol-5-ylmethyl)-2,3,4,9-tetrahy...)Show InChI InChI=1S/C19H18N2O2/c1-2-4-15-13(3-1)14-7-8-20-16(19(14)21-15)9-12-5-6-17-18(10-12)23-11-22-17/h1-6,10,16,20-21H,7-9,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde
Curated by ChEMBL
| Assay Description
Displacement of [3H]5CT from 5HT7 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 2649-55 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.093
BindingDB Entry DOI: 10.7270/Q2WQ03F0 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Microtus ochrogaster) | BDBM50380759

(CHEMBL2017869)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OCCF)c1 Show InChI InChI=1S/C30H38FN3O6S/c1-41(37,38)33-17-12-25(13-18-33)40-26-8-6-23(28(21-26)39-19-14-31)20-30(36)32-15-10-24(11-16-32)34-27-5-3-2-4-22(27)7-9-29(34)35/h2-6,8,21,24-25H,7,9-20H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-LVA from prairie vole vasopressin V1A receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097388

(CHEMBL3586426)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C23H24N4OS/c1-15-10-16(2)25-23(24-15)27-13-17-11-26(12-18(17)14-27)22(28)20-7-4-3-6-19(20)21-8-5-9-29-21/h3-10,17-18H,11-14H2,1-2H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092814
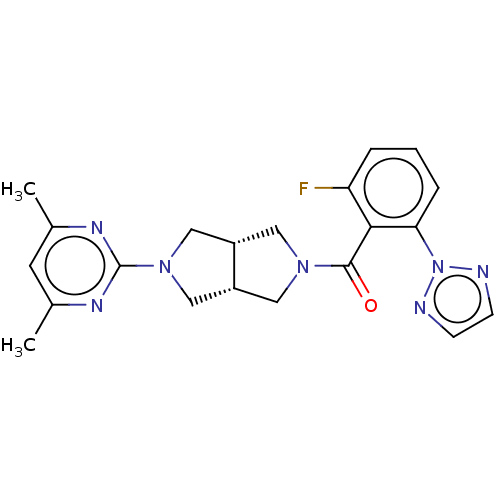
(CHEMBL3586436)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(F)cccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)19-17(22)4-3-5-18(19)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380758

(CHEMBL2017867)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C\I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092813
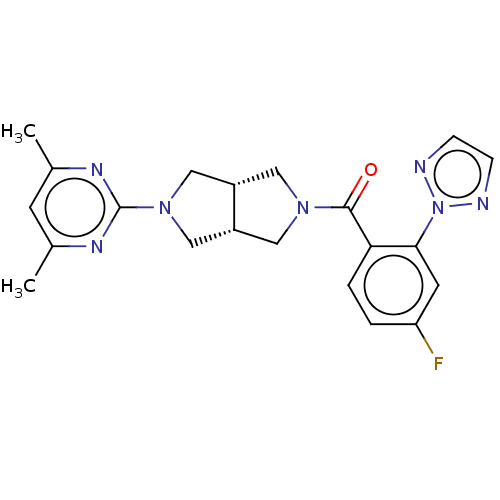
(CHEMBL3586434)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccc(F)cc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-4-3-17(22)8-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50092812
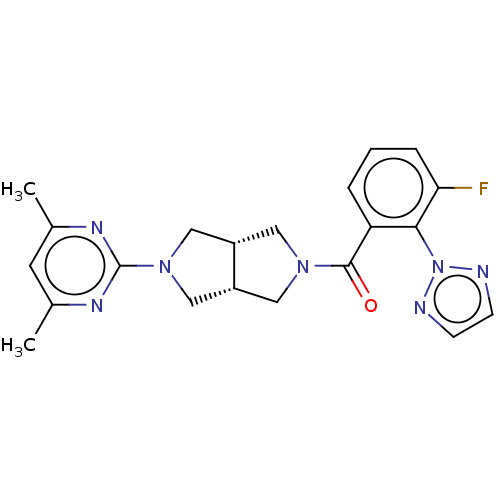
(CHEMBL3586433)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cccc(F)c1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)17-4-3-5-18(22)19(17)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50092814

(CHEMBL3586436)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(F)cccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)19-17(22)4-3-5-18(19)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059499
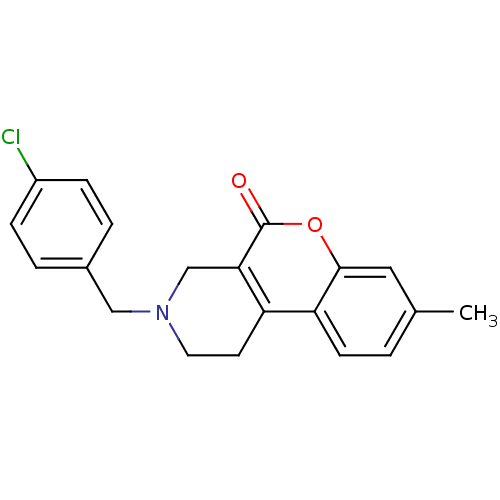
(3-(4-Chloro-benzyl)-8-methyl-1,2,3,4-tetrahydro-ch...)Show InChI InChI=1S/C20H18ClNO2/c1-13-2-7-17-16-8-9-22(11-14-3-5-15(21)6-4-14)12-18(16)20(23)24-19(17)10-13/h2-7,10H,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097360

(CHEMBL3586440)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(C)cccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O/c1-14-5-4-6-19(29-23-7-8-24-29)20(14)21(30)27-10-17-12-28(13-18(17)11-27)22-25-15(2)9-16(3)26-22/h4-9,17-18H,10-13H2,1-3H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097380

(CHEMBL3586432)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-n1nccn1 |r| Show InChI InChI=1S/C21H23N7O/c1-14-9-15(2)25-21(24-14)27-12-16-10-26(11-17(16)13-27)20(29)18-5-3-4-6-19(18)28-22-7-8-23-28/h3-9,16-17H,10-13H2,1-2H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50326719
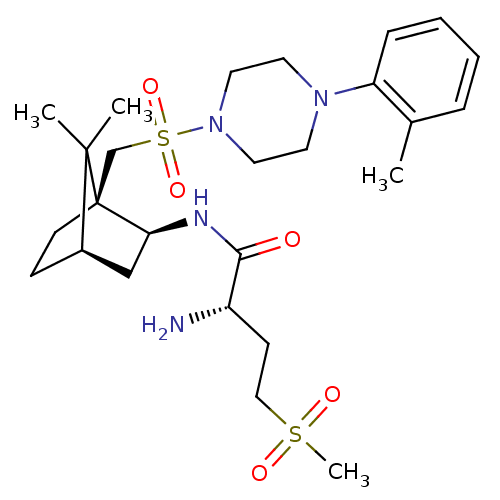
((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...)Show SMILES Cc1ccccc1N1CCN(CC1)S(=O)(=O)C[C@]12CC[C@H](C[C@@H]1NC(=O)[C@@H](N)CCS(C)(=O)=O)C2(C)C |r,TLB:23:22:34:18.19| Show InChI InChI=1S/C26H42N4O5S2/c1-19-7-5-6-8-22(19)29-12-14-30(15-13-29)37(34,35)18-26-11-9-20(25(26,2)3)17-23(26)28-24(31)21(27)10-16-36(4,32)33/h5-8,20-21,23H,9-18,27H2,1-4H3,(H,28,31)/t20-,21+,23+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yerkes National Primate Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to human OT receptor |
Bioorg Med Chem Lett 23: 902-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.116
BindingDB Entry DOI: 10.7270/Q29Z9690 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50092813

(CHEMBL3586434)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccc(F)cc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-4-3-17(22)8-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092812

(CHEMBL3586433)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cccc(F)c1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)17-4-3-5-18(22)19(17)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50440392
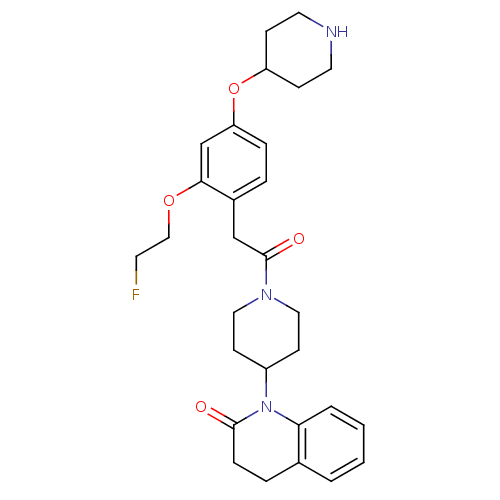
(CHEMBL2424668)Show SMILES FCCOc1cc(OC2CCNCC2)ccc1CC(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C29H36FN3O4/c30-13-18-36-27-20-25(37-24-9-14-31-15-10-24)7-5-22(27)19-29(35)32-16-11-23(12-17-32)33-26-4-2-1-3-21(26)6-8-28(33)34/h1-5,7,20,23-24,31H,6,8-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Binding affinity to human OTR by competitive binding assay |
Bioorg Med Chem Lett 23: 5415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.045
BindingDB Entry DOI: 10.7270/Q20R9QVH |
More data for this
Ligand-Target Pair | |
Inositol phosphorylceramide synthase
(Candida albicans) | BDBM50333120
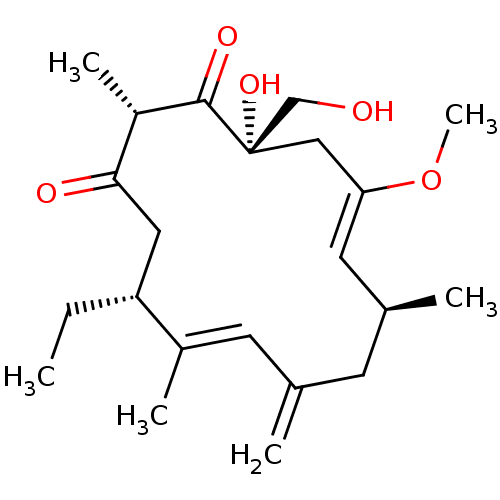
(CHEMBL1631581 | Rustmicin)Show SMILES CC[C@@H]1CC(=O)[C@H](C)C(=O)[C@@](O)(CO)C\C(OC)=C\[C@@H](C)CC(=C)\C=C1/C |r,t:17,24| Show InChI InChI=1S/C22H34O5/c1-7-18-11-20(24)17(5)21(25)22(26,13-23)12-19(27-6)10-15(3)8-14(2)9-16(18)4/h9-10,15,17-18,23,26H,2,7-8,11-13H2,1,3-6H3/b16-9+,19-10-/t15-,17-,18+,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp.
Curated by ChEMBL
| Assay Description
Inhibition of Candida albicans ATCC 38247 inositol phosphorylceramide synthase preincubated for 30 mins |
Antimicrob Agents Chemother 53: 496-504 (2009)
Article DOI: 10.1128/AAC.00633-08
BindingDB Entry DOI: 10.7270/Q27H1JV5 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380759

(CHEMBL2017869)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OCCF)c1 Show InChI InChI=1S/C30H38FN3O6S/c1-41(37,38)33-17-12-25(13-18-33)40-26-8-6-23(28(21-26)39-19-14-31)20-30(36)32-15-10-24(11-16-32)34-27-5-3-2-4-22(27)7-9-29(34)35/h2-6,8,21,24-25H,7,9-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Binding affinity to human OTR by competitive binding assay |
Bioorg Med Chem Lett 23: 5415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.045
BindingDB Entry DOI: 10.7270/Q20R9QVH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380759

(CHEMBL2017869)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OCCF)c1 Show InChI InChI=1S/C30H38FN3O6S/c1-41(37,38)33-17-12-25(13-18-33)40-26-8-6-23(28(21-26)39-19-14-31)20-30(36)32-15-10-24(11-16-32)34-27-5-3-2-4-22(27)7-9-29(34)35/h2-6,8,21,24-25H,7,9-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Microtus ochrogaster) | BDBM50380757

(CHEMBL2017868)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C/I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-LVA from prairie vole vasopressin V1A receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380759

(CHEMBL2017869)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OCCF)c1 Show InChI InChI=1S/C30H38FN3O6S/c1-41(37,38)33-17-12-25(13-18-33)40-26-8-6-23(28(21-26)39-19-14-31)20-30(36)32-15-10-24(11-16-32)34-27-5-3-2-4-22(27)7-9-29(34)35/h2-6,8,21,24-25H,7,9-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Oxytocin from human OXTR expressed in CHO cell membranes after 90 mins by liquid scintillation counting method |
Bioorg Med Chem 25: 305-315 (2017)
Article DOI: 10.1016/j.bmc.2016.10.035
BindingDB Entry DOI: 10.7270/Q24T6MNC |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524393
(CHEMBL4448092)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(ccnc12)N1CCN(CC1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H29N5O4/c33-16-22-24(34)25(35)27(36-22)32-17-29-23-21(10-11-28-26(23)32)31-14-12-30(13-15-31)20-8-6-19(7-9-20)18-4-2-1-3-5-18/h1-11,17,22,24-25,27,33-35H,12-16H2/t22-,24-,25-,27-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... |
J Med Chem 62: 4483-4499 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00020
BindingDB Entry DOI: 10.7270/Q2028VZ7 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50233853

(CHEMBL3392901)Show SMILES Cc1ccccc1N1CCN(CC1)S(=O)(=O)CC12CCC(CC1NC(=O)C(N)CCS(C)(=O)=O)C2(C)C |TLB:23:22:34:18.19| Show InChI InChI=1S/C26H42N4O5S2/c1-19-7-5-6-8-22(19)29-12-14-30(15-13-29)37(34,35)18-26-11-9-20(25(26,2)3)17-23(26)28-24(31)21(27)10-16-36(4,32)33/h5-8,20-21,23H,9-18,27H2,1-4H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Oxytocin from human OXTR expressed in CHO cell membranes after 90 mins by liquid scintillation counting method |
Bioorg Med Chem 25: 305-315 (2017)
Article DOI: 10.1016/j.bmc.2016.10.035
BindingDB Entry DOI: 10.7270/Q24T6MNC |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50059494
(3-(3-Chloro-benzyl)-8-methoxy-1,2,3,4-tetrahydro-c...)Show InChI InChI=1S/C20H18ClNO3/c1-24-15-5-6-17-16-7-8-22(11-13-3-2-4-14(21)9-13)12-18(16)20(23)25-19(17)10-15/h2-6,9-10H,7-8,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data