 Found 228 hits with Last Name = 'am ende' and Initial = 'c'
Found 228 hits with Last Name = 'am ende' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0510 | -58.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36543
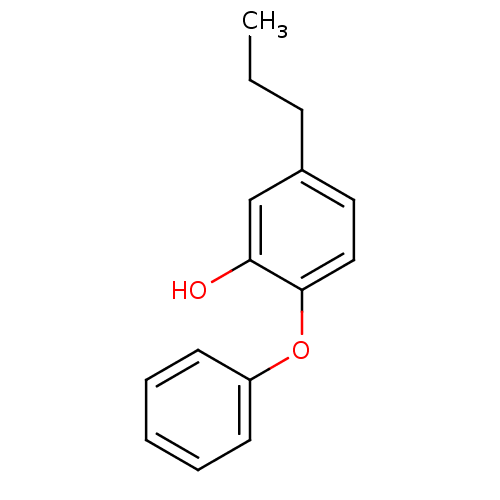
(5-propyl-2-phenoxyphenol | PT02)Show InChI InChI=1S/C15H16O2/c1-2-6-12-9-10-15(14(16)11-12)17-13-7-4-3-5-8-13/h3-5,7-11,16H,2,6H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.440 | -53.4 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50373348
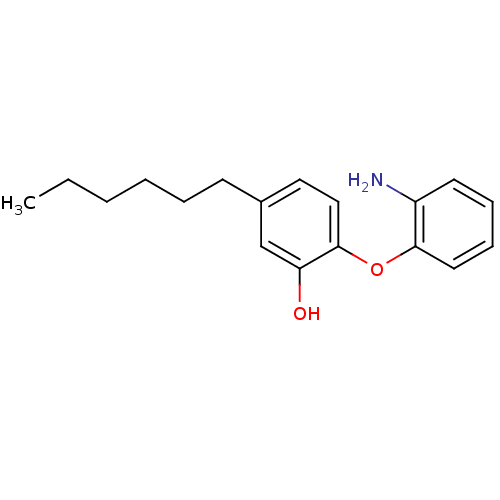
(CHEMBL264434 | PT13)Show InChI InChI=1S/C18H23NO2/c1-2-3-4-5-8-14-11-12-18(16(20)13-14)21-17-10-7-6-9-15(17)19/h6-7,9-13,20H,2-5,8,19H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis H37RV InhA |
Bioorg Med Chem Lett 18: 3029-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.038
BindingDB Entry DOI: 10.7270/Q2SB46KT |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50373347
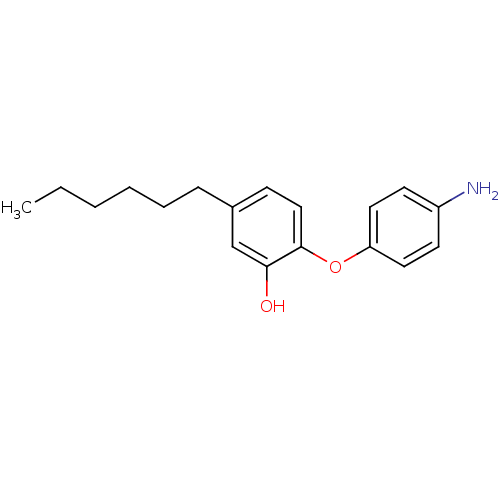
(CHEMBL264175 | PT15)Show InChI InChI=1S/C18H23NO2/c1-2-3-4-5-6-14-7-12-18(17(20)13-14)21-16-10-8-15(19)9-11-16/h7-13,20H,2-6,19H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis H37RV InhA |
Bioorg Med Chem Lett 18: 3029-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.038
BindingDB Entry DOI: 10.7270/Q2SB46KT |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM16296
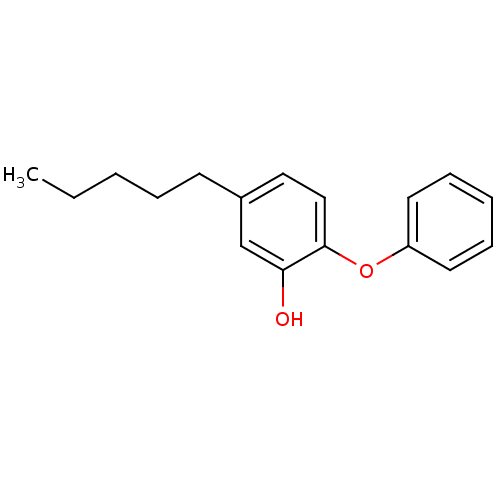
(5-Pentyl-2-phenoxy-phenol | 5-pentyl-2-phenoxylphe...)Show InChI InChI=1S/C17H20O2/c1-2-3-5-8-14-11-12-17(16(18)13-14)19-15-9-6-4-7-10-15/h4,6-7,9-13,18H,2-3,5,8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.30 | -50.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36542
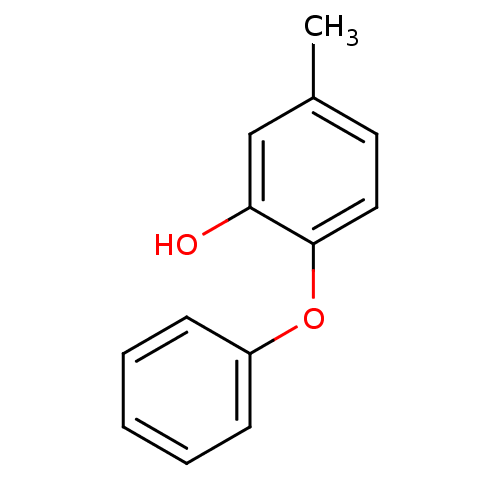
(5-methyl-2-phenoxylphenol | PT53 | US10071965, Com...)Show InChI InChI=1S/C13H12O2/c1-10-7-8-13(12(14)9-10)15-11-5-3-2-4-6-11/h2-9,14H,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | -49.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36539
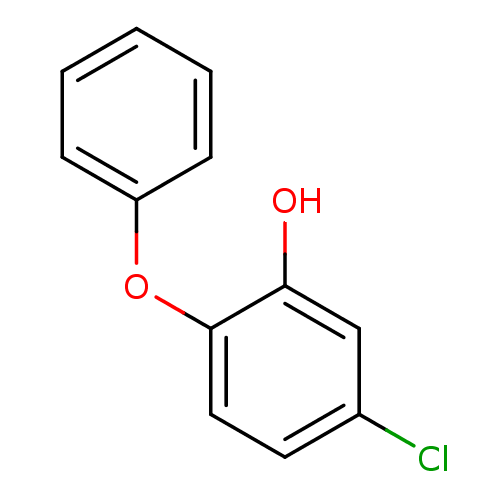
(5-chloro-2-phenoxylphenol | PT52 | US10071965, Com...)Show InChI InChI=1S/C12H9ClO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | -49.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM16294
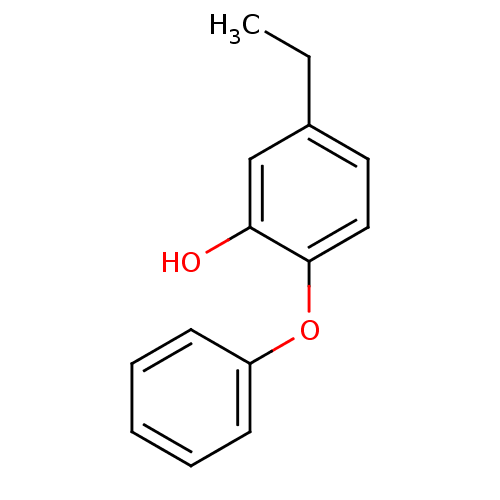
(2PP | 5-Ethyl-2-phenoxy-phenol | 5-butyl-2-phenoxy...)Show InChI InChI=1S/C14H14O2/c1-2-11-8-9-14(13(15)10-11)16-12-6-4-3-5-7-12/h3-10,15H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.10 | -49.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM16297
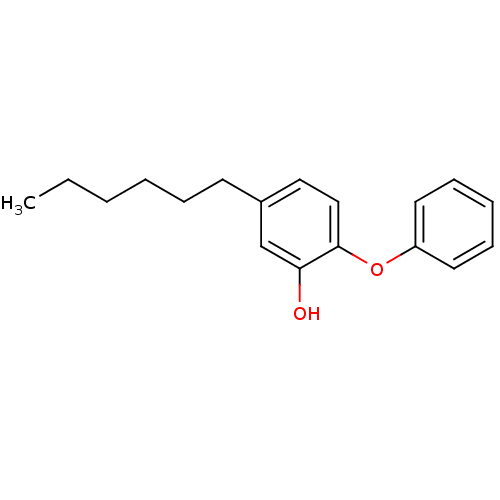
(5-Hexyl-2-phenoxy-phenol | 5-hexyl-2-phenoxylpheno...)Show InChI InChI=1S/C18H22O2/c1-2-3-4-6-9-15-12-13-18(17(19)14-15)20-16-10-7-5-8-11-16/h5,7-8,10-14,19H,2-4,6,9H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.70 | -48.9 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36540
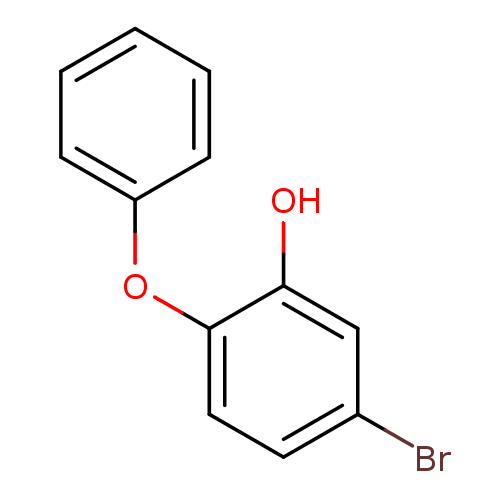
(5-bromo-2-phenoxylphenol | PT103)Show InChI InChI=1S/C12H9BrO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | -46.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM16298
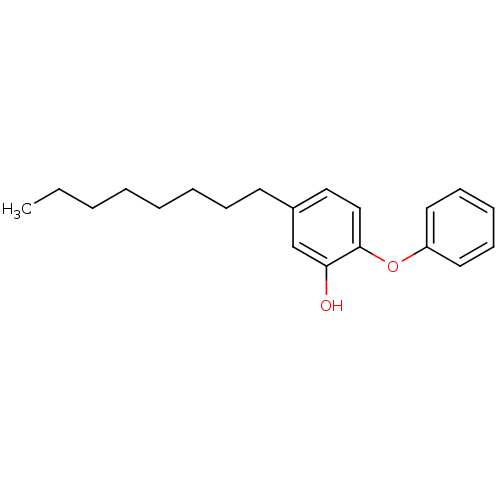
(5-Octyl-2-phenoxy-phenol | 5-heptyl-2-phenoxylphen...)Show InChI InChI=1S/C20H26O2/c1-2-3-4-5-6-8-11-17-14-15-20(19(21)16-17)22-18-12-9-7-10-13-18/h7,9-10,12-16,21H,2-6,8,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 23.6 | -43.5 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36540

(5-bromo-2-phenoxylphenol | PT103)Show InChI InChI=1S/C12H9BrO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 149 | -39.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NADH |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM16298

(5-Octyl-2-phenoxy-phenol | 5-heptyl-2-phenoxylphen...)Show InChI InChI=1S/C20H26O2/c1-2-3-4-5-6-8-11-17-14-15-20(19(21)16-17)22-18-12-9-7-10-13-18/h7,9-10,12-16,21H,2-6,8,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 192 | -38.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NADH |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis H37RV InhA |
Bioorg Med Chem Lett 18: 3029-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.038
BindingDB Entry DOI: 10.7270/Q2SB46KT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36542

(5-methyl-2-phenoxylphenol | PT53 | US10071965, Com...)Show InChI InChI=1S/C13H12O2/c1-10-7-8-13(12(14)9-10)15-11-5-3-2-4-6-11/h2-9,14H,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 247 | -37.7 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NADH |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36538
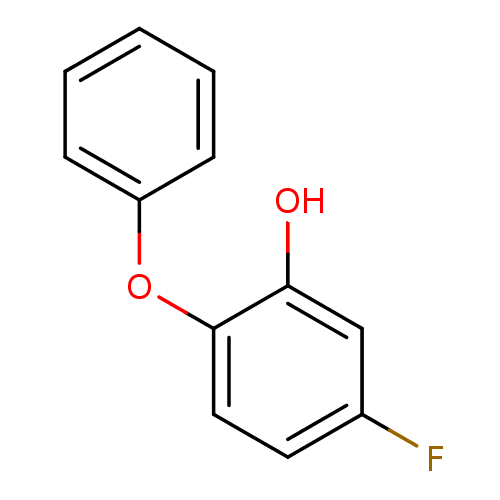
(5-fluoro-2-phenoxylphenol | PT55 | US10071965, Com...)Show InChI InChI=1S/C12H9FO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 289 | -37.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36539

(5-chloro-2-phenoxylphenol | PT52 | US10071965, Com...)Show InChI InChI=1S/C12H9ClO2/c13-9-6-7-12(11(14)8-9)15-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 308 | -37.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NADH |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM16294

(2PP | 5-Ethyl-2-phenoxy-phenol | 5-butyl-2-phenoxy...)Show InChI InChI=1S/C14H14O2/c1-2-11-8-9-14(13(15)10-11)16-12-6-4-3-5-7-12/h3-10,15H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 311 | -37.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NADH |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM16297

(5-Hexyl-2-phenoxy-phenol | 5-hexyl-2-phenoxylpheno...)Show InChI InChI=1S/C18H22O2/c1-2-3-4-6-9-15-12-13-18(17(19)14-15)20-16-10-7-5-8-11-16/h5,7-8,10-14,19H,2-4,6,9H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 331 | -37.0 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NADH |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36543

(5-propyl-2-phenoxyphenol | PT02)Show InChI InChI=1S/C15H16O2/c1-2-6-12-9-10-15(14(16)11-12)17-13-7-4-3-5-8-13/h3-5,7-11,16H,2,6H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 696 | -35.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NADH |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36541
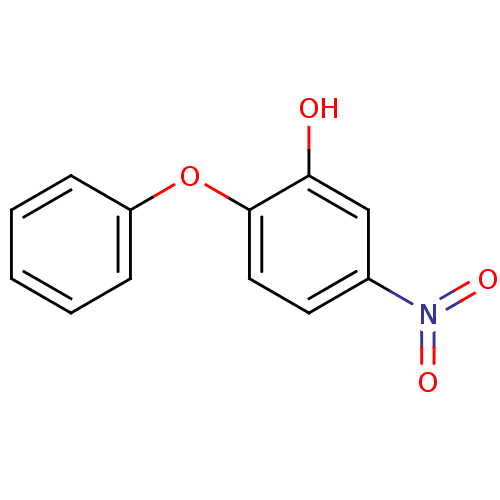
(5-nitro-2-phenoxylphenol | PT104)Show InChI InChI=1S/C12H9NO4/c14-11-8-9(13(15)16)6-7-12(11)17-10-4-2-1-3-5-10/h1-8,14H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 789 | -34.8 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM36537

(2-phenoxylphenol | PT51 | US10071965, Compound PT5...)Show InChI InChI=1S/C12H10O2/c13-11-8-4-5-9-12(11)14-10-6-2-1-3-7-10/h1-9,13H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.26E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NAD+ |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Francisella tularensis) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.00E+3 | -30.3 | n/a | n/a | n/a | n/a | n/a | 8.0 | 25 |
Stony Brook University
| Assay Description
Inhibition constant binding to E-NADH |
ACS Chem Biol 4: 221-31 (2009)
Article DOI: 10.1021/cb800306y
BindingDB Entry DOI: 10.7270/Q2T72FTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50250852
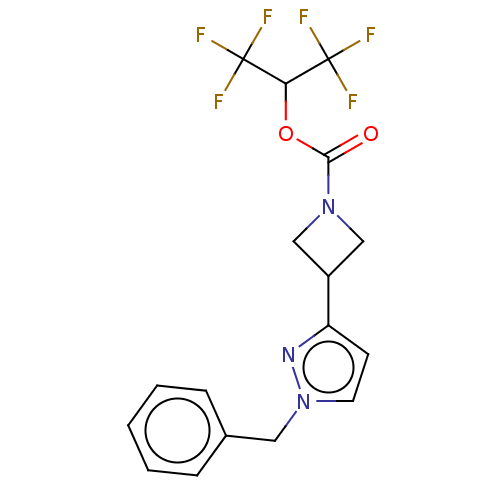
(CHEMBL4078217)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(Cc2ccccc2)n1)C(F)(F)F Show InChI InChI=1S/C17H15F6N3O2/c18-16(19,20)14(17(21,22)23)28-15(27)25-9-12(10-25)13-6-7-26(24-13)8-11-4-2-1-3-5-11/h1-7,12,14H,8-10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250848
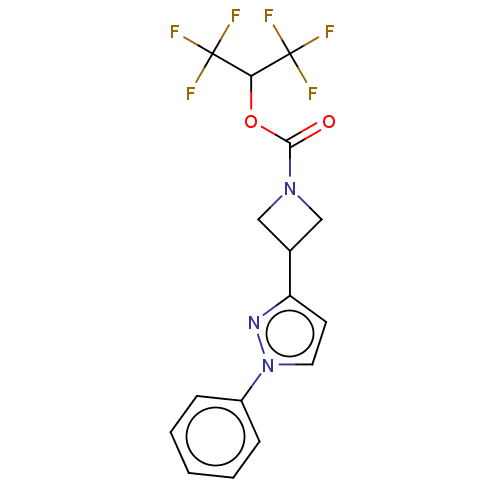
(CHEMBL4059676)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(n1)-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C16H13F6N3O2/c17-15(18,19)13(16(20,21)22)27-14(26)24-8-10(9-24)12-6-7-25(23-12)11-4-2-1-3-5-11/h1-7,10,13H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250859
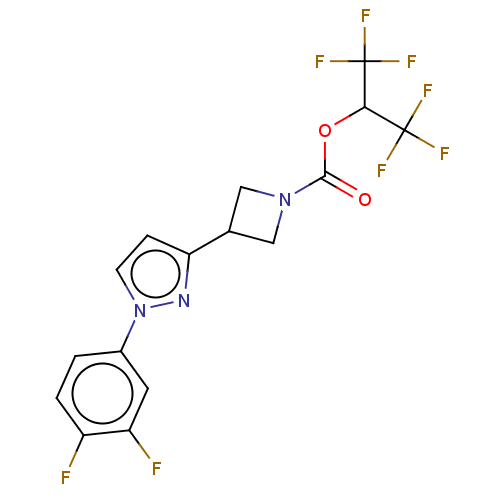
(CHEMBL4097203)Show SMILES Fc1ccc(cc1F)-n1ccc(n1)C1CN(C1)C(=O)OC(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C16H11F8N3O2/c17-10-2-1-9(5-11(10)18)27-4-3-12(25-27)8-6-26(7-8)14(28)29-13(15(19,20)21)16(22,23)24/h1-5,8,13H,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250855
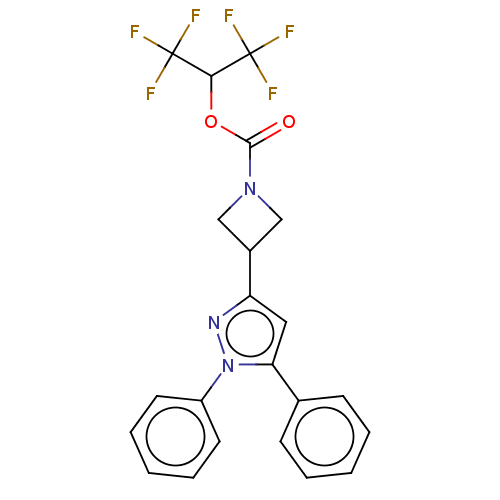
(CHEMBL4077745)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1cc(-c2ccccc2)n(n1)-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C22H17F6N3O2/c23-21(24,25)19(22(26,27)28)33-20(32)30-12-15(13-30)17-11-18(14-7-3-1-4-8-14)31(29-17)16-9-5-2-6-10-16/h1-11,15,19H,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250850
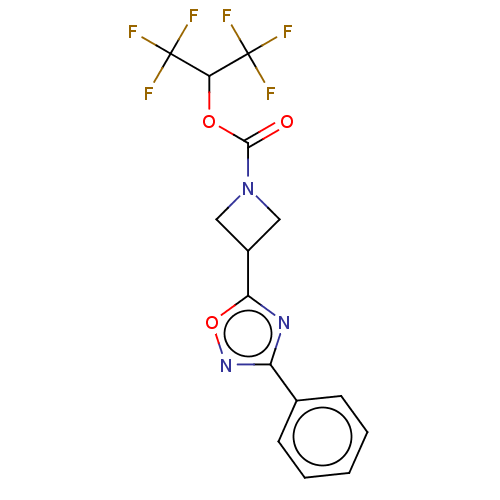
(CHEMBL4089505)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1nc(no1)-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C15H11F6N3O3/c16-14(17,18)12(15(19,20)21)26-13(25)24-6-9(7-24)11-22-10(23-27-11)8-4-2-1-3-5-8/h1-5,9,12H,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250852

(CHEMBL4078217)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(Cc2ccccc2)n1)C(F)(F)F Show InChI InChI=1S/C17H15F6N3O2/c18-16(19,20)14(17(21,22)23)28-15(27)25-9-12(10-25)13-6-7-26(24-13)8-11-4-2-1-3-5-11/h1-7,12,14H,8-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50250855

(CHEMBL4077745)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1cc(-c2ccccc2)n(n1)-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C22H17F6N3O2/c23-21(24,25)19(22(26,27)28)33-20(32)30-12-15(13-30)17-11-18(14-7-3-1-4-8-14)31(29-17)16-9-5-2-6-10-16/h1-11,15,19H,12-13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496623

(CHEMBL3134498)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:38| Show InChI InChI=1S/C42H53N5O6/c1-27(38(49)47-37-40(51)44-33-23-15-14-22-32(33)36(46-37)30-20-12-7-13-21-30)43-39(50)31(24-28-16-8-5-9-17-28)26-35(48)34(25-29-18-10-6-11-19-29)45-41(52)53-42(2,3)4/h5-6,8-11,14-19,22-23,27,30-31,34-35,37,48H,7,12-13,20-21,24-26H2,1-4H3,(H,43,50)(H,44,51)(H,45,52)(H,47,49)/t27-,31+,34-,35+,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using Notch1 substrate assessed as Notch1-NICD production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50250859

(CHEMBL4097203)Show SMILES Fc1ccc(cc1F)-n1ccc(n1)C1CN(C1)C(=O)OC(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C16H11F8N3O2/c17-10-2-1-9(5-11(10)18)27-4-3-12(25-27)8-6-26(7-8)14(28)29-13(15(19,20)21)16(22,23)24/h1-5,8,13H,6-7H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496623

(CHEMBL3134498)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:38| Show InChI InChI=1S/C42H53N5O6/c1-27(38(49)47-37-40(51)44-33-23-15-14-22-32(33)36(46-37)30-20-12-7-13-21-30)43-39(50)31(24-28-16-8-5-9-17-28)26-35(48)34(25-29-18-10-6-11-19-29)45-41(52)53-42(2,3)4/h5-6,8-11,14-19,22-23,27,30-31,34-35,37,48H,7,12-13,20-21,24-26H2,1-4H3,(H,43,50)(H,44,51)(H,45,52)(H,47,49)/t27-,31+,34-,35+,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using APP substrate assessed as amyloid-beta40 production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250854
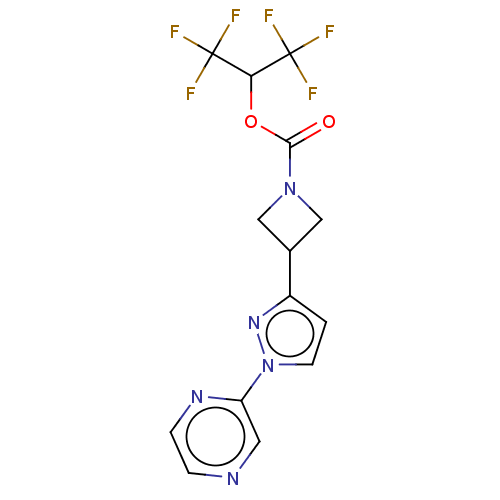
(CHEMBL4079190)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(n1)-c1cnccn1)C(F)(F)F Show InChI InChI=1S/C14H11F6N5O2/c15-13(16,17)11(14(18,19)20)27-12(26)24-6-8(7-24)9-1-4-25(23-9)10-5-21-2-3-22-10/h1-5,8,11H,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496624

(CHEMBL3134497)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(=O)c1ccc(OCC#C)cc1)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:53| Show InChI InChI=1S/C54H55N5O7/c1-3-31-66-43-29-27-40(28-30-43)49(61)39-23-25-41(26-24-39)52(63)57-46(33-37-17-9-5-10-18-37)47(60)34-42(32-36-15-7-4-8-16-36)53(64)55-35(2)51(62)59-50-54(65)56-45-22-14-13-21-44(45)48(58-50)38-19-11-6-12-20-38/h1,4-5,7-10,13-18,21-30,35,38,42,46-47,50,60H,6,11-12,19-20,31-34H2,2H3,(H,55,64)(H,56,65)(H,57,63)(H,59,62)/t35-,42+,46-,47+,50-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using APP substrate assessed as amyloid-beta40 production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50496624

(CHEMBL3134497)Show SMILES C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(=O)c1ccc(OCC#C)cc1)Cc1ccccc1)C(=O)N[C@@H]1N=C(C2CCCCC2)c2ccccc2NC1=O |r,t:53| Show InChI InChI=1S/C54H55N5O7/c1-3-31-66-43-29-27-40(28-30-43)49(61)39-23-25-41(26-24-39)52(63)57-46(33-37-17-9-5-10-18-37)47(60)34-42(32-36-15-7-4-8-16-36)53(64)55-35(2)51(62)59-50-54(65)56-45-22-14-13-21-44(45)48(58-50)38-19-11-6-12-20-38/h1,4-5,7-10,13-18,21-30,35,38,42,46-47,50,60H,6,11-12,19-20,31-34H2,2H3,(H,55,64)(H,56,65)(H,57,63)(H,59,62)/t35-,42+,46-,47+,50-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) using Notch1 substrate assessed as Notch1-NICD production |
Medchemcomm 5: 338-341 (2014)
Article DOI: 10.1039/c3md00281k
BindingDB Entry DOI: 10.7270/Q2ZP493S |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50250854

(CHEMBL4079190)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(n1)-c1cnccn1)C(F)(F)F Show InChI InChI=1S/C14H11F6N5O2/c15-13(16,17)11(14(18,19)20)27-12(26)24-6-8(7-24)9-1-4-25(23-9)10-5-21-2-3-22-10/h1-5,8,11H,6-7H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250853
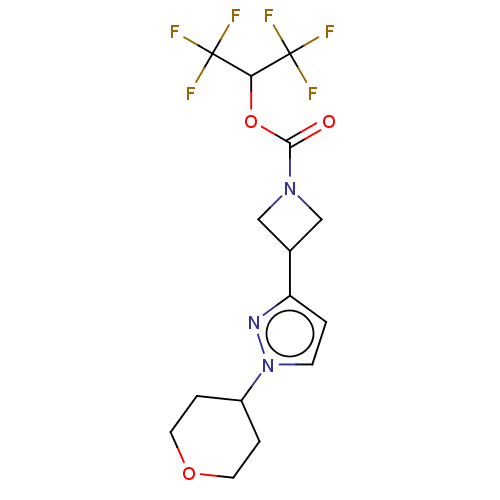
(CHEMBL4102496)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(n1)C1CCOCC1)C(F)(F)F Show InChI InChI=1S/C15H17F6N3O3/c16-14(17,18)12(15(19,20)21)27-13(25)23-7-9(8-23)11-1-4-24(22-11)10-2-5-26-6-3-10/h1,4,9-10,12H,2-3,5-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250849
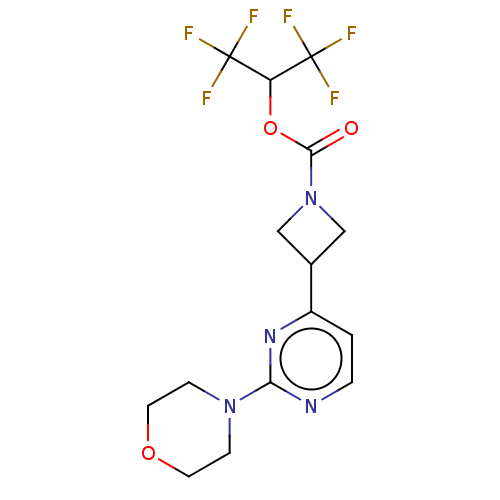
(CHEMBL4068332)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccnc(n1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C15H16F6N4O3/c16-14(17,18)11(15(19,20)21)28-13(26)25-7-9(8-25)10-1-2-22-12(23-10)24-3-5-27-6-4-24/h1-2,9,11H,3-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250851
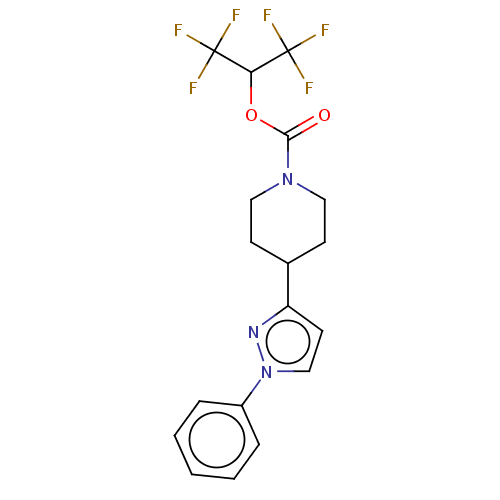
(CHEMBL4096459)Show SMILES FC(F)(F)C(OC(=O)N1CCC(CC1)c1ccn(n1)-c1ccccc1)C(F)(F)F Show InChI InChI=1S/C18H17F6N3O2/c19-17(20,21)15(18(22,23)24)29-16(28)26-9-6-12(7-10-26)14-8-11-27(25-14)13-4-2-1-3-5-13/h1-5,8,11-12,15H,6-7,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250858

(CHEMBL4078417)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)c1ccn(n1)-c1ccc(cc1)C#C)C(F)(F)F Show InChI InChI=1S/C18H13F6N3O2/c1-2-11-3-5-13(6-4-11)27-8-7-14(25-27)12-9-26(10-12)16(28)29-15(17(19,20)21)18(22,23)24/h1,3-8,12,15H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50250857

(CHEMBL4081625)Show SMILES FC(F)(F)C(OC(=O)N1CC(C1)N1CCN(CC1)c1ncccn1)C(F)(F)F Show InChI InChI=1S/C15H17F6N5O2/c16-14(17,18)11(15(19,20)21)28-13(27)26-8-10(9-26)24-4-6-25(7-5-24)12-22-2-1-3-23-12/h1-3,10-11H,4-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method |
J Med Chem 60: 9860-9873 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01531
BindingDB Entry DOI: 10.7270/Q2N300CG |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM193022

(US9193726, 10)Show SMILES C[C@@H]([C@@H]1CC[C@@H](O1)c1cc(F)c(c(F)c1)C(F)(F)F)N1CCn2c(ccc(-n3cnc(C)c3)c2=O)C1=O Show InChI InChI=1S/C25H23F5N4O3/c1-13-11-32(12-31-13)18-3-4-19-24(36)33(7-8-34(19)23(18)35)14(2)20-5-6-21(37-20)15-9-16(26)22(17(27)10-15)25(28,29)30/h3-4,9-12,14,20-21H,5-8H2,1-2H3/t14-,20-,21+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.34 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
The ability of compounds to modulate production of amyloid beta protein
Abeta(1-42) was determined using human WT-APP overexpressing CHO cells.
Cel... |
US Patent US9193726 (2015)
BindingDB Entry DOI: 10.7270/Q22B8WT7 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50484974
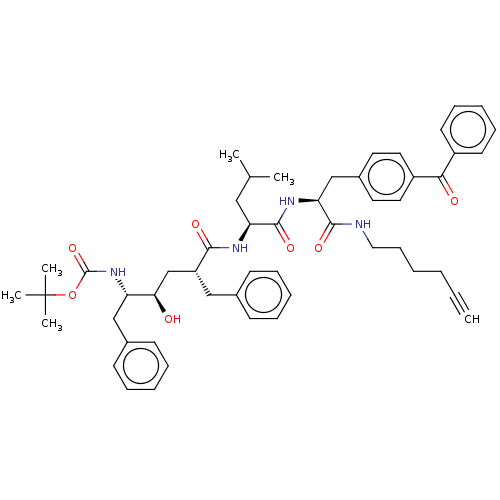
(CHEMBL2017499)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(cc1)C(=O)c1ccccc1)C(=O)NCCCCC#C |r| Show InChI InChI=1S/C52H64N4O7/c1-7-8-9-19-30-53-49(60)45(34-39-26-28-41(29-27-39)47(58)40-24-17-12-18-25-40)55-50(61)44(31-36(2)3)54-48(59)42(32-37-20-13-10-14-21-37)35-46(57)43(33-38-22-15-11-16-23-38)56-51(62)63-52(4,5)6/h1,10-18,20-29,36,42-46,57H,8-9,19,30-35H2,2-6H3,(H,53,60)(H,54,59)(H,55,61)(H,56,62)/t42-,43+,44+,45+,46-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase in cell-free human HeLa membrane assessed as amyloid beta-42 production using APP as substrate |
Bioorg Med Chem Lett 22: 2997-3000 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.027
BindingDB Entry DOI: 10.7270/Q2SB48KN |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B
(Homo sapiens (Human)) | BDBM50272575
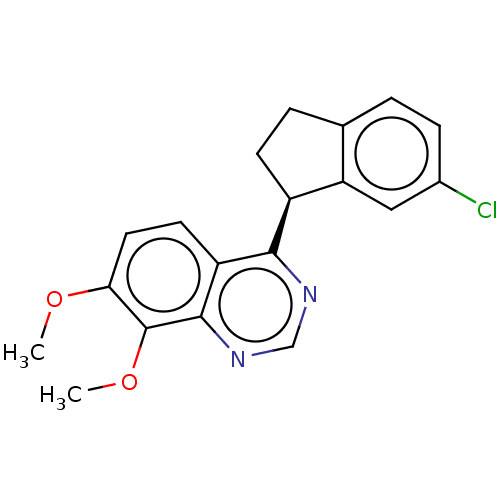
(CHEMBL4126270)Show SMILES COc1ccc2c(ncnc2c1OC)[C@H]1CCc2ccc(Cl)cc12 |r| Show InChI InChI=1S/C19H17ClN2O2/c1-23-16-8-7-14-17(21-10-22-18(14)19(16)24-2)13-6-4-11-3-5-12(20)9-15(11)13/h3,5,7-10,13H,4,6H2,1-2H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human PDE1B1 using 3',5'-[3H]cAMP as substrate after 30 mins by scintillation proximity assay |
J Med Chem 61: 4635-4640 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00374
BindingDB Entry DOI: 10.7270/Q2J67KDQ |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM193067
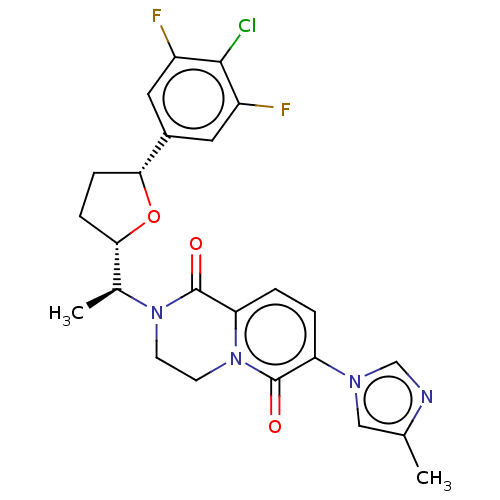
(US9193726, 55)Show SMILES C[C@@H]([C@@H]1CC[C@@H](O1)c1cc(F)c(Cl)c(F)c1)N1CCn2c(ccc(-n3cnc(C)c3)c2=O)C1=O Show InChI InChI=1S/C24H23ClF2N4O3/c1-13-11-29(12-28-13)18-3-4-19-24(33)30(7-8-31(19)23(18)32)14(2)20-5-6-21(34-20)15-9-16(26)22(25)17(27)10-15/h3-4,9-12,14,20-21H,5-8H2,1-2H3/t14-,20-,21+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.48 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
The ability of compounds to modulate production of amyloid beta protein
Abeta(1-42) was determined using human WT-APP overexpressing CHO cells.
Cel... |
US Patent US9193726 (2015)
BindingDB Entry DOI: 10.7270/Q22B8WT7 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM193079
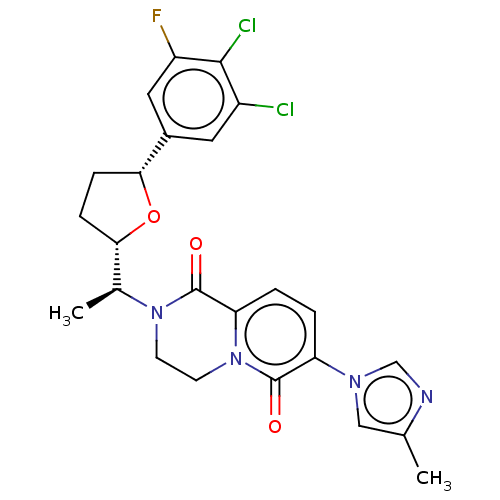
(US9193726, 67)Show SMILES C[C@@H]([C@@H]1CC[C@@H](O1)c1cc(F)c(Cl)c(Cl)c1)N1CCn2c(ccc(-n3cnc(C)c3)c2=O)C1=O Show InChI InChI=1S/C24H23Cl2FN4O3/c1-13-11-29(12-28-13)18-3-4-19-24(33)30(7-8-31(19)23(18)32)14(2)20-5-6-21(34-20)15-9-16(25)22(26)17(27)10-15/h3-4,9-12,14,20-21H,5-8H2,1-2H3/t14-,20-,21+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.78 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
The ability of compounds to modulate production of amyloid beta protein
Abeta(1-42) was determined using human WT-APP overexpressing CHO cells.
Cel... |
US Patent US9193726 (2015)
BindingDB Entry DOI: 10.7270/Q22B8WT7 |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50101747
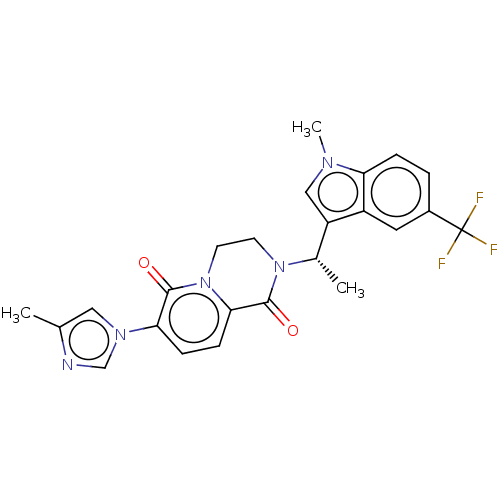
(CHEMBL3394946)Show SMILES C[C@H](N1CCn2c(ccc(-n3cnc(C)c3)c2=O)C1=O)c1cn(C)c2ccc(cc12)C(F)(F)F |r| Show InChI InChI=1S/C24H22F3N5O2/c1-14-11-30(13-28-14)20-6-7-21-23(34)31(8-9-32(21)22(20)33)15(2)18-12-29(3)19-5-4-16(10-17(18)19)24(25,26)27/h4-7,10-13,15H,8-9H2,1-3H3/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase (unknown origin) expressed in CHO cells overexpressing human wild type APP assessed as reduction of amyloid beta 42 lev... |
Bioorg Med Chem Lett 25: 908-13 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.059
BindingDB Entry DOI: 10.7270/Q2QV3P8R |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50096851

(CHEMBL3580774)Show SMILES C[C@@H](COc1ccc(F)cc1[C@@H](C)C(F)(F)F)N1CCn2c(ccc(-n3cnc(C)c3)c2=O)C1=O |r| Show InChI InChI=1S/C24H24F4N4O3/c1-14-11-30(13-29-14)19-5-6-20-23(34)31(8-9-32(20)22(19)33)15(2)12-35-21-7-4-17(25)10-18(21)16(3)24(26,27)28/h4-7,10-11,13,15-16H,8-9,12H2,1-3H3/t15-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Modulation of human gamma secretase overexpressed in CHO cells co-expressing wild type human APP assessed as inhibition of amyloid beta-42 production... |
ACS Med Chem Lett 6: 596-601 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00070
BindingDB Entry DOI: 10.7270/Q26975BS |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM193081
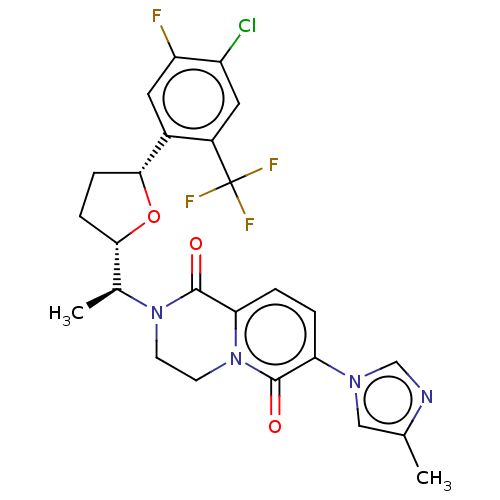
(US9193726, 69)Show SMILES C[C@@H]([C@@H]1CC[C@@H](O1)c1cc(F)c(Cl)cc1C(F)(F)F)N1CCn2c(ccc(-n3cnc(C)c3)c2=O)C1=O Show InChI InChI=1S/C25H23ClF4N4O3/c1-13-11-32(12-31-13)19-3-4-20-24(36)33(7-8-34(20)23(19)35)14(2)21-5-6-22(37-21)15-9-18(27)17(26)10-16(15)25(28,29)30/h3-4,9-12,14,21-22H,5-8H2,1-2H3/t14-,21-,22+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.84 | n/a | n/a | n/a | n/a | n/a | 37 |
Pfizer Inc.
US Patent
| Assay Description
The ability of compounds to modulate production of amyloid beta protein
Abeta(1-42) was determined using human WT-APP overexpressing CHO cells.
Cel... |
US Patent US9193726 (2015)
BindingDB Entry DOI: 10.7270/Q22B8WT7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data