 Found 7516 hits with Last Name = 'young' and Initial = 'k'
Found 7516 hits with Last Name = 'young' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50128358

(CHEMBL3629347)Show SMILES CCCCC(NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52?,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant melanocortin 4 receptor expressed in CHO cells |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM10755
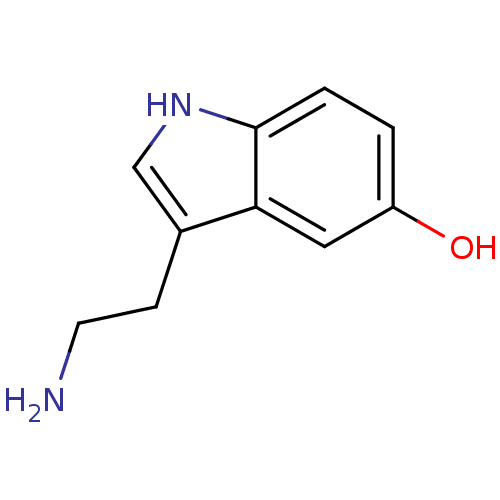
(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT7 receptor expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50176065
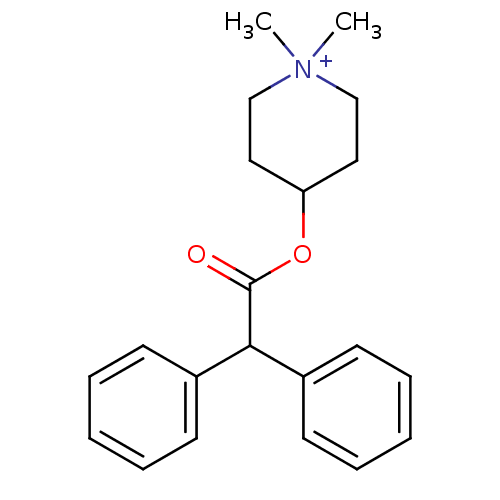
(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]4-DAMP from human recombinant Muscarinic acetylcholine receptor M3 expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50008735
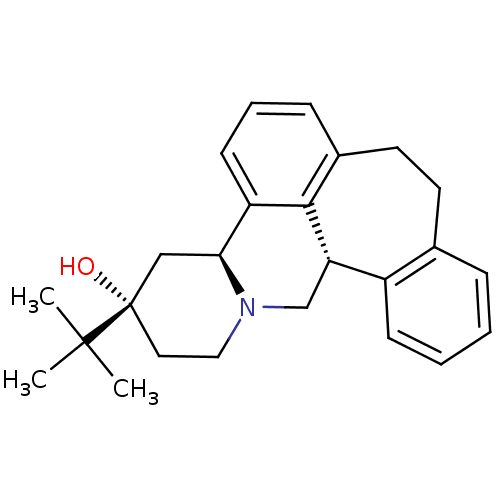
((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyl-spiperone from human recombinant D2S receptor in HEK293 cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21395
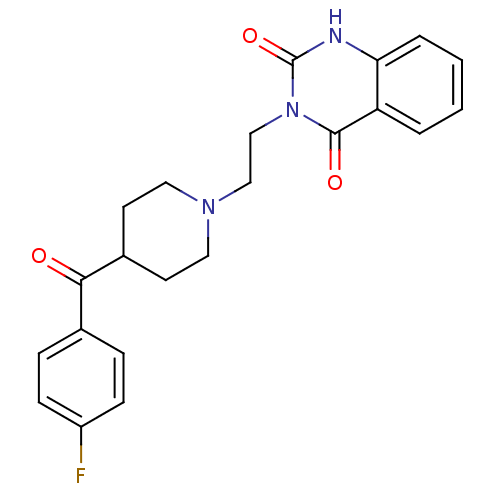
(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor in HEK293 cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000296
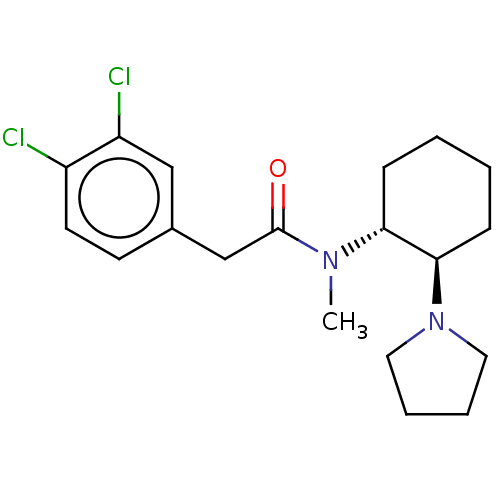
(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]U 69593 from rat recombinant kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50028059
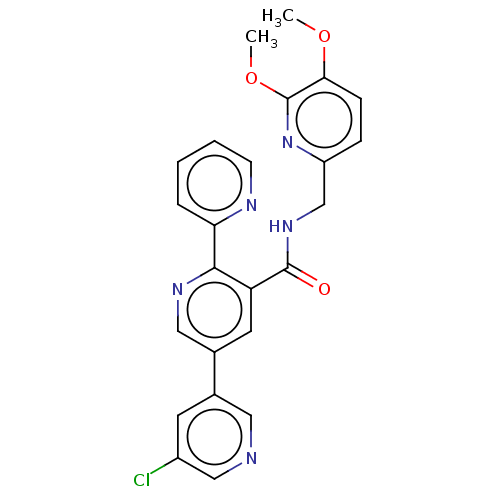
(CHEMBL3338866)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2ccccn2)-c2cncc(Cl)c2)nc1OC Show InChI InChI=1S/C24H20ClN5O3/c1-32-21-7-6-18(30-24(21)33-2)14-29-23(31)19-10-16(15-9-17(25)13-26-11-15)12-28-22(19)20-5-3-4-8-27-20/h3-13H,14H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec... |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22567
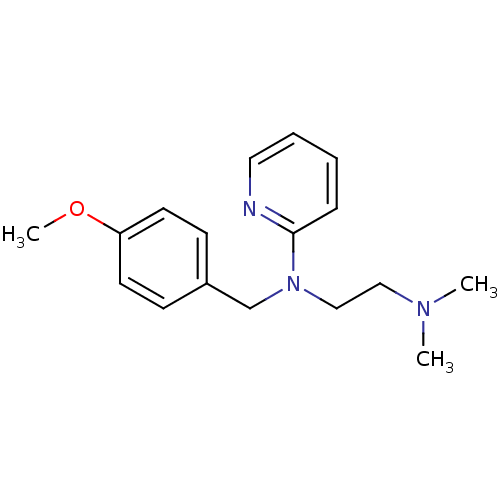
(3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...)Show InChI InChI=1S/C17H23N3O/c1-19(2)12-13-20(17-6-4-5-11-18-17)14-15-7-9-16(21-3)10-8-15/h4-11H,12-14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor in HEK293 cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM28582
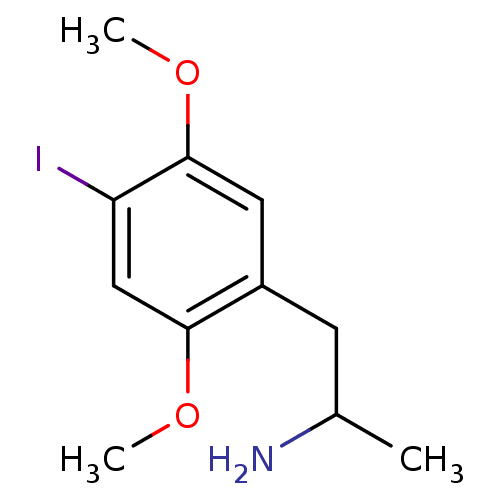
(1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...)Show InChI InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [125I](+/-)DOI from human recombinant 5-HT2B receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50233962
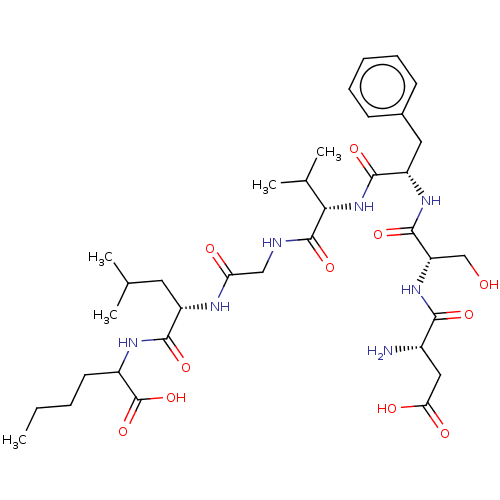
(CHEMBL4068783)Show SMILES CCCCC(NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(O)=O |r| Show InChI InChI=1S/C35H55N7O11/c1-6-7-13-23(35(52)53)39-31(48)24(14-19(2)3)38-27(44)17-37-34(51)29(20(4)5)42-32(49)25(15-21-11-9-8-10-12-21)40-33(50)26(18-43)41-30(47)22(36)16-28(45)46/h8-12,19-20,22-26,29,43H,6-7,13-18,36H2,1-5H3,(H,37,51)(H,38,44)(H,39,48)(H,40,50)(H,41,47)(H,42,49)(H,45,46)(H,52,53)/t22-,23?,24-,25-,26-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [125I]NKA from human recombinant tachykinin NK2 receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097380
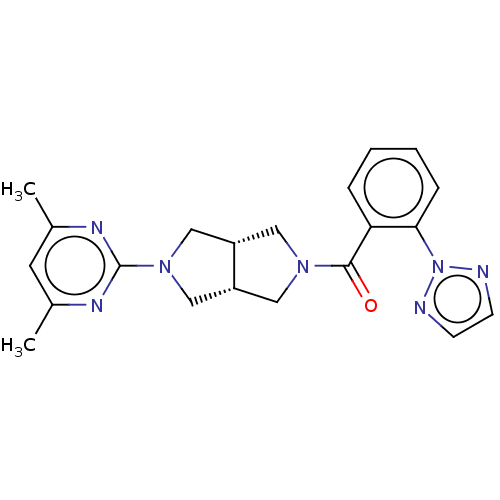
(CHEMBL3586432)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-n1nccn1 |r| Show InChI InChI=1S/C21H23N7O/c1-14-9-15(2)25-21(24-14)27-12-16-10-26(11-17(16)13-27)20(29)18-5-3-4-6-19(18)28-22-7-8-23-28/h3-9,16-17H,10-13H2,1-2H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50176062
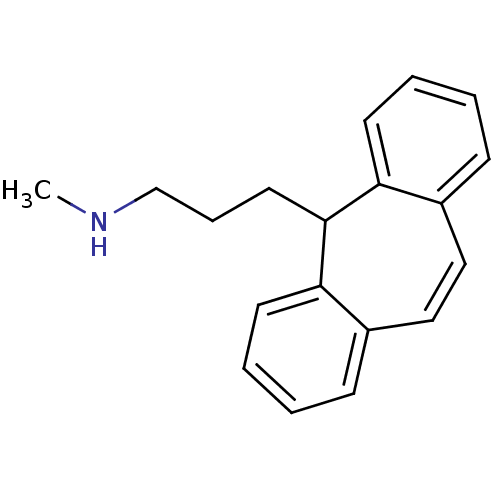
(3-(5H-dibenzo[a,d][7]annulen-5-yl)-N-methylpropan-...)Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-10,12-13,19-20H,6,11,14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from human recombinant norepinephrine transporter expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50412863
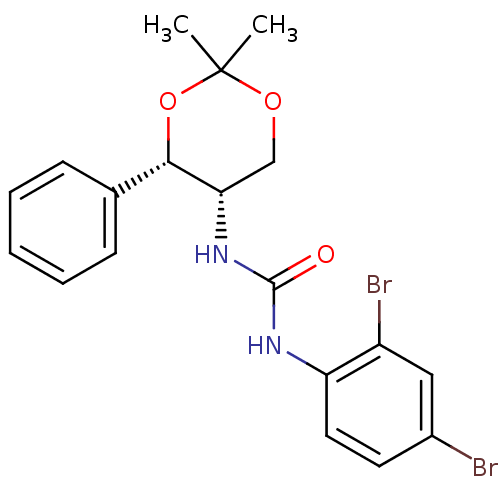
(CHEMBL359632 | JNJ-10397049)Show SMILES CC1(C)OC[C@H](NC(=O)Nc2ccc(Br)cc2Br)[C@@H](O1)c1ccccc1 |r| Show InChI InChI=1S/C19H20Br2N2O3/c1-19(2)25-11-16(17(26-19)12-6-4-3-5-7-12)23-18(24)22-15-9-8-13(20)10-14(15)21/h3-10,16-17H,11H2,1-2H3,(H2,22,23,24)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC-MS/MS analysis |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50005534
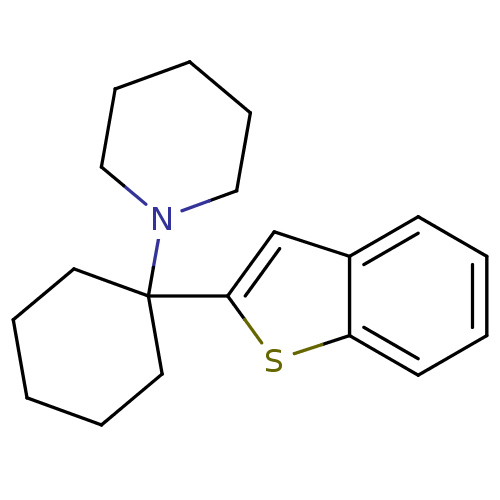
(1-(1-(benzo[b]thiophen-2-yl)cyclohexyl)piperidine ...)Show InChI InChI=1S/C19H25NS/c1-5-11-19(12-6-1,20-13-7-2-8-14-20)18-15-16-9-3-4-10-17(16)21-18/h3-4,9-10,15H,1-2,5-8,11-14H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]BTCP from human recombinant dopamine transporter expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097388
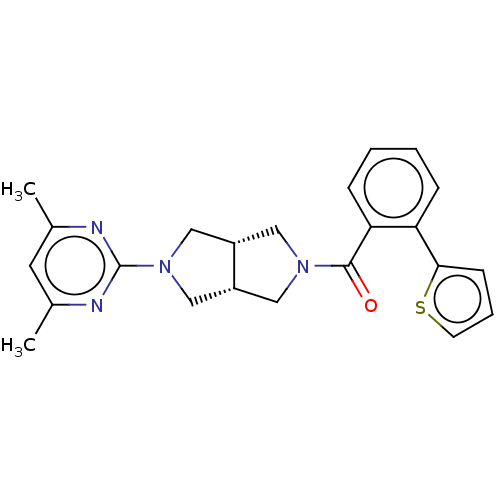
(CHEMBL3586426)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C23H24N4OS/c1-15-10-16(2)25-23(24-15)27-13-17-11-26(12-18(17)14-27)22(28)20-7-4-3-6-19(20)21-8-5-9-29-21/h3-10,17-18H,11-14H2,1-2H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097360
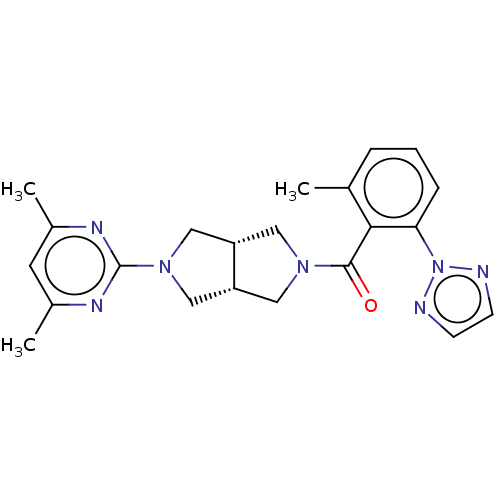
(CHEMBL3586440)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(C)cccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O/c1-14-5-4-6-19(29-23-7-8-24-29)20(14)21(30)27-10-17-12-28(13-18(17)11-27)22-25-15(2)9-16(3)26-22/h4-9,17-18H,10-13H2,1-3H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097388

(CHEMBL3586426)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C23H24N4OS/c1-15-10-16(2)25-23(24-15)27-13-17-11-26(12-18(17)14-27)22(28)20-7-4-3-6-19(20)21-8-5-9-29-21/h3-10,17-18H,11-14H2,1-2H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50092814
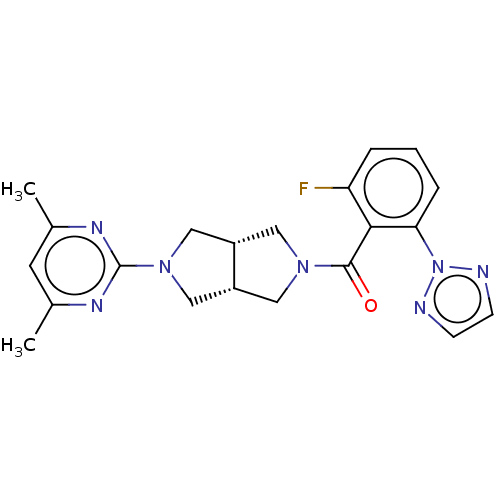
(CHEMBL3586436)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(F)cccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)19-17(22)4-3-5-18(19)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092814

(CHEMBL3586436)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(F)cccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)19-17(22)4-3-5-18(19)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50092812
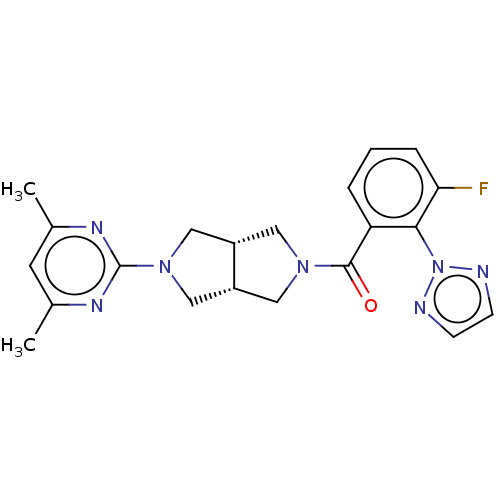
(CHEMBL3586433)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cccc(F)c1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)17-4-3-5-18(22)19(17)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092813
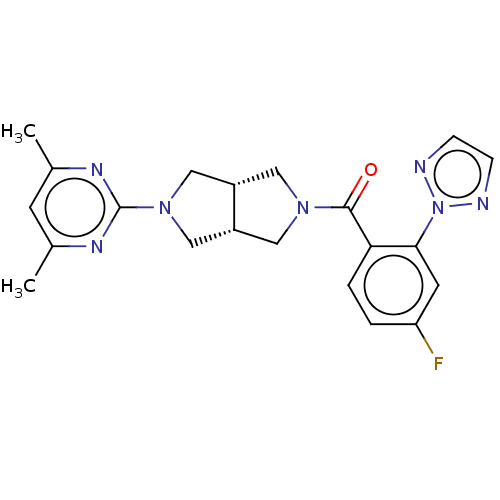
(CHEMBL3586434)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccc(F)cc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-4-3-17(22)8-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097360

(CHEMBL3586440)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(C)cccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O/c1-14-5-4-6-19(29-23-7-8-24-29)20(14)21(30)27-10-17-12-28(13-18(17)11-27)22-25-15(2)9-16(3)26-22/h4-9,17-18H,10-13H2,1-3H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097380

(CHEMBL3586432)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-n1nccn1 |r| Show InChI InChI=1S/C21H23N7O/c1-14-9-15(2)25-21(24-14)27-12-16-10-26(11-17(16)13-27)20(29)18-5-3-4-6-19(18)28-22-7-8-23-28/h3-9,16-17H,10-13H2,1-2H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50092813

(CHEMBL3586434)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccc(F)cc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-4-3-17(22)8-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092812

(CHEMBL3586433)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cccc(F)c1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-8-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)17-4-3-5-18(22)19(17)29-23-6-7-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(Mus musculus) | BDBM50403726
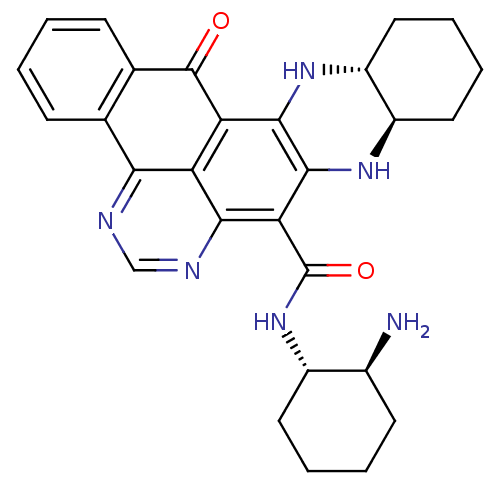
(CHEMBL2112936)Show SMILES N[C@H]1CCCC[C@@H]1NC(=O)c1c2N[C@@H]3CCCC[C@H]3Nc2c2C(=O)c3ccccc3-c3ncnc1c23 Show InChI InChI=1S/C28H30N6O2/c29-16-9-3-4-10-17(16)34-28(36)22-24-20-21(25-26(22)33-19-12-6-5-11-18(19)32-25)27(35)15-8-2-1-7-14(15)23(20)30-13-31-24/h1-2,7-8,13,16-19,32-33H,3-6,9-12,29H2,(H,34,36)/t16-,17-,18+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Subsidiary of Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Antagonistic activity against [125I]-Tyr-ovine CRF binding to Corticotropin releasing factor receptor 2 beta |
Bioorg Med Chem Lett 9: 765-70 (1999)
BindingDB Entry DOI: 10.7270/Q2TD9WJJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM39341
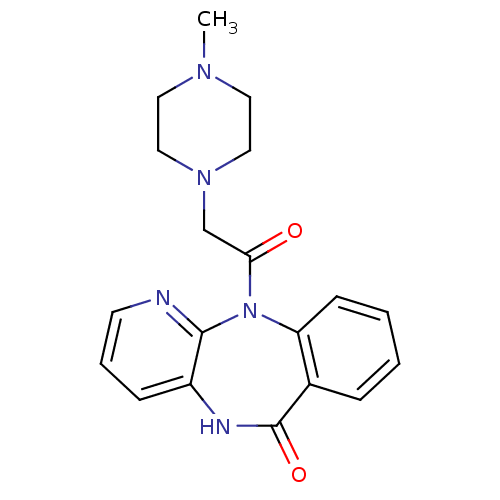
(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]pirenzepine from human recombinant Muscarinic acetylcholine receptor M1 expressed in CHO cells after 60 mins by scintillation cou... |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092810
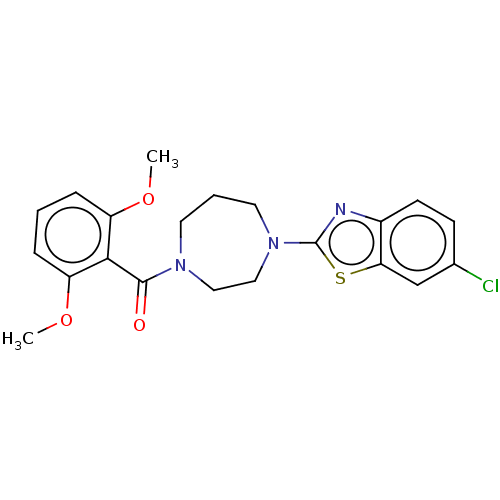
(CHEMBL3586412)Show SMILES COc1cccc(OC)c1C(=O)N1CCCN(CC1)c1nc2ccc(Cl)cc2s1 Show InChI InChI=1S/C21H22ClN3O3S/c1-27-16-5-3-6-17(28-2)19(16)20(26)24-9-4-10-25(12-11-24)21-23-15-8-7-14(22)13-18(15)29-21/h3,5-8,13H,4,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-((3H)-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2 rec... |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097384
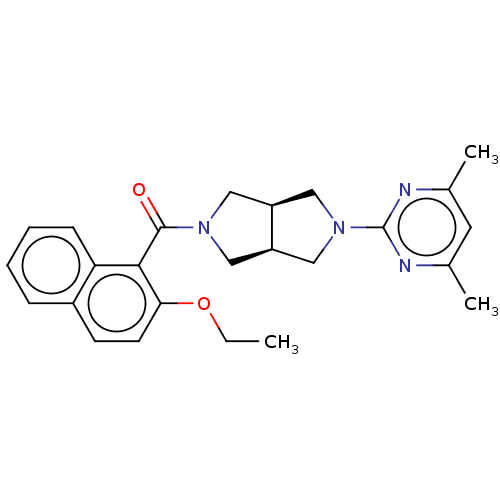
(CHEMBL3586430)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(OCC)ccc2ccccc12 |r| Show InChI InChI=1S/C25H28N4O2/c1-4-31-22-10-9-18-7-5-6-8-21(18)23(22)24(30)28-12-19-14-29(15-20(19)13-28)25-26-16(2)11-17(3)27-25/h5-11,19-20H,4,12-15H2,1-3H3/t19-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097379
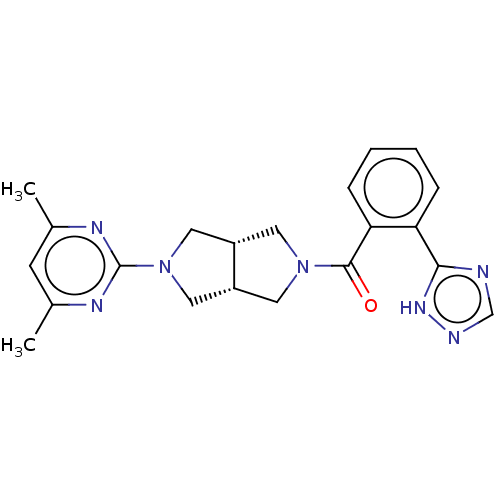
(CHEMBL3586437)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-c1ncn[nH]1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097376
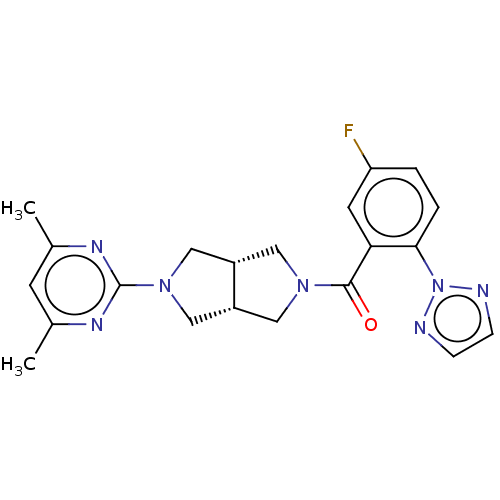
(CHEMBL3586435)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(F)ccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-8-17(22)3-4-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097392

(CHEMBL3586441)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(OC)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O2/c1-14-8-15(2)26-22(25-14)28-12-16-10-27(11-17(16)13-28)21(30)19-9-18(31-3)4-5-20(19)29-23-6-7-24-29/h4-9,16-17H,10-13H2,1-3H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM50097379

(CHEMBL3586437)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1ccccc1-c1ncn[nH]1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097381
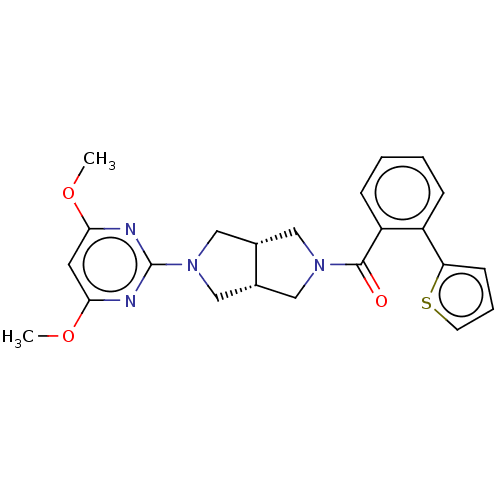
(CHEMBL3586431)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(OC)cc(OC)n1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C23H24N4O3S/c1-29-20-10-21(30-2)25-23(24-20)27-13-15-11-26(12-16(15)14-27)22(28)18-7-4-3-6-17(18)19-8-5-9-31-19/h3-10,15-16H,11-14H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(Mus musculus) | BDBM50075917
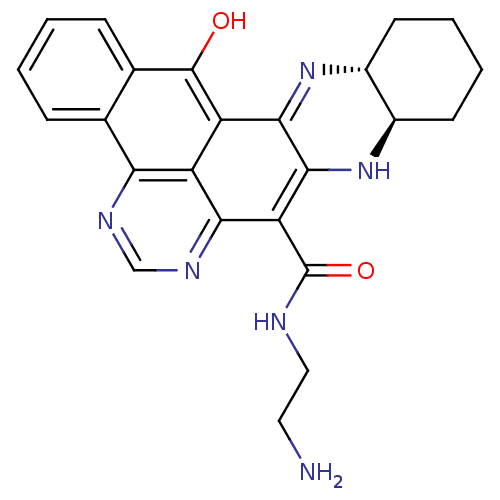
(8N-(2-aminoethyl)-15-oxo-9a,10,11,12,13,13a,14,15-...)Show SMILES NCCNC(=O)c1c2N[C@@H]3CCCC[C@H]3N=c2c2c(O)c3ccccc3c3ncnc1c23 |c:16| Show InChI InChI=1S/C24H24N6O2/c25-9-10-26-24(32)18-20-16-17(21-22(18)30-15-8-4-3-7-14(15)29-21)23(31)13-6-2-1-5-12(13)19(16)27-11-28-20/h1-2,5-6,11,14-15,30-31H,3-4,7-10,25H2,(H,26,32)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Subsidiary of Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Antagonistic activity against [125I]-Tyr-ovine CRF binding to Corticotropin releasing factor receptor 2 beta |
Bioorg Med Chem Lett 9: 765-70 (1999)
BindingDB Entry DOI: 10.7270/Q2TD9WJJ |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097392

(CHEMBL3586441)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(OC)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O2/c1-14-8-15(2)26-22(25-14)28-12-16-10-27(11-17(16)13-28)21(30)19-9-18(31-3)4-5-20(19)29-23-6-7-24-29/h4-9,16-17H,10-13H2,1-3H3/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
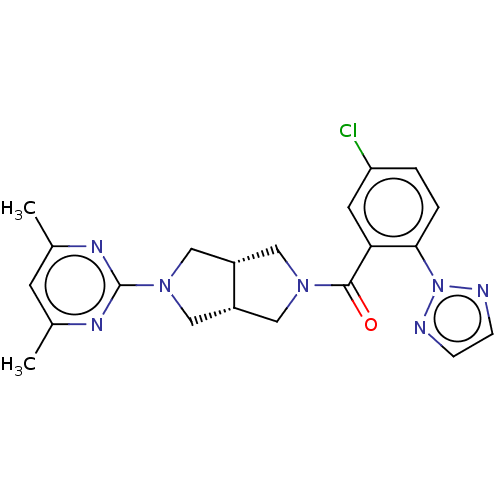
(Rattus norvegicus (Rat)) | BDBM50092811
(CHEMBL3586442)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(Cl)ccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22ClN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-8-17(22)3-4-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
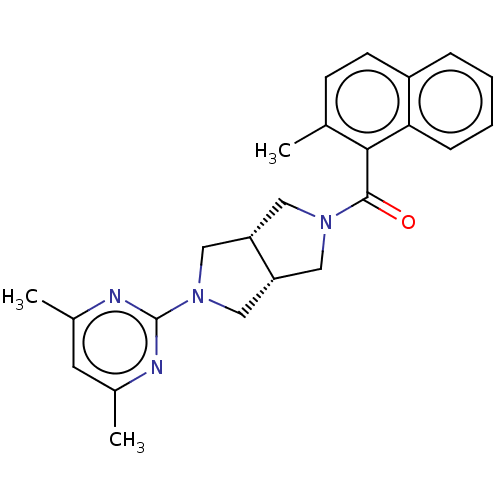
(Homo sapiens (Human)) | BDBM50097387
(CHEMBL3586427)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1c(C)ccc2ccccc12 |r| Show InChI InChI=1S/C24H26N4O/c1-15-8-9-18-6-4-5-7-21(18)22(15)23(29)27-11-19-13-28(14-20(19)12-27)24-25-16(2)10-17(3)26-24/h4-10,19-20H,11-14H2,1-3H3/t19-,20+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097374
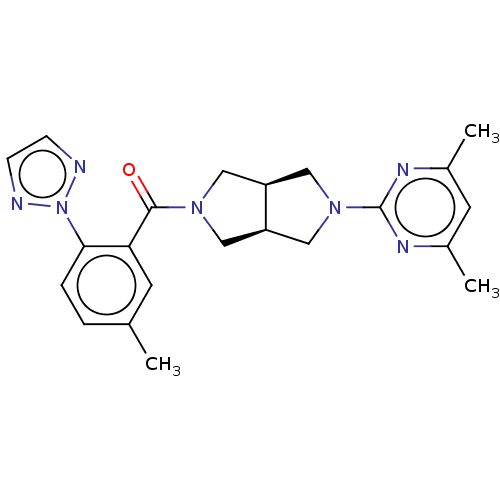
(CHEMBL3586439)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C18H23N3O2/c1-21(2)14-7-12(8-14)16-9-19-17-4-3-11(6-15(16)17)5-13-10-23-18(22)20-13/h3-4,6,9,12-14,19H,5,7-8,10H2,1-2H3,(H,20,22)/t12?,13?,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT6 receptor expressed in CHO cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Corticotropin-releasing factor receptor 2
(Mus musculus) | BDBM50403725

(CHEMBL2112326)Show SMILES NCCNC(=O)c1c2N[C@H]3CCCC[C@@H]3Nc2c2C(=O)c3ccccc3-c3ncnc1c23 Show InChI InChI=1S/C24H24N6O2/c25-9-10-26-24(32)18-20-16-17(21-22(18)30-15-8-4-3-7-14(15)29-21)23(31)13-6-2-1-5-12(13)19(16)27-11-28-20/h1-2,5-6,11,14-15,29-30H,3-4,7-10,25H2,(H,26,32)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Subsidiary of Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Antagonistic activity against [125I]-Tyr-ovine CRF binding to Corticotropin releasing factor receptor 2 beta |
Bioorg Med Chem Lett 9: 765-70 (1999)
BindingDB Entry DOI: 10.7270/Q2TD9WJJ |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092811

(CHEMBL3586442)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cc(Cl)ccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22ClN7O/c1-13-7-14(2)26-21(25-13)28-11-15-9-27(10-16(15)12-28)20(30)18-8-17(22)3-4-19(18)29-23-5-6-24-29/h3-8,15-16H,9-12H2,1-2H3/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 2
(Mus musculus) | BDBM50075918
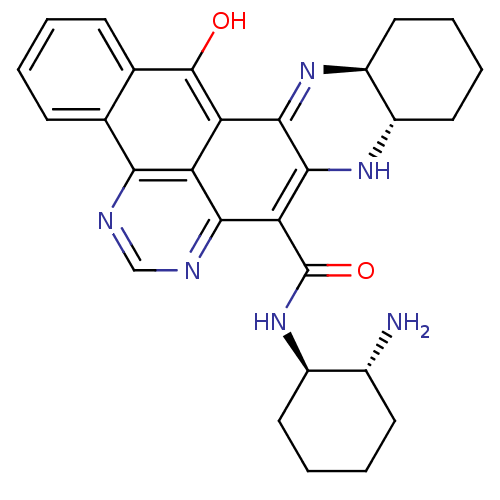
(8N-[2-amino-(1R,2R)-cyclohexyl]-15-oxo-(9aS,13aS)-...)Show SMILES N[C@@H]1CCCC[C@H]1NC(=O)c1c2N[C@H]3CCCC[C@@H]3N=c2c2c(O)c3ccccc3c3ncnc1c23 |c:21| Show InChI InChI=1S/C28H30N6O2/c29-16-9-3-4-10-17(16)34-28(36)22-24-20-21(25-26(22)33-19-12-6-5-11-18(19)32-25)27(35)15-8-2-1-7-14(15)23(20)30-13-31-24/h1-2,7-8,13,16-19,33,35H,3-6,9-12,29H2,(H,34,36)/t16-,17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Subsidiary of Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Antagonistic activity against [125I]-Tyr-ovine CRF binding to Corticotropin releasing factor receptor 2 beta |
Bioorg Med Chem Lett 9: 765-70 (1999)
BindingDB Entry DOI: 10.7270/Q2TD9WJJ |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097385
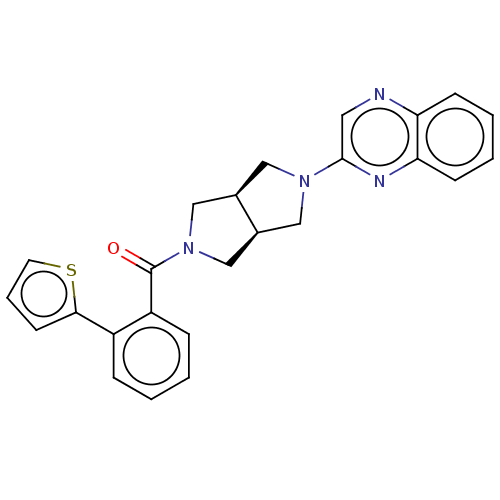
(CHEMBL3586425)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1cnc2ccccc2n1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C25H22N4OS/c30-25(20-7-2-1-6-19(20)23-10-5-11-31-23)29-15-17-13-28(14-18(17)16-29)24-12-26-21-8-3-4-9-22(21)27-24/h1-12,17-18H,13-16H2/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50403726

(CHEMBL2112936)Show SMILES N[C@H]1CCCC[C@@H]1NC(=O)c1c2N[C@@H]3CCCC[C@H]3Nc2c2C(=O)c3ccccc3-c3ncnc1c23 Show InChI InChI=1S/C28H30N6O2/c29-16-9-3-4-10-17(16)34-28(36)22-24-20-21(25-26(22)33-19-12-6-5-11-18(19)32-25)27(35)15-8-2-1-7-14(15)23(20)30-13-31-24/h1-2,7-8,13,16-19,32-33H,3-6,9-12,29H2,(H,34,36)/t16-,17-,18+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Subsidiary of Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Antagonistic activity against [125I]-Tyr-ovine CRF binding to Corticotropin releasing factor receptor 1 |
Bioorg Med Chem Lett 9: 765-70 (1999)
BindingDB Entry DOI: 10.7270/Q2TD9WJJ |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097377
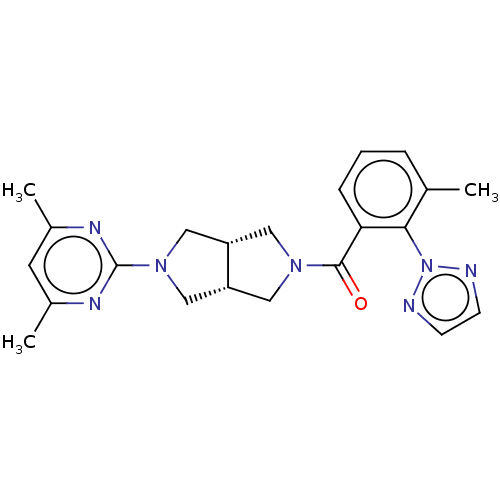
(CHEMBL3586438)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc(C)cc(C)n1)C(=O)c1cccc(C)c1-n1nccn1 |r| Show InChI InChI=1S/C22H25N7O/c1-14-5-4-6-19(20(14)29-23-7-8-24-29)21(30)27-10-17-12-28(13-18(17)11-27)22-25-15(2)9-16(3)26-22/h4-9,17-18H,10-13H2,1-3H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 5A
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5-HT5a receptor in HEK293 cells after 120 mins by scintillation counting |
J Med Chem 60: 349-361 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01422
BindingDB Entry DOI: 10.7270/Q27M0B6T |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092819
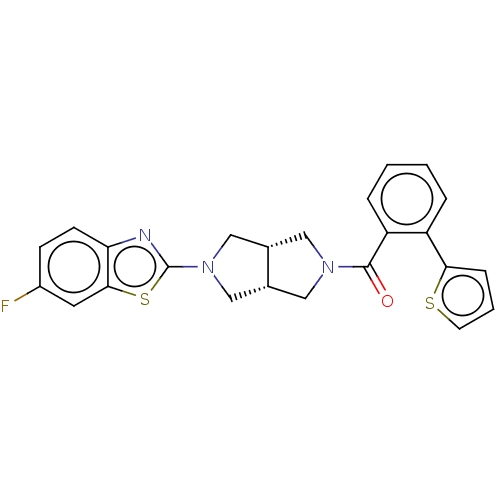
(CHEMBL3586420)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nc2ccc(F)cc2s1)C(=O)c1ccccc1-c1cccs1 |r| Show InChI InChI=1S/C24H20FN3OS2/c25-17-7-8-20-22(10-17)31-24(26-20)28-13-15-11-27(12-16(15)14-28)23(29)19-5-2-1-4-18(19)21-6-3-9-30-21/h1-10,15-16H,11-14H2/t15-,16+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate by LC-MS/MS analysis |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50097383

(CHEMBL3586428)Show SMILES [H][C@@]12CN(C[C@]1([H])CN(C2)c1nccc(OC)n1)C(=O)c1c(C)ccc2ccccc12 |r| Show InChI InChI=1S/C23H24N4O2/c1-15-7-8-16-5-3-4-6-19(16)21(15)22(28)26-11-17-13-27(14-18(17)12-26)23-24-10-9-20(25-23)29-2/h3-10,17-18H,11-14H2,1-2H3/t17-,18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor |
J Med Chem 58: 5620-36 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00742
BindingDB Entry DOI: 10.7270/Q2QR4ZWG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM50075917

(8N-(2-aminoethyl)-15-oxo-9a,10,11,12,13,13a,14,15-...)Show SMILES NCCNC(=O)c1c2N[C@@H]3CCCC[C@H]3N=c2c2c(O)c3ccccc3c3ncnc1c23 |c:16| Show InChI InChI=1S/C24H24N6O2/c25-9-10-26-24(32)18-20-16-17(21-22(18)30-15-8-4-3-7-14(15)29-21)23(31)13-6-2-1-5-12(13)19(16)27-11-28-20/h1-2,5-6,11,14-15,30-31H,3-4,7-10,25H2,(H,26,32)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
A Subsidiary of Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Antagonistic activity against [125I]-Tyr-ovine CRF binding to Corticotropin releasing factor receptor 1 |
Bioorg Med Chem Lett 9: 765-70 (1999)
BindingDB Entry DOI: 10.7270/Q2TD9WJJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data