

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM14073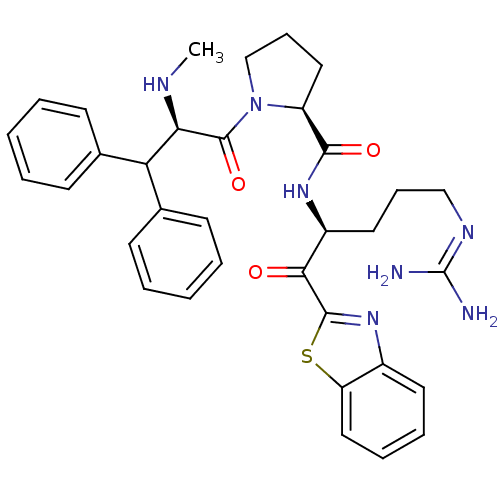 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | -72.4 | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM194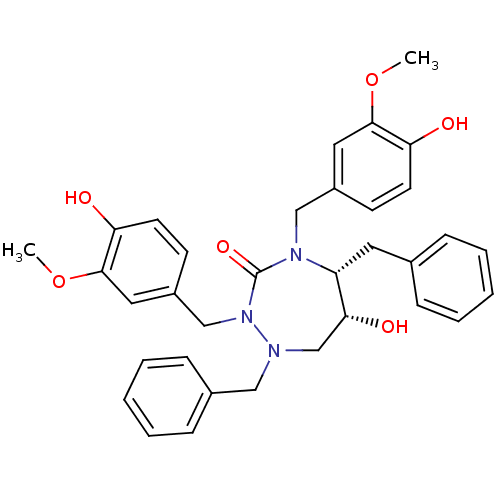 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | -65.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14065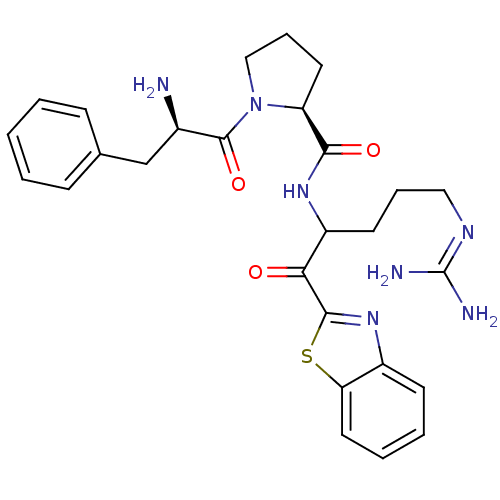 ((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00550 | -66.9 | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14127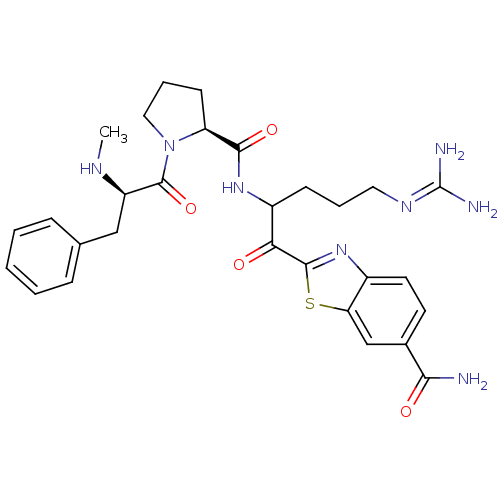 (2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14076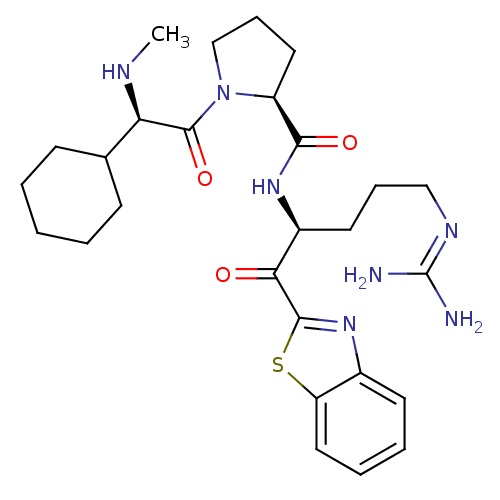 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -63.8 | 5.30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50121249 (CHEMBL415845 | F-G-G-F-T-G-A-R-K-S-A-R-K-L-Aib-N-Q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P. Curated by ChEMBL | Assay Description Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells | J Med Chem 45: 5280-6 (2002) BindingDB Entry DOI: 10.7270/Q2474BKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM370133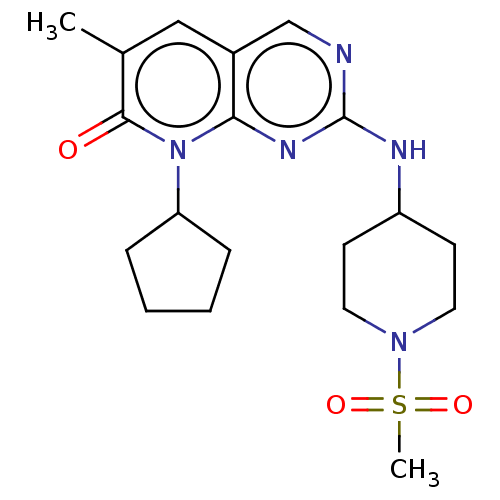 (US10233188, Example 22 | US10800783, Example 22 | ...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174619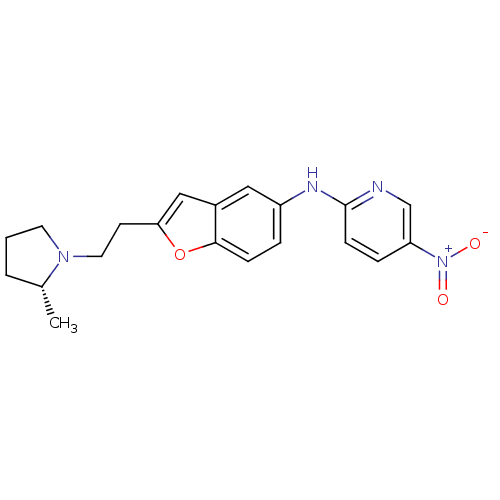 (CHEMBL197747 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50121247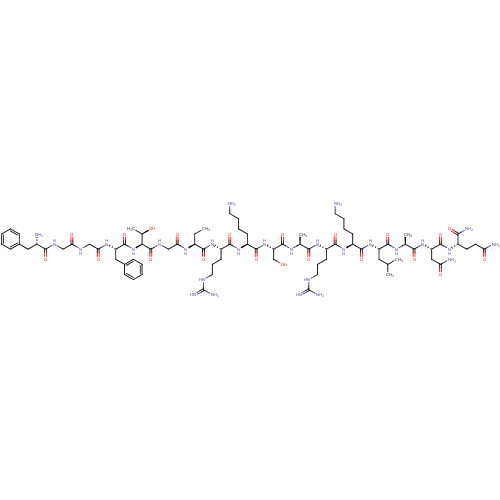 (CHEMBL414542 | F-G-G-F-T-G-Aib-R-K-S-A-R-K-L-A-N-Q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P. Curated by ChEMBL | Assay Description Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells | J Med Chem 45: 5280-6 (2002) BindingDB Entry DOI: 10.7270/Q2474BKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50121245 (CHEMBL266191 | F-G-G-F-T-G-Aib-R-K-S-Aib-R-K-L-A-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P. Curated by ChEMBL | Assay Description Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells | J Med Chem 45: 5280-6 (2002) BindingDB Entry DOI: 10.7270/Q2474BKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50121246 (CHEMBL438537 | F-G-G-F-T-G-A-R-K-S-A-R-K-L-MeA-N-Q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P. Curated by ChEMBL | Assay Description Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells | J Med Chem 45: 5280-6 (2002) BindingDB Entry DOI: 10.7270/Q2474BKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM195 ((5R,6R)-1,5-dibenzyl-2-(cyclopropylmethyl)-6-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | -59.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM467013 ((-)-6-(2,2-difluoroethyl)-8-[(1R*,2R*)-2-hydroxy-2...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM192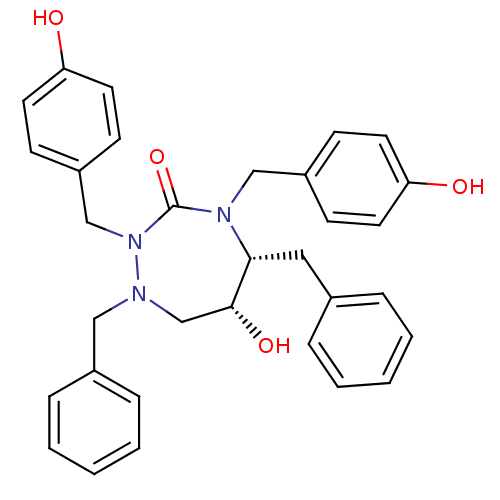 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxyp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | -58.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description In vitro binding affinity by inhibiting [3H]cytisine binding in rat brain tissue at Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 45: 3041-7 (2002) Article DOI: 10.1021/jm0103561 BindingDB Entry DOI: 10.7270/Q2GQ71H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174627 (CHEMBL199245 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50121253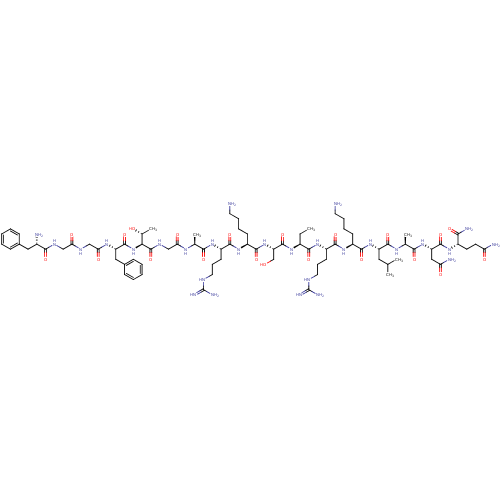 (CHEMBL408356 | F-G-G-F-T-G-A-R-K-S-Aib-R-K-L-A-N-Q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P. Curated by ChEMBL | Assay Description Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells | J Med Chem 45: 5280-6 (2002) BindingDB Entry DOI: 10.7270/Q2474BKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM370147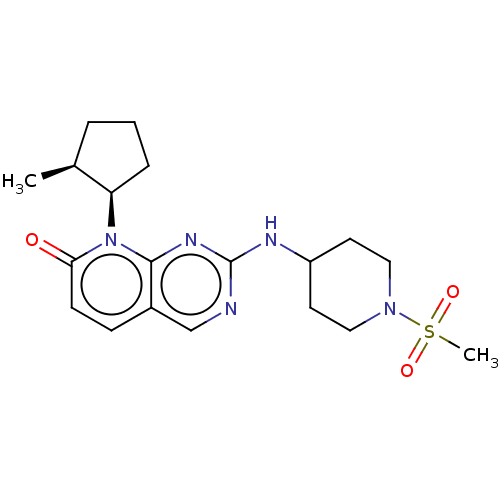 (US10233188, Example 36 | US10800783, Example 36 | ...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50121244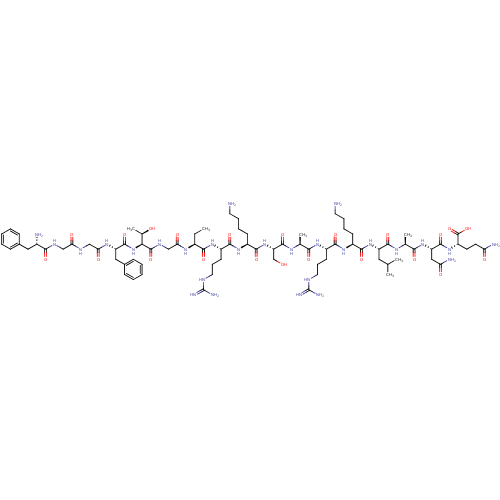 (CHEMBL264084 | F-G-G-F-T-G-Aib-R-K-S-A-R-K-L-A-N-Q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P. Curated by ChEMBL | Assay Description Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells | J Med Chem 45: 5280-6 (2002) BindingDB Entry DOI: 10.7270/Q2474BKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM50609170 (CHEMBL5266837) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | UniChem | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174621 (CHEMBL196467 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM467013 ((-)-6-(2,2-difluoroethyl)-8-[(1R*,2R*)-2-hydroxy-2...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM370121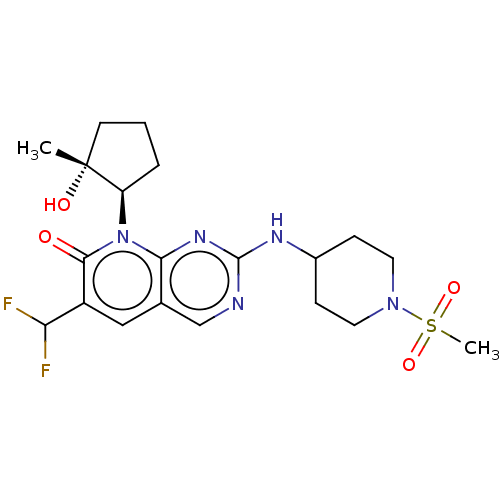 (6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50174619 (CHEMBL197747 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for rat histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14066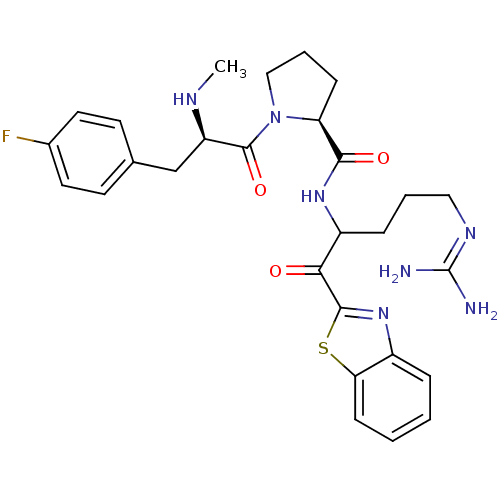 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -58.9 | 15 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM370205 (US10233188, Example 92 | US10800783, Example 92 | ...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174613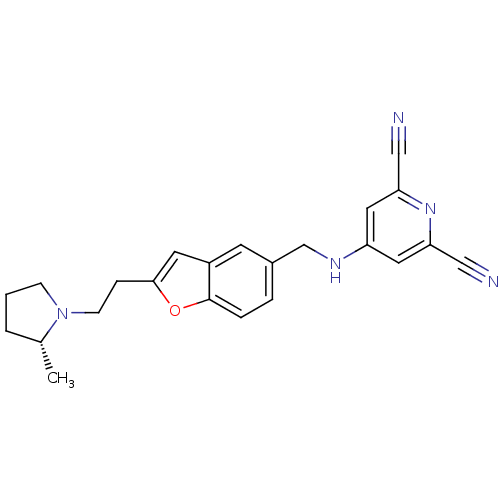 (4-({2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM370121 (6-(difluoromethyl)-8-[(1R,2R)-2-hydroxy-2-methylcy...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14123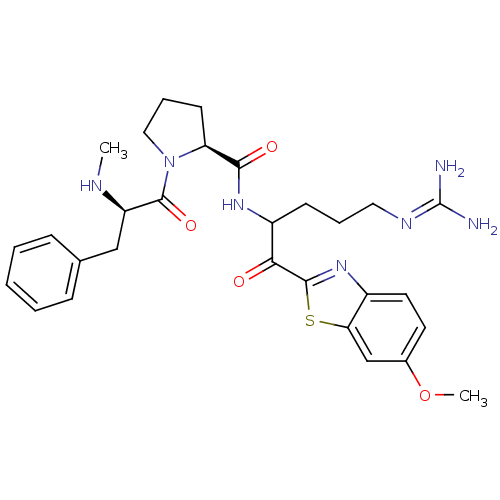 ((2S)-N-[5-carbamimidamido-1-(6-methoxy-1,3-benzoth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description in vitro binding affinity by inhibiting [3H]cytisine binding in rat brain tissue at Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 45: 3041-7 (2002) Article DOI: 10.1021/jm0103561 BindingDB Entry DOI: 10.7270/Q2GQ71H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174637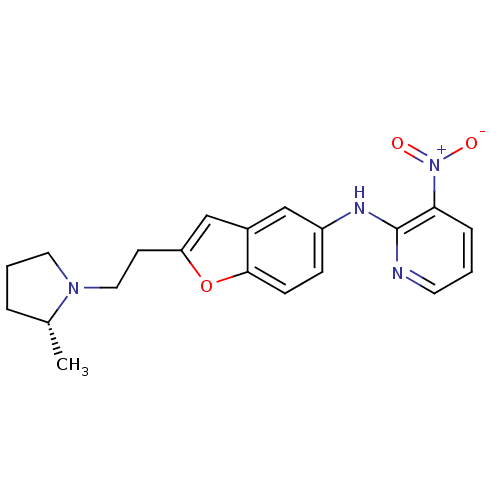 (CHEMBL196294 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14125 ((2S)-N-[5-carbamimidamido-1-(6-fluoro-1,3-benzothi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50121256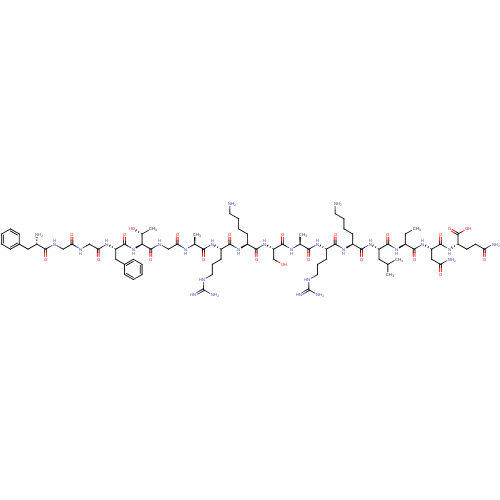 (CHEMBL410979 | F-G-G-F-T-G-A-R-K-S-A-R-K-L-Aib-N-Q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P. Curated by ChEMBL | Assay Description Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells | J Med Chem 45: 5280-6 (2002) BindingDB Entry DOI: 10.7270/Q2474BKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM15131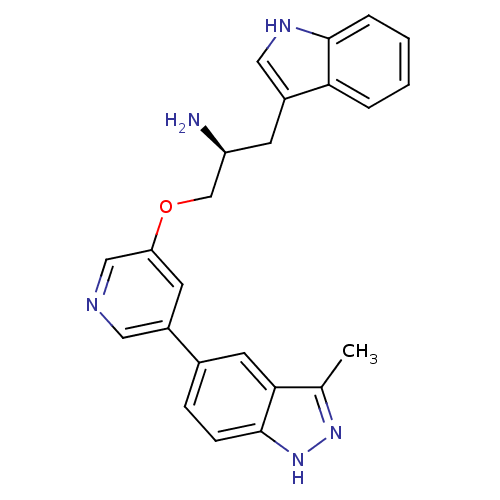 (5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of AKT1 | Nat Chem Biol 5: 484-93 (2009) Article DOI: 10.1038/nchembio.183 BindingDB Entry DOI: 10.7270/Q2D21XTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50143320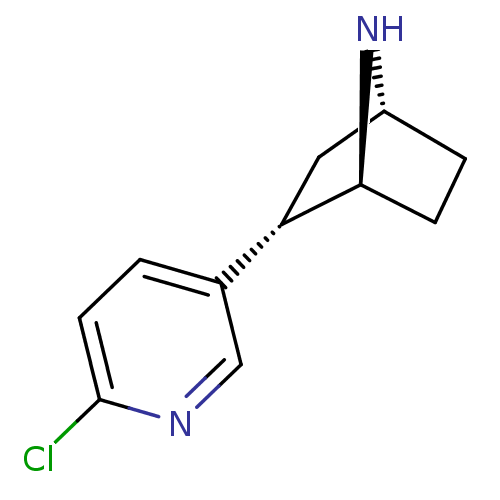 ((+)-epibatidine | (-)-1-epidatidine | (1S,2S,4R)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Orleans Curated by ChEMBL | Assay Description in vitro binding affinity by inhibiting [3H]cytisine binding in rat brain tissue at Nicotinic acetylcholine receptor alpha4-beta2 | J Med Chem 45: 3041-7 (2002) Article DOI: 10.1021/jm0103561 BindingDB Entry DOI: 10.7270/Q2GQ71H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14068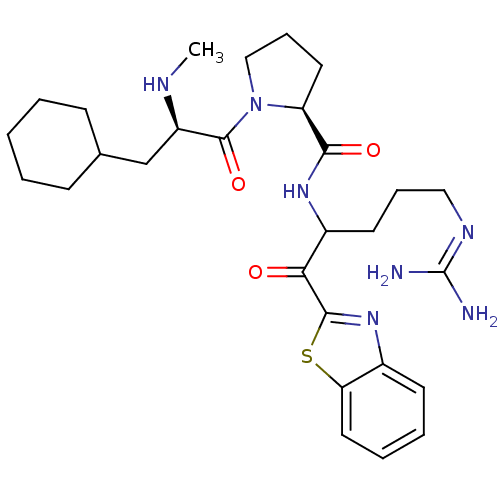 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.180 | -57.9 | 48 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM370256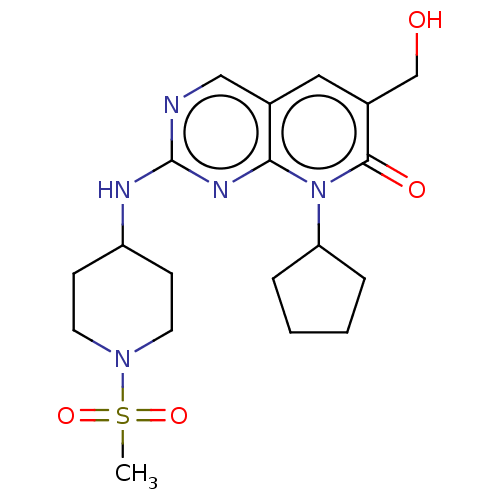 (BDBM467151 | US10233188, Example 143) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | UniChem | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM467013 ((-)-6-(2,2-difluoroethyl)-8-[(1R*,2R*)-2-hydroxy-2...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50174615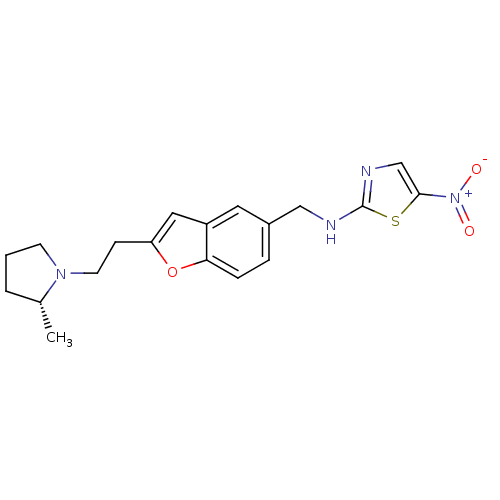 (CHEMBL424842 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity for human histamine H3 receptor using [3H]-N-alpha-methylhistamine | J Med Chem 48: 6482-90 (2005) Article DOI: 10.1021/jm0504398 BindingDB Entry DOI: 10.7270/Q25D8RDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50228853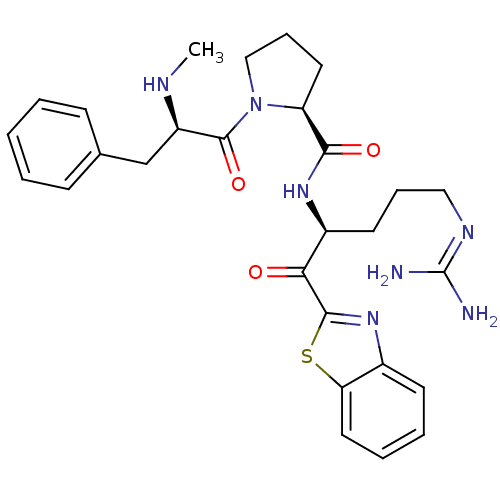 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin. | J Med Chem 39: 3039-43 (1996) Article DOI: 10.1021/jm9603274 BindingDB Entry DOI: 10.7270/Q24B3208 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50224191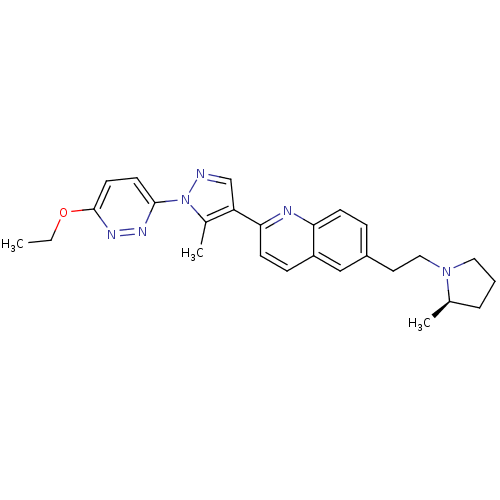 ((R)-2-(1-(6-ethoxypyridazin-3-yl)-5-methyl-1H-pyra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha methyl histamine from human H3 receptor expressed in C6 cells | J Med Chem 50: 5439-48 (2007) Article DOI: 10.1021/jm0705051 BindingDB Entry DOI: 10.7270/Q25M65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM370257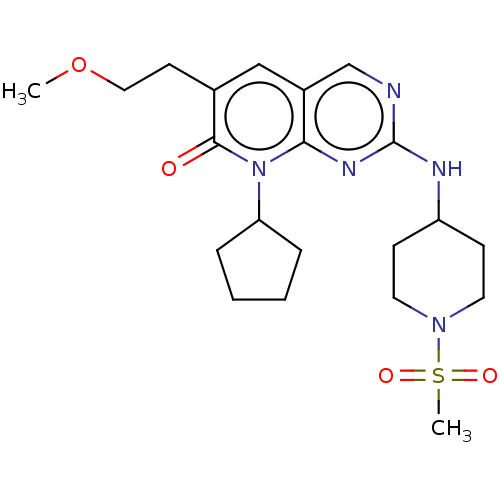 (BDBM467152 | US10233188, Example 144) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | UniChem | 0.196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14071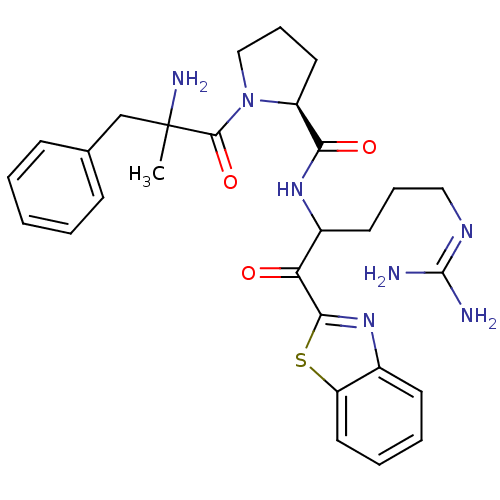 ((2S)-1-(2-amino-2-benzylpropanoyl)-N-[1-(1,3-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | -57.6 | 3.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14063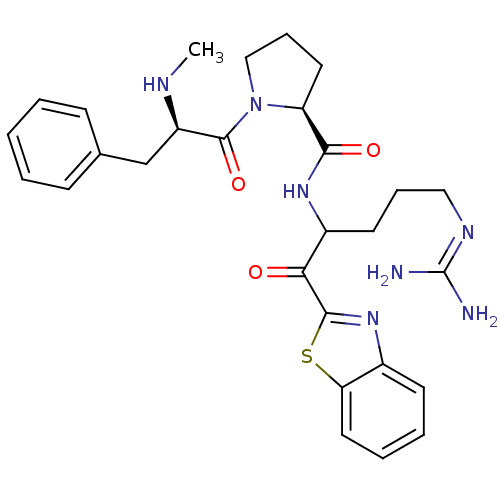 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -57.6 | 29 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM370113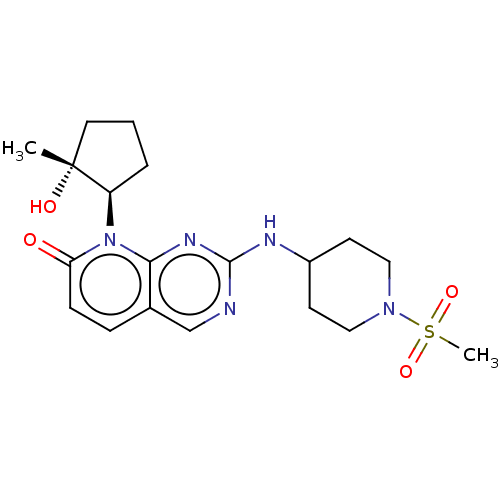 (8-[(1R,2R)-2-hydroxy-2-methylcyclopentyl]-2-{[1-(m...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM370120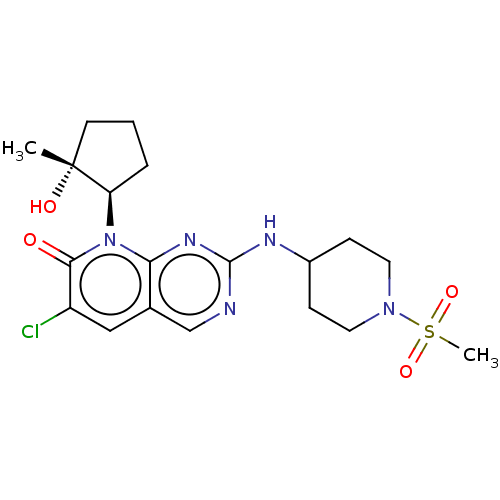 (6-chloro-8-[(1R,2R)-2-hydroxy-2-methylcyclopentyl]...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM50609166 (CHEMBL5280928) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | UniChem | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM50609166 (CHEMBL5280928) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | UniChem | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Mus musculus (mouse)) | BDBM6309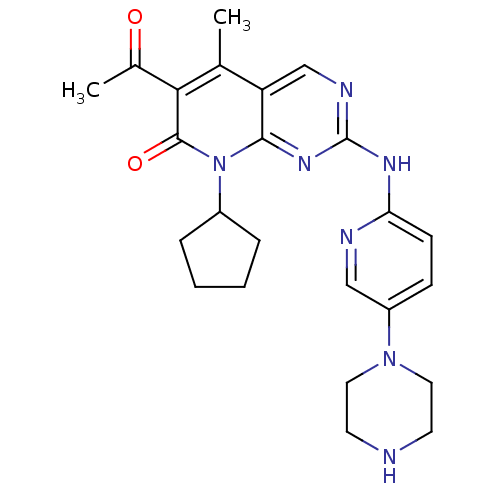 (6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...) | MMDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM27210 ((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... | J Med Chem 51: 5423-30 (2008) Article DOI: 10.1021/jm8003625 BindingDB Entry DOI: 10.7270/Q21G0JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 17859 total ) | Next | Last >> |