 Found 850 hits for monomerid = 7966,22880,22881,22883,22566,22891,22893,22542,26739,27213,27193,26226,31005,35229,77970
Found 850 hits for monomerid = 7966,22880,22881,22883,22566,22891,22893,22542,26739,27213,27193,26226,31005,35229,77970 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM77970
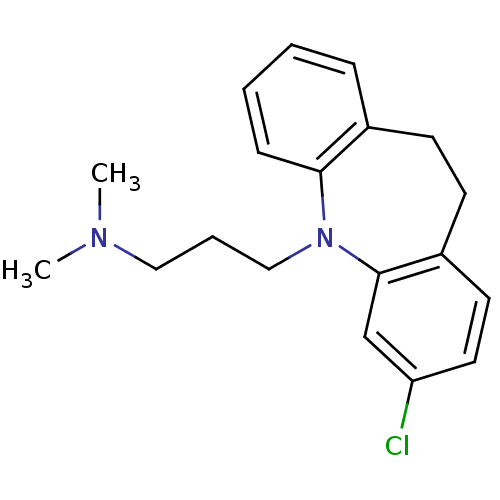
(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM35229
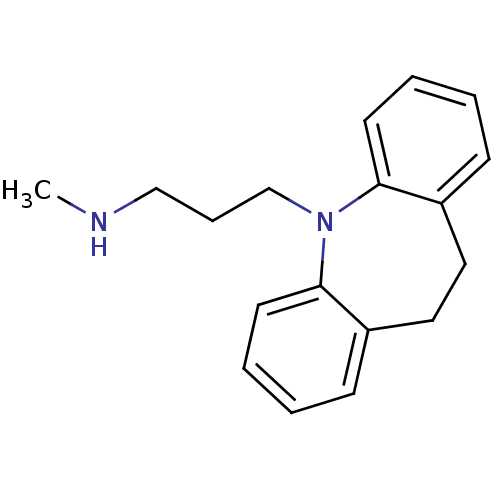
(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 301: 1097-102 (2002)
Article DOI: 10.1124/jpet.301.3.1097
BindingDB Entry DOI: 10.7270/Q2ZW1JH0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM77970

(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Turku
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 126: 234-40 (1996)
Article DOI: 10.1007/bf02246453
BindingDB Entry DOI: 10.7270/Q2BP0192 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM77970

(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boots Pharmaceuticals
Curated by PDSP Ki Database
| |
Neuropharmacology 32: 737-43 (1993)
Article DOI: 10.1016/0028-3908(93)90181-2
BindingDB Entry DOI: 10.7270/Q22J69D4 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM77970

(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 565-80 (2001)
BindingDB Entry DOI: 10.7270/Q2QJ7FVW |
More data for this
Ligand-Target Pair | |
Histamine receptor H4
(GUINEA PIG) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
Mol Pharmacol 59: 434-41 (2001)
Article DOI: 10.1124/mol.59.3.434
BindingDB Entry DOI: 10.7270/Q2Z899ZR |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Laboratories, Inc.
Curated by PDSP Ki Database
| |
Biochem Pharmacol 35: 4493-7 (1986)
Article DOI: 10.1016/0006-2952(86)90769-0
BindingDB Entry DOI: 10.7270/Q22J69CP |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM77970

(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Eur J Pharmacol 340: 249-58 (1997)
Article DOI: 10.1016/s0014-2999(97)01393-9
BindingDB Entry DOI: 10.7270/Q2V69H3D |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM77970

(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mt. Sinai
Curated by ChEMBL
| Assay Description
Displacement of [3H]-imipramine from human serotonin transporter expressed in HEK293 cells after 30 mins by liquid scintillation counting analysis |
Bioorg Med Chem 23: 6528-34 (2015)
Article DOI: 10.1016/j.bmc.2015.07.007
BindingDB Entry DOI: 10.7270/Q22J6DNH |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542
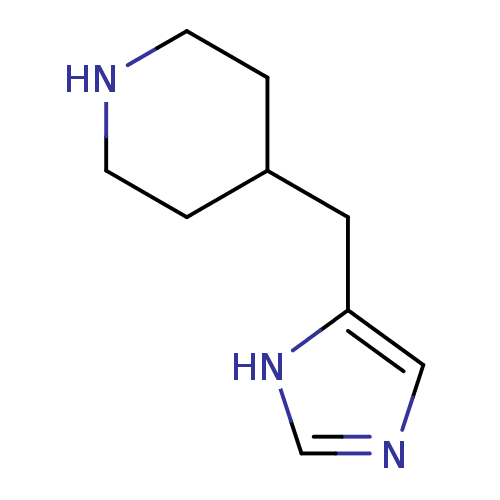
(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)-alpha-methylhistamine from human histamine H3 receptor expressed in CHO cells by liquid scintillation counting |
J Med Chem 53: 6445-56 (2010)
Article DOI: 10.1021/jm100643t
BindingDB Entry DOI: 10.7270/Q2QF8T3X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Seika Kaisha, Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H] (R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation... |
Bioorg Med Chem 18: 5441-8 (2010)
Article DOI: 10.1016/j.bmc.2010.04.052
BindingDB Entry DOI: 10.7270/Q2C82B88 |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
| |
Mol Pharmacol 59: 420-6 (2001)
Article DOI: 10.1124/mol.59.3.420
BindingDB Entry DOI: 10.7270/Q26Q1VSR |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in guinea pig brain |
Bioorg Med Chem Lett 16: 395-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.076
BindingDB Entry DOI: 10.7270/Q29S1QK1 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.407 | -12.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
VU University Amsterdam
| Assay Description
Ligand displacement assays were performed on CHO cells membranes expressing hH3R. Retained radioactivity was determined by liquid scintillation count... |
J Med Chem 51: 2944-53 (2008)
Article DOI: 10.1021/jm7014149
BindingDB Entry DOI: 10.7270/Q24F1P2W |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM77970

(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin
Curated by ChEMBL
| Assay Description
In vitro binding affinity against rat histamine H3 receptor |
J Med Chem 47: 2678-87 (2004)
Article DOI: 10.1021/jm031065q
BindingDB Entry DOI: 10.7270/Q2SQ8ZTC |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Invitro competitive binding versus [N-methyl-3H]-WIN 35428 in murine kidney cells transfected with cDNA for human dopamine transporter (DAT) |
J Med Chem 46: 925-35 (2003)
Article DOI: 10.1021/jm0100180
BindingDB Entry DOI: 10.7270/Q2W66K4V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin
Curated by ChEMBL
| Assay Description
Histamine H3 receptor antagonist activity in an assay with K+ evoked depolarization-induced release of [3H]-histamine from rat synaptosomes. |
J Med Chem 43: 3335-43 (2000)
BindingDB Entry DOI: 10.7270/Q2RR1XGV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universit£t Berlin
Curated by ChEMBL
| Assay Description
Effect on histamine H3 receptors in vitro, on synaptosomes of rat cerebral cortex for the release of [3H]histamine |
J Med Chem 43: 3987-94 (2000)
BindingDB Entry DOI: 10.7270/Q2833SRN |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universität Berlin
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Histamine H3 receptor for K+ evoked depolarization-induced release of [3H]-histamine from synaptosomes of rat ce... |
Bioorg Med Chem Lett 10: 2379-82 (2001)
BindingDB Entry DOI: 10.7270/Q28G8JZ4 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 293: 771-8 (2000)
BindingDB Entry DOI: 10.7270/Q2348HX8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
| Assay Description
Ligand displacement assays were performed on The SK-N-MC/hH3R cell homogenates. Retained radioactivity was determined by liquid scintillation countin... |
J Pharmacol Exp Ther 314: 1310-21 (2005)
Article DOI: 10.1124/jpet.105.087965
BindingDB Entry DOI: 10.7270/Q2KD1W6V |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human H3 receptor expressed in HEK293 cells after 1 to 1.5 hrs by scintillation counting |
J Med Chem 54: 8136-47 (2011)
Article DOI: 10.1021/jm201042n
BindingDB Entry DOI: 10.7270/Q2MW2JDX |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing rat histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 349: 129-32 (1998)
Article DOI: 10.1016/s0014-2999(98)00241-6
BindingDB Entry DOI: 10.7270/Q279436B |
More data for this
Ligand-Target Pair | |
Norepinephrine transporter
(RAT) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.551 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by PDSP Ki Database
| |
Synapse 32: 198-211 (1999)
Article DOI: 10.1002/(SICI)1098-2396(19990601)32:3
BindingDB Entry DOI: 10.7270/Q2JH3JQX |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.600 | -13.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research
| Assay Description
Compounds were evaluated the inhibition of [3H] nisoxetine binding to MDCK-Net6 cells, stably transfected with the human norepinephrine transporter (... |
Bioorg Med Chem 17: 7802-15 (2009)
Article DOI: 10.1016/j.bmc.2009.09.023
BindingDB Entry DOI: 10.7270/Q26Q1VK2 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 1305-22 (1997)
BindingDB Entry DOI: 10.7270/Q25Q4TMX |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of norepinephrine (NE) into rat brain synaptosomes |
J Med Chem 27: 943-6 (1984)
BindingDB Entry DOI: 10.7270/Q2VT1SPH |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of uptake of tritiated norepinephrine (NE) in rat synaptosomes |
J Med Chem 30: 1433-54 (1987)
BindingDB Entry DOI: 10.7270/Q2D50NJ3 |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.654 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from rat NET in rat cerebral cortex |
J Med Chem 53: 7869-73 (2010)
Article DOI: 10.1021/jm100666w
BindingDB Entry DOI: 10.7270/Q2P84C4F |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from human NET |
J Med Chem 53: 5549-57 (2010)
Article DOI: 10.1021/jm100269c
BindingDB Entry DOI: 10.7270/Q2KK9CRK |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic Jacksonville
Curated by PDSP Ki Database
| |
Eur J Pharmacol 340: 249-58 (1997)
Article DOI: 10.1016/s0014-2999(97)01393-9
BindingDB Entry DOI: 10.7270/Q2V69H3D |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity at NET |
Bioorg Med Chem 15: 616-25 (2006)
Article DOI: 10.1016/j.bmc.2006.10.065
BindingDB Entry DOI: 10.7270/Q22B8XPV |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM31005
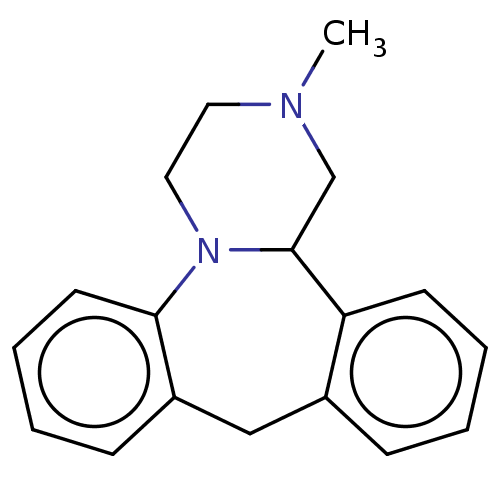
(2-methyl-1,2,3,4,10,14b-hexahydrodibenzo[c,f]pyraz...)Show InChI InChI=1S/C18H20N2.ClH/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19;/h2-9,18H,10-13H2,1H3;1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 2 receptor at 1.0 uM concentration |
J Med Chem 30: 1433-54 (1987)
BindingDB Entry DOI: 10.7270/Q2D50NJ3 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Mus musculus (mouse)) | BDBM26739
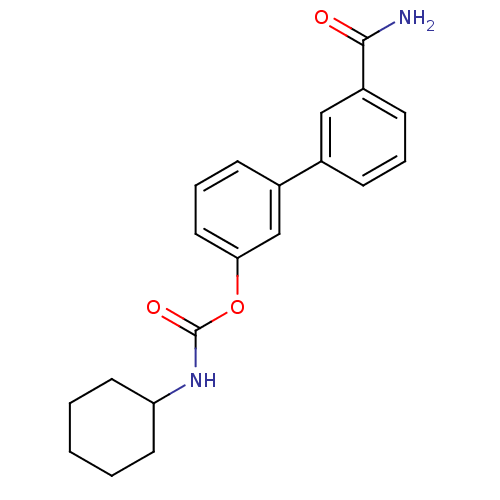
(3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...)Show InChI InChI=1S/C20H22N2O3/c21-19(23)16-8-4-6-14(12-16)15-7-5-11-18(13-15)25-20(24)22-17-9-2-1-3-10-17/h4-8,11-13,17H,1-3,9-10H2,(H2,21,23)(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... |
J Med Chem 55: 6898-915 (2012)
Article DOI: 10.1021/jm300689c
BindingDB Entry DOI: 10.7270/Q2ZK5HSK |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM77970

(3-(2-chloranyl-5,6-dihydrobenzo[b][1]benzazepin-11...)Show InChI InChI=1S/C19H23ClN2/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 298: 565-80 (2001)
BindingDB Entry DOI: 10.7270/Q2QJ7FVW |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 293: 771-8 (2000)
BindingDB Entry DOI: 10.7270/Q2348HX8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM31005

(2-methyl-1,2,3,4,10,14b-hexahydrodibenzo[c,f]pyraz...)Show InChI InChI=1S/C18H20N2.ClH/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19;/h2-9,18H,10-13H2,1H3;1H | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Center for Pharmacy
Curated by ChEMBL
| Assay Description
The binding affinity of the compound at 5-hydroxytryptamine 2C receptor was determined using [3H]-mesulergine |
J Med Chem 45: 3280-5 (2002)
BindingDB Entry DOI: 10.7270/Q28051ZZ |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM31005

(2-methyl-1,2,3,4,10,14b-hexahydrodibenzo[c,f]pyraz...)Show InChI InChI=1S/C18H20N2.ClH/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19;/h2-9,18H,10-13H2,1H3;1H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to rat cortical membranes at 5-hydroxytryptamine 2 (5-HT2) receptor using [3H]KET as a radioligand |
J Med Chem 30: 1-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K4BS7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM31005

(2-methyl-1,2,3,4,10,14b-hexahydrodibenzo[c,f]pyraz...)Show InChI InChI=1S/C18H20N2.ClH/c1-19-10-11-20-17-9-5-3-7-15(17)12-14-6-2-4-8-16(14)18(20)13-19;/h2-9,18H,10-13H2,1H3;1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Center for Pharmacy
Curated by ChEMBL
| Assay Description
The binding affinity of the compound at 5-hydroxytryptamine 2A receptor was determined using [3H]-ketanserin |
J Med Chem 45: 3280-5 (2002)
BindingDB Entry DOI: 10.7270/Q28051ZZ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22542

(4-(1H-imidazol-4-ylmethyl)piperidine | 4-(1H-imida...)Show InChI InChI=1S/C9H15N3/c1-3-10-4-2-8(1)5-9-6-11-7-12-9/h6-8,10H,1-5H2,(H,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 293: 771-8 (2000)
BindingDB Entry DOI: 10.7270/Q2348HX8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase