

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Wt: 162.1 BDBM23988 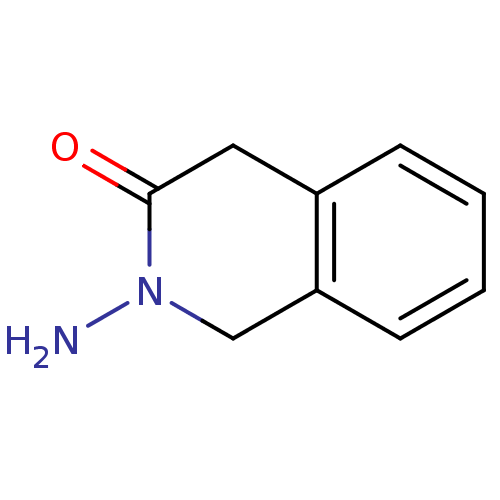 Purchase Purchase | Wt: 146.1 BDBM23989 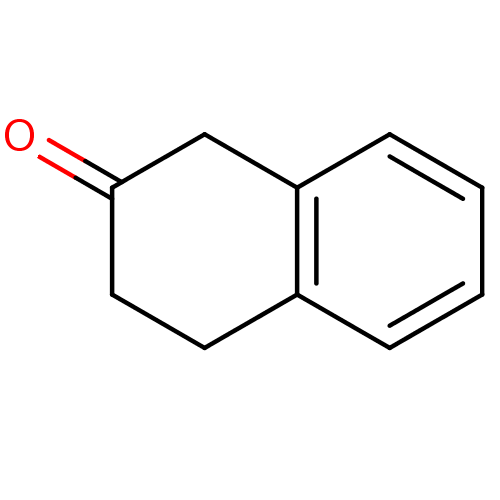 Purchase Purchase | Wt: 162.1 BDBM23990 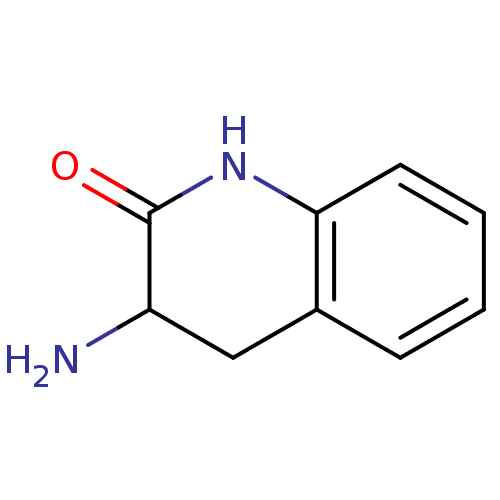 Purchase Purchase | Wt: 265.1 BDBM23715 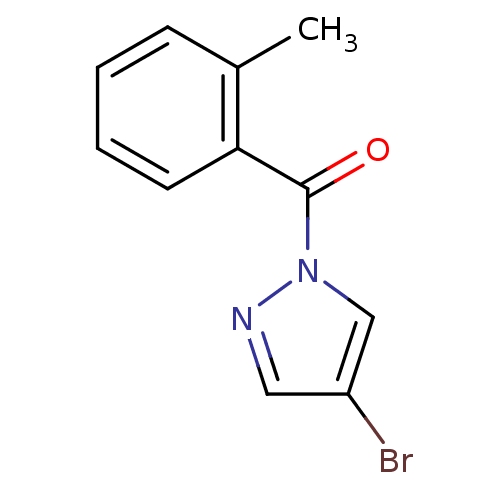 Purchase Purchase | Wt: 243.2 BDBM23716 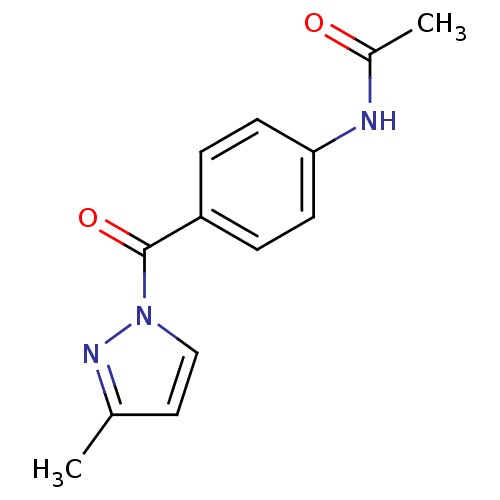 Purchase Purchase |
| Wt: 241.0 BDBM23717  Purchase Purchase | Wt: 186.2 BDBM23718 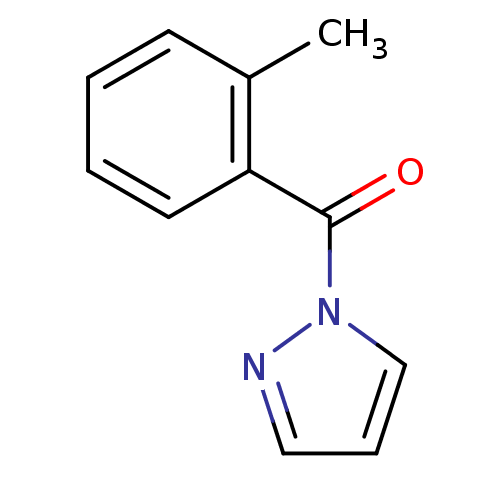 Purchase Purchase | Wt: 308.3 BDBM23971  Purchase Purchase | Wt: 321.5 BDBM23719 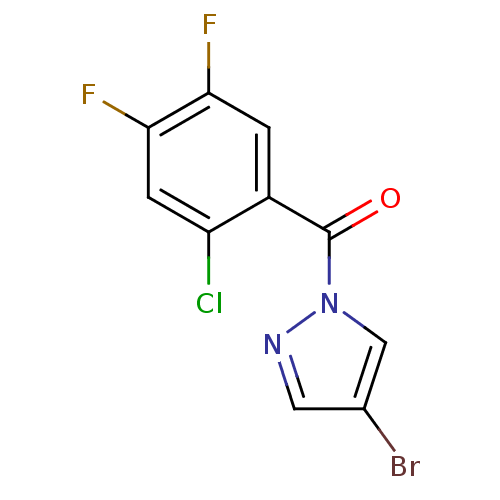 Purchase Purchase | Wt: 262.7 BDBM23720  Purchase Purchase |
| Wt: 252.6 BDBM23721 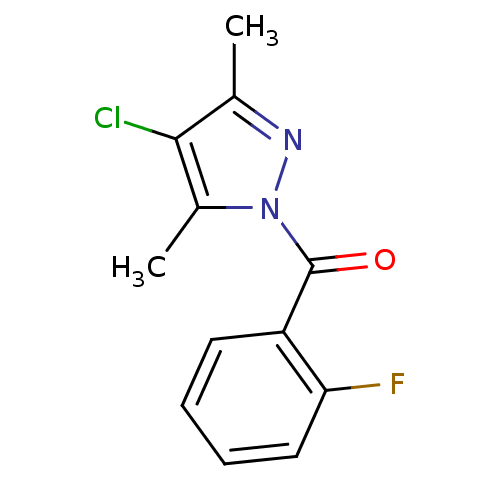 Purchase Purchase | Wt: 269.1 BDBM23722  Purchase Purchase | Wt: 309.1 BDBM23723 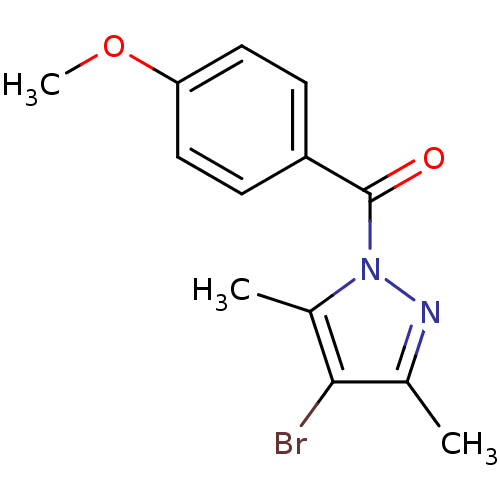 Purchase Purchase | Wt: 251.0 BDBM23724 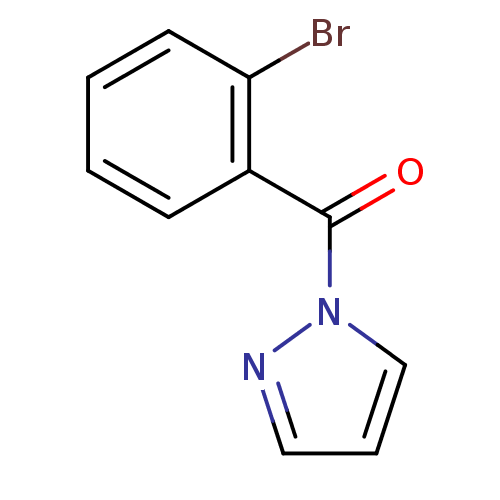 Purchase Purchase | Wt: 304.3 BDBM23725  Purchase Purchase |
| << First | Previous | Displayed 16 to 30 (of 77 total ) | Next | Last >> |
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace Curated by ChEMBL | Assay Description Competitive inhibition of pig APN using L-leucine-p-nitroanilide as substrate by Dixon-plot analysis | Bioorg Med Chem 20: 4942-53 (2012) Article DOI: 10.1016/j.bmc.2012.06.041 BindingDB Entry DOI: 10.7270/Q2RB75Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity for cytosolic leucine aminopeptidase (LAP) from porcine kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace Curated by ChEMBL | Assay Description Inhibition of Aeromonas proteolytica leucyl aminopeptidase using L-leucine para-notroanilide as substrate assessed as release of para-nitroaniline by... | Bioorg Med Chem 21: 6447-55 (2013) Article DOI: 10.1016/j.bmc.2013.08.044 BindingDB Entry DOI: 10.7270/Q2PR7XF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibitory activity against Leucine aminopeptidase | J Med Chem 31: 2193-9 (1988) BindingDB Entry DOI: 10.7270/Q2Z038RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of leucine aminopeptidase | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Rattus norvegicus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibitory activity against aminopeptidase B | J Med Chem 31: 2193-9 (1988) BindingDB Entry DOI: 10.7270/Q2Z038RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M1 family aminopeptidase (Plasmodium falciparum (isolate FcB1 / Columbia)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant M1-aminopeptidase expressed in Escherichia coli after 40 mins uisng fluorigenic substrate L-Leucyl-7-... | J Med Chem 54: 1655-66 (2011) Article DOI: 10.1021/jm101227t BindingDB Entry DOI: 10.7270/Q23X86ZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23715 (4-bromo-1-[(2-methylphenyl)carbonyl]-1H-pyrazole |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 300 | -8.89 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23716 (N-Benzoylpyrazole deriv., 24 | N-{4-[(3-methyl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 300 | -8.89 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt Curated by ChEMBL | Assay Description Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... | Bioorg Med Chem 24: 5243-5248 (2016) Article DOI: 10.1016/j.bmc.2016.08.047 BindingDB Entry DOI: 10.7270/Q2959KH9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytosol aminopeptidase (Bos taurus (bovine)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace Curated by ChEMBL | Assay Description Competitive inhibition of bovine LAPc using L-leucine-p-nitroanilide as substrate by spectrophotometric analysis | Bioorg Med Chem 20: 4942-53 (2012) Article DOI: 10.1016/j.bmc.2012.06.041 BindingDB Entry DOI: 10.7270/Q2RB75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23717 (4-chloro-1-[(2-chlorophenyl)carbonyl]-1H-pyrazole ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.00E+3 | -8.18 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de La Habana Curated by ChEMBL | Assay Description Inhibition of human cytomegalovirus DNA polymerase (95 uL) activity in a solution containing 6.4 mM HEPES (pH 7.5), incubation for 12 minutes at 26 d... | Bioorg Med Chem 25: 4628-4636 (2017) Article DOI: 10.1016/j.bmc.2017.06.047 BindingDB Entry DOI: 10.7270/Q2MS3W75 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Inhibition of soluble human APN ectodomain stably expressed in HEK293 GnTI(-) cells using H-Leu-NHMec as substrate preincubated for 10 mins followed ... | J Med Chem 62: 7185-7209 (2019) Article DOI: 10.1021/acs.jmedchem.9b00757 BindingDB Entry DOI: 10.7270/Q2KK9G8J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase N (Mus musculus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of mouse APN | ACS Med Chem Lett 3: 959-964 (2012) Article DOI: 10.1021/ml3000758 BindingDB Entry DOI: 10.7270/Q2QJ7JFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23718 (1-[(2-methylphenyl)carbonyl]-1H-pyrazole | N-Benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | -7.46 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Haute Alsace Curated by ChEMBL | Assay Description Inhibition of porcine kidney APN using L-leucine para-notroanilide as substrate assessed as release of para-nitroaniline by Dixon plot analysis | Bioorg Med Chem 21: 6447-55 (2013) Article DOI: 10.1016/j.bmc.2013.08.044 BindingDB Entry DOI: 10.7270/Q2PR7XF2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of porcine kidney microsome aminopeptidase using L-Leu-p-nitroanilide as substrate by Dixon method | Bioorg Med Chem Lett 22: 5863-9 (2012) Article DOI: 10.1016/j.bmcl.2012.07.086 BindingDB Entry DOI: 10.7270/Q2VD70KS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leucyl-cystinyl aminopeptidase (Rattus norvegicus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of aminopeptidase M or membrane leucine aminopeptidase | J Med Chem 32: 1378-92 (1989) BindingDB Entry DOI: 10.7270/Q20G3KQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23719 (4-bromo-1-[(2-chloro-4,5-difluorophenyl)carbonyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 7.20E+3 | -7.01 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin Curated by ChEMBL | Assay Description Inhibitory activity against Aminopeptidase M | J Med Chem 31: 2193-9 (1988) BindingDB Entry DOI: 10.7270/Q2Z038RM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23720 (1-[(4-tert-butylphenyl)carbonyl]-4-chloro-1H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | -6.88 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23721 (4-chloro-1-[(2-fluorophenyl)carbonyl]-3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | -6.88 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23722 (4-chloro-1-[(4-chlorophenyl)carbonyl]-3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.07E+4 | -6.78 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 2 (Rattus norvegicus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Hospital Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Gly-Sar uptake in PEPT2-expressing LLC-PK1 cells | Pflugers Arch 440: 679-84 (2000) Article DOI: 10.1007/s004240000339 BindingDB Entry DOI: 10.7270/Q2CR5X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23723 (4-bromo-1-[(4-methoxyphenyl)carbonyl]-3,5-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.45E+4 | -6.29 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23724 (1-[(2-bromophenyl)carbonyl]-1H-pyrazole | N-Benzoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.99E+4 | -6.17 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM23725 (3,4,5-trimethyl-1-[(3,4,5-trimethoxyphenyl)carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.09E+4 | -5.85 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Montana State University | Assay Description HTS was performed in black flat-bottom 96-well microtiter plates. The reaction was initiated by addition of elastase substrate to the reaction buffer... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bacterial leucyl aminopeptidase (Vibrio proteolyticus) | BDBM23990 (3-amino-1,2,3,4-tetrahydroquinolin-2-one hydrochlo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu | Assay Description Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... | Bioorg Med Chem 14: 7241-57 (2006) Article DOI: 10.1016/j.bmc.2006.06.050 BindingDB Entry DOI: 10.7270/Q2N29V73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition constant (Ki) for human intestinal peptide carrier | J Med Chem 46: 5725-34 (2003) Article DOI: 10.1021/jm030976x BindingDB Entry DOI: 10.7270/Q22N531C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Rattus norvegicus) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Hospital Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Gly-Sar uptake in PEPT1-expressing LLC-PK1 cells | Pflugers Arch 440: 679-84 (2000) Article DOI: 10.1007/s004240000339 BindingDB Entry DOI: 10.7270/Q2CR5X6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of aminopeptidase N (unknown origin) | Bioorg Med Chem Lett 23: 4948-52 (2013) Article DOI: 10.1016/j.bmcl.2013.06.058 BindingDB Entry DOI: 10.7270/Q2377B4J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of pig microsomal aminopeptidase N using L-leu-p-nitroanilide as substrate incubated for 5 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 4948-52 (2013) Article DOI: 10.1016/j.bmcl.2013.06.058 BindingDB Entry DOI: 10.7270/Q2377B4J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23715 (4-bromo-1-[(2-methylphenyl)carbonyl]-1H-pyrazole |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23716 (N-Benzoylpyrazole deriv., 24 | N-{4-[(3-methyl-1H-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23717 (4-chloro-1-[(2-chlorophenyl)carbonyl]-1H-pyrazole ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23718 (1-[(2-methylphenyl)carbonyl]-1H-pyrazole | N-Benzo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23719 (4-bromo-1-[(2-chloro-4,5-difluorophenyl)carbonyl]-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23720 (1-[(4-tert-butylphenyl)carbonyl]-4-chloro-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23721 (4-chloro-1-[(2-fluorophenyl)carbonyl]-3,5-dimethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23722 (4-chloro-1-[(4-chlorophenyl)carbonyl]-3,5-dimethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23723 (4-bromo-1-[(4-methoxyphenyl)carbonyl]-3,5-dimethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23724 (1-[(2-bromophenyl)carbonyl]-1H-pyrazole | N-Benzoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM23725 (3,4,5-trimethyl-1-[(3,4,5-trimethoxyphenyl)carbony...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Chymotrypsin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentrati... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM23715 (4-bromo-1-[(2-methylphenyl)carbonyl]-1H-pyrazole |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM23716 (N-Benzoylpyrazole deriv., 24 | N-{4-[(3-methyl-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM23717 (4-chloro-1-[(2-chlorophenyl)carbonyl]-1H-pyrazole ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM23718 (1-[(2-methylphenyl)carbonyl]-1H-pyrazole | N-Benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM23719 (4-bromo-1-[(2-chloro-4,5-difluorophenyl)carbonyl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Montana State University | Assay Description Thrombin activity was monitored at excitation and emission wavelengths of 355 and 460 nm, respectively. For all compounds tested, the concentration o... | J Med Chem 50: 4928-38 (2007) Article DOI: 10.1021/jm070600+ BindingDB Entry DOI: 10.7270/Q2ST7N5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 242 total ) | Next | Last >> |