

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chitinase (Ostrinia furnacalis) | BDBM50514507 (CHEMBL1583158) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Ostrinia furnacalis chitinase h catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by flu... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50514508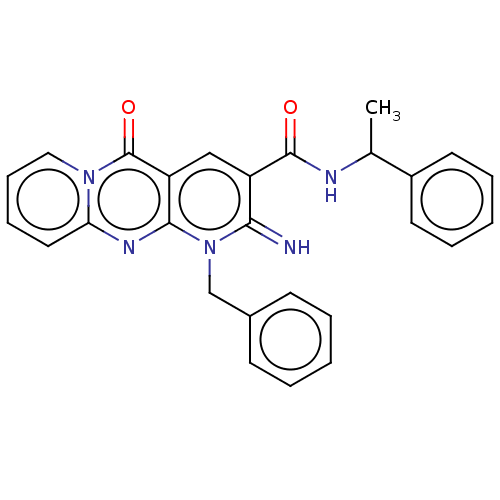 (CHEMBL4549449) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of mouse CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50514508 (CHEMBL4549449) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of human CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50514507 (CHEMBL1583158) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase b catalytic domain overexpressed in Escherichia coli BL21(DE3) cells using 4MU-(GlcNAc)2 as substrate aft... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Ostrinia furnacalis) | BDBM50514508 (CHEMBL4549449) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Ostrinia furnacalis chitinase h catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by flu... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50514508 (CHEMBL4549449) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase b catalytic domain overexpressed in Escherichia coli BL21(DE3) cells using 4MU-(GlcNAc)2 as substrate aft... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50514508 (CHEMBL4549449) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of mouse acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50514507 (CHEMBL1583158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of human CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50514507 (CHEMBL1583158) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of mouse acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50514507 (CHEMBL1583158) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of mouse CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50514508 (CHEMBL4549449) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of human acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50514507 (CHEMBL1583158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 9.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Inhibition of human acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227634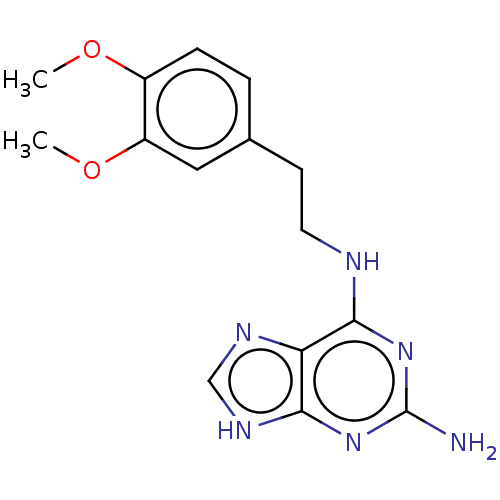 (MTH1 inhibitor, 121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227638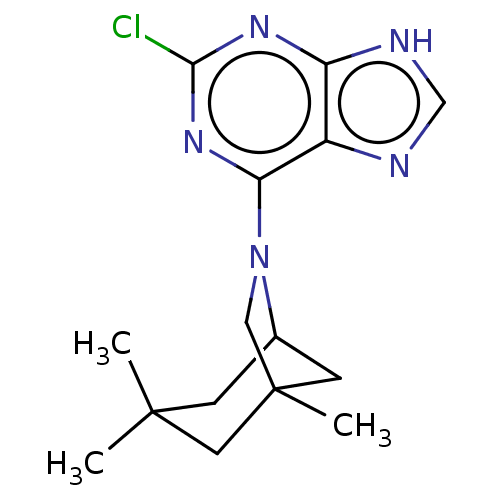 (MTH1 inhibitor, 132) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227638 (MTH1 inhibitor, 132) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227634 (MTH1 inhibitor, 121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227632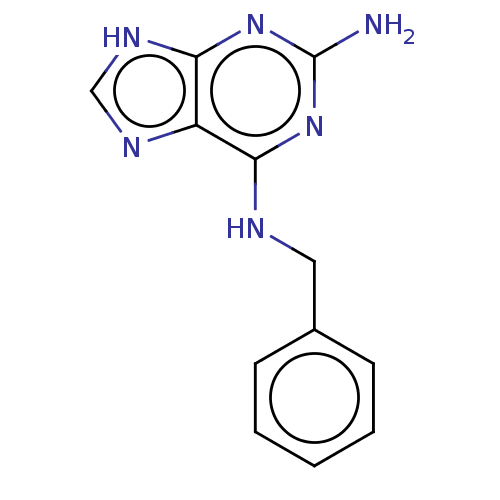 (MTH1 inhibitor, 53) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227632 (MTH1 inhibitor, 53) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227636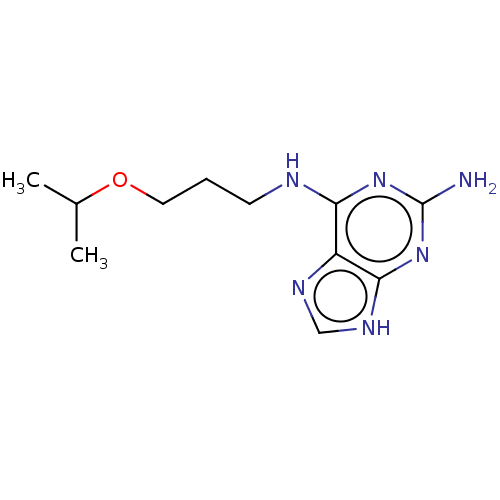 (MTH1 inhibitor, 123) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227636 (MTH1 inhibitor, 123) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227631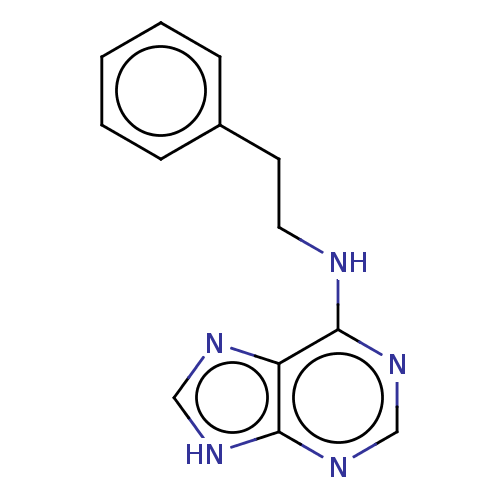 (MTH1 inhibitor, 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227631 (MTH1 inhibitor, 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227635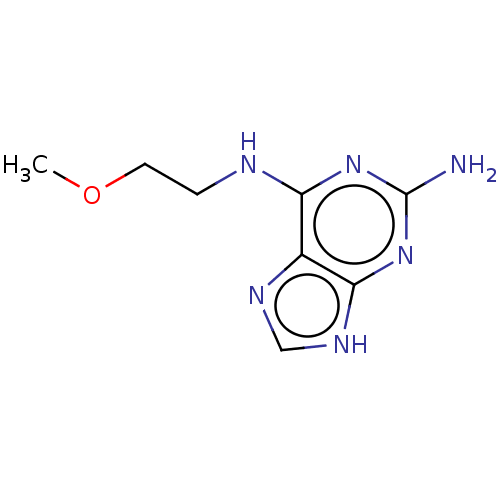 (MTH1 inhibitor, 122) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227635 (MTH1 inhibitor, 122) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227637 (MTH1 inhibitor, 131) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM181147 (MTH1 inhibitor, 88 | US9138393, Kinetin | US914453...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227633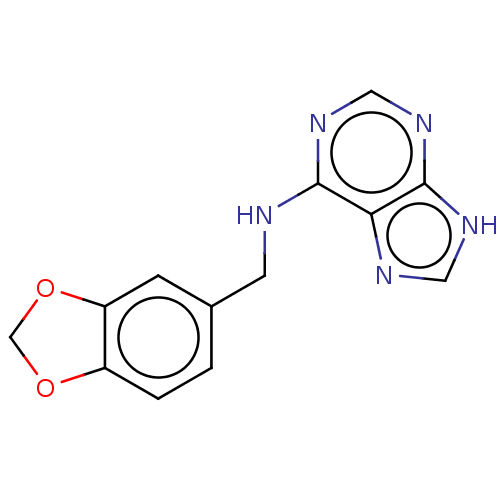 (MTH1 inhibitor, 120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227637 (MTH1 inhibitor, 131) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM227633 (MTH1 inhibitor, 120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM181147 (MTH1 inhibitor, 88 | US9138393, Kinetin | US914453...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
RIKEN Center for Life Science Technologies | Assay Description Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r... | Chem Biol Drug Des 89: 862-869 (2017) Article DOI: 10.1111/cbdd.12909 BindingDB Entry DOI: 10.7270/Q24748RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50514507 (CHEMBL1583158) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Binding affinity to Serratia marcescens chitinase b catalytic domain overexpressed in Escherichia coli BL21(DE3) cells by isothermal titration calori... | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase (Ostrinia furnacalis) | BDBM50514507 (CHEMBL1583158) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Binding affinity to Ostrinia furnacalis chitinase h catalytic domain overexpressed in Pichia pastoris by isothermal titration calorimetry | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50514507 (CHEMBL1583158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a |
Dalian University of Technology Curated by ChEMBL | Assay Description Binding affinity to human CHIT1 catalytic domain overexpressed in Pichia pastoris assessed by isothermal titration calorimetry | J Med Chem 63: 987-1001 (2020) Article DOI: 10.1021/acs.jmedchem.9b01154 BindingDB Entry DOI: 10.7270/Q2N019W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||