

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50049820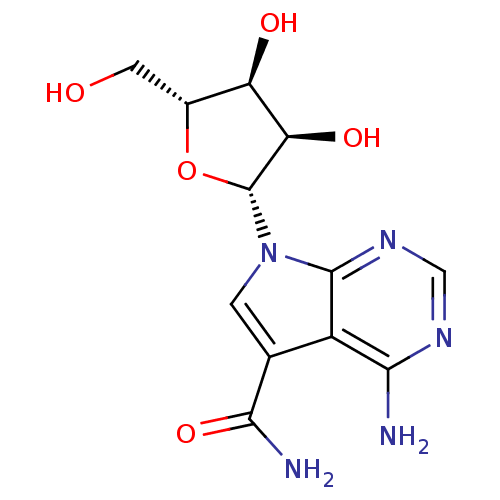 (4-Amino-5-carboxamide-7-(D-ribofuranosyl)pyrrolo[2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center Curated by ChEMBL | Assay Description Inhibition of rhodopsin kinase (unknown origin) | J Biol Chem 282: 15271-83 (2007) Article DOI: 10.1074/jbc.M701362200 BindingDB Entry DOI: 10.7270/Q2XD11FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50566949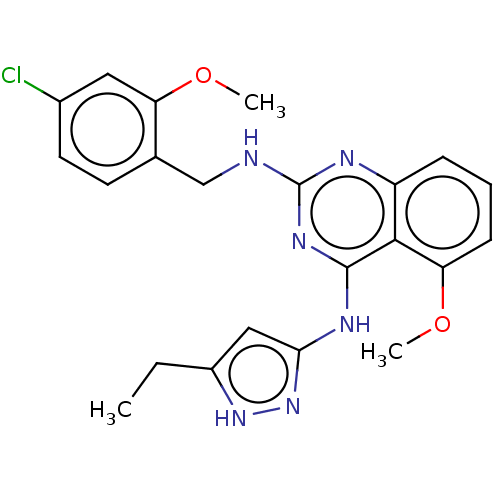 (CHEMBL4877302) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GRK1 using casein as substrate in presence of [gamma33P]ATP by radiometric hotspot kinase assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00506 BindingDB Entry DOI: 10.7270/Q20K2D97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 57.8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human GRK1 using casein as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173307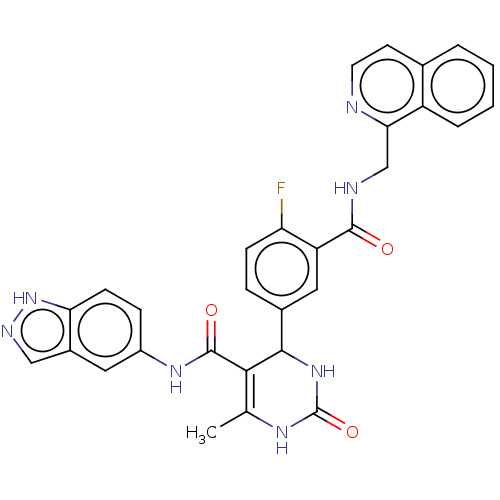 (CHEMBL3809796 | US10023564, Example 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 78.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GRK1 using casein as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173307 (CHEMBL3809796 | US10023564, Example 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50265871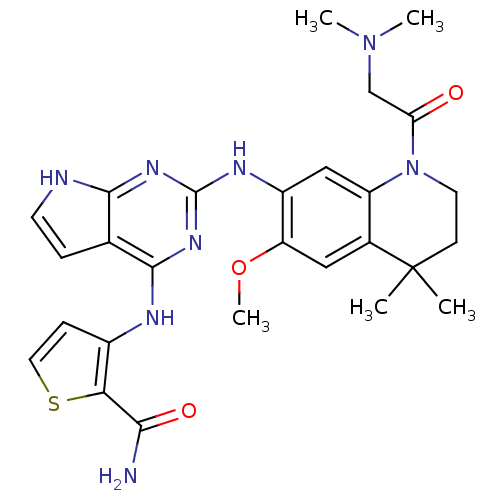 (3-(2-(1-(2-(dimethylamino)acetyl)-6-methoxy-4,4-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of BODIPY TR-ADP from bovine GRK1 (1 to 535 residues) preincubated for 10 mins followed by compound addition and measured after 10 to 15... | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50566946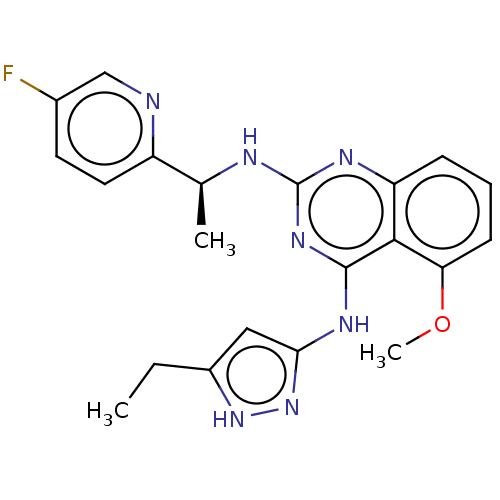 (CHEMBL4854871) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GRK1 using casein as substrate in presence of [gamma33P]ATP by radiometric hotspot kinase assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00506 BindingDB Entry DOI: 10.7270/Q20K2D97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50257340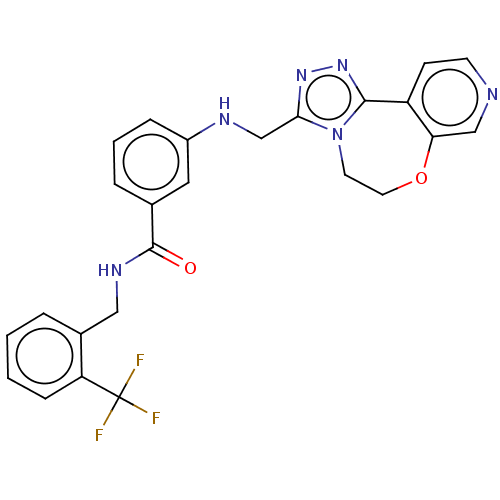 (CHEMBL4072828) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged GRK1 expressed in baculovirus infected fall armyworm Sf9 cells after 60 mins by LanthaScreen eu kinase bin... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM3149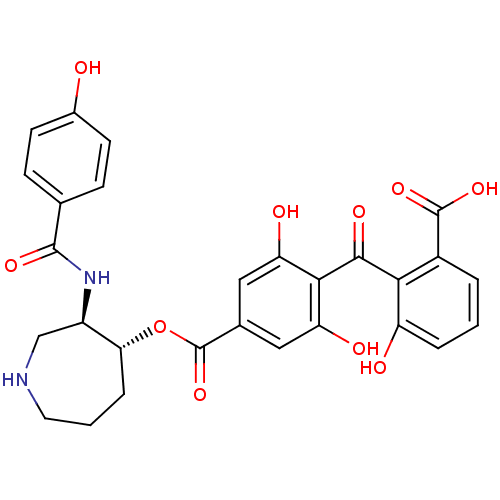 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of GRK1-mediated bovine tubulin phosphorylation by scintillation counting | J Med Chem 53: 1867-70 (2010) Article DOI: 10.1021/jm9017515 BindingDB Entry DOI: 10.7270/Q2P26Z8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50566947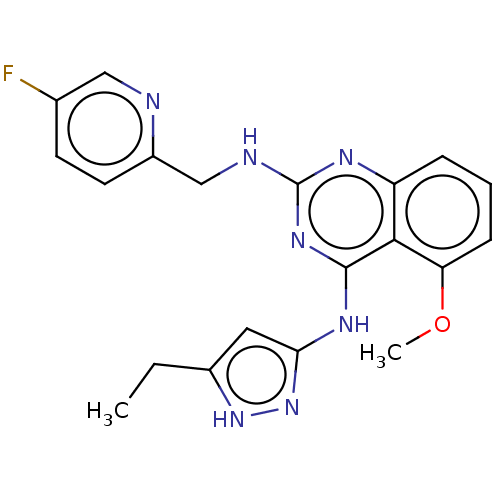 (CHEMBL4847703) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GRK1 using casein as substrate in presence of [gamma33P]ATP by radiometric hotspot kinase assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00506 BindingDB Entry DOI: 10.7270/Q20K2D97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50529469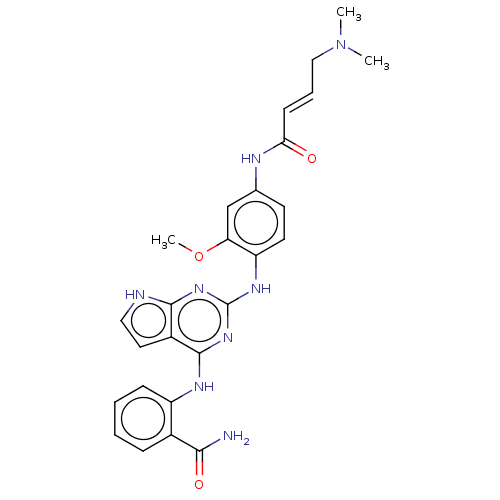 (CHEMBL4456539) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 using tubulin as substrate measured after 4 hrs by [gamma-32P]-ATP assay | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50529470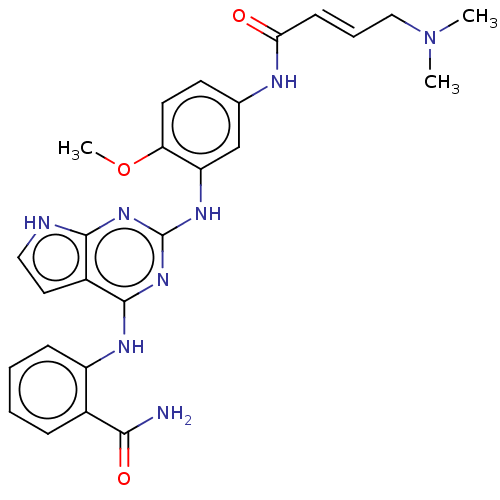 (CHEMBL4475305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 using tubulin as substrate measured after 4 hrs by [gamma-32P]-ATP assay | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM283996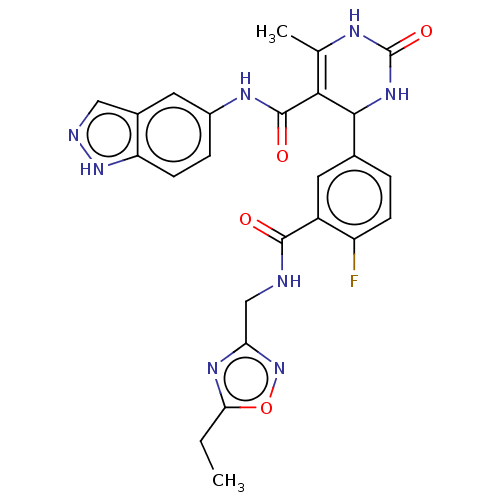 (E22 | US10023564, Example 22) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) preincubated for 10 mins followed by peptide substrate and ATP addition measured after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 28: 1507-1515 (2018) Article DOI: 10.1016/j.bmcl.2018.03.082 BindingDB Entry DOI: 10.7270/Q22Z1868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50257350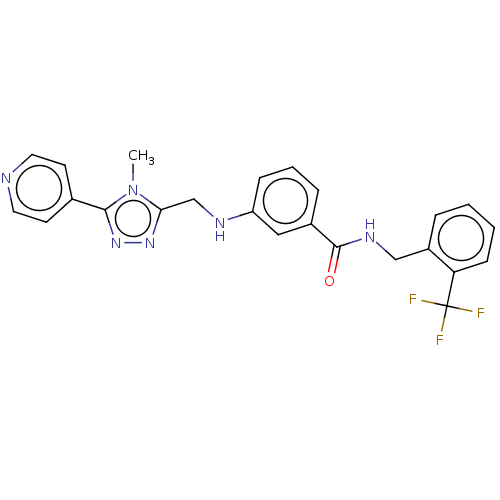 (CHEMBL1738877) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01511 BindingDB Entry DOI: 10.7270/Q22Z19M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50257350 (CHEMBL1738877) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged GRK1 expressed in baculovirus infected fall armyworm Sf9 cells after 60 mins by LanthaScreen eu kinase bin... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM283996 (E22 | US10023564, Example 22) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173310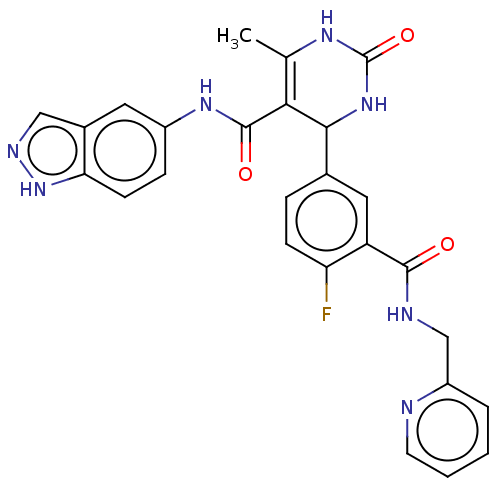 (CHEMBL3808660 | US10023564, Example 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173310 (CHEMBL3808660 | US10023564, Example 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | US Patent | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM3149 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of bovine C-terminal truncated GRK1 (535 residues) assessed as decrease in phosphorylation of urea-washed bovine rod outer segments preinc... | J Med Chem 59: 9277-9294 (2016) BindingDB Entry DOI: 10.7270/Q2348NB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173313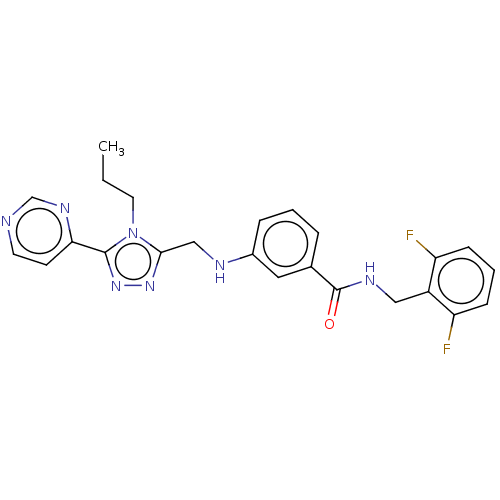 (CHEMBL1738878) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50173313 (CHEMBL1738878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50554258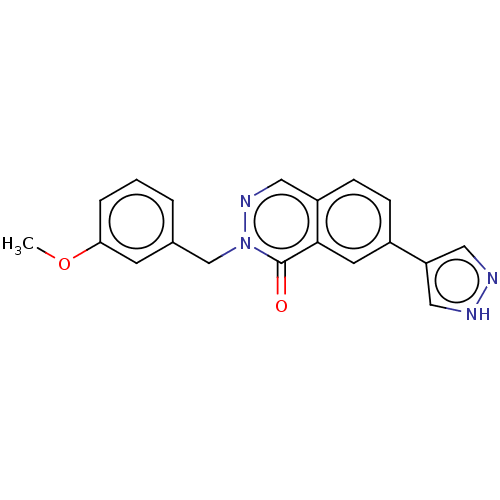 (CHEMBL4744858) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GRK1 | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127602 BindingDB Entry DOI: 10.7270/Q2H135PS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50135286 (CHEMBL3745885) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human GRK1 using casein as substrate | Bioorg Med Chem 24: 521-44 (2016) Article DOI: 10.1016/j.bmc.2015.11.045 BindingDB Entry DOI: 10.7270/Q24Q7WT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50519662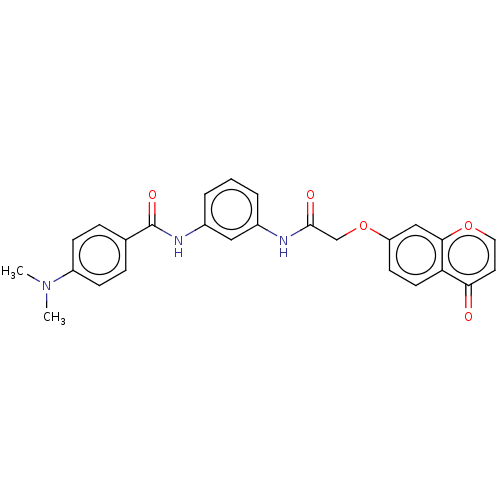 (CHEMBL4438748) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant full length human GRK1 using KKKKERLLDDRHD as substrate after 40 mins in presence of [gamma-33ATP] by radiometric scintilla... | J Med Chem 62: 10691-10710 (2019) Article DOI: 10.1021/acs.jmedchem.9b01143 BindingDB Entry DOI: 10.7270/Q2MC93FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50554257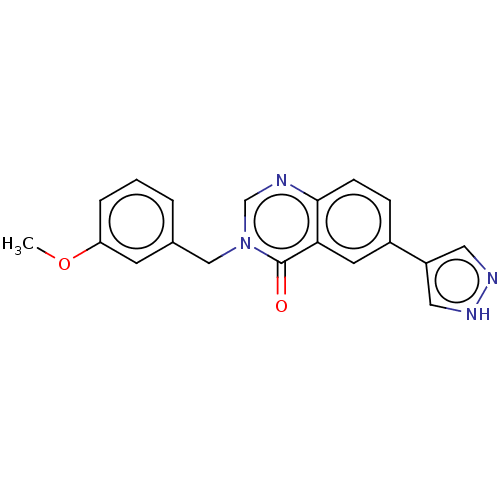 (CHEMBL4742990) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GRK1 | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127602 BindingDB Entry DOI: 10.7270/Q2H135PS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173315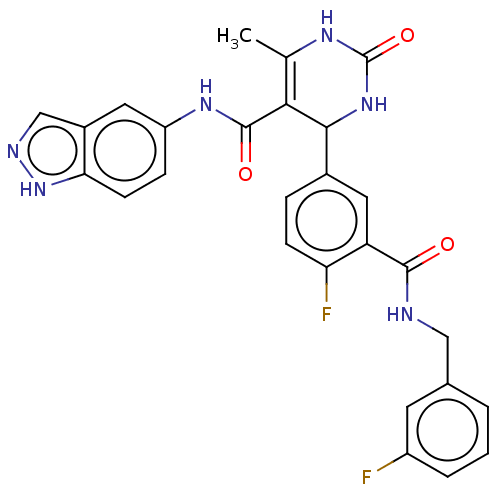 (CHEMBL3809100 | US10023564, Example 4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173315 (CHEMBL3809100 | US10023564, Example 4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM27791 (2-{[2-({4-chloro-2-methoxy-5-[(1-propylpiperidin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of BODIPY TR-ADP from bovine GRK1 (1 to 535 residues) preincubated for 10 mins followed by compound addition and measured after 10 to 15... | ACS Med Chem Lett 10: 1628-1634 (2019) Article DOI: 10.1021/acsmedchemlett.9b00365 BindingDB Entry DOI: 10.7270/Q28W3HR8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM284001 (E27 | US10023564, Example 27) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM284001 (E27 | US10023564, Example 27) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) preincubated for 10 mins followed by peptide substrate and ATP addition measured after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 28: 1507-1515 (2018) Article DOI: 10.1016/j.bmcl.2018.03.082 BindingDB Entry DOI: 10.7270/Q22Z1868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173325 (CHEMBL3809965 | US10023564, Example 5) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173325 (CHEMBL3809965 | US10023564, Example 5) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50461321 (CHEMBL4227138) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) preincubated for 10 mins followed by peptide substrate and ATP addition measured after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 28: 1507-1515 (2018) Article DOI: 10.1016/j.bmcl.2018.03.082 BindingDB Entry DOI: 10.7270/Q22Z1868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM283998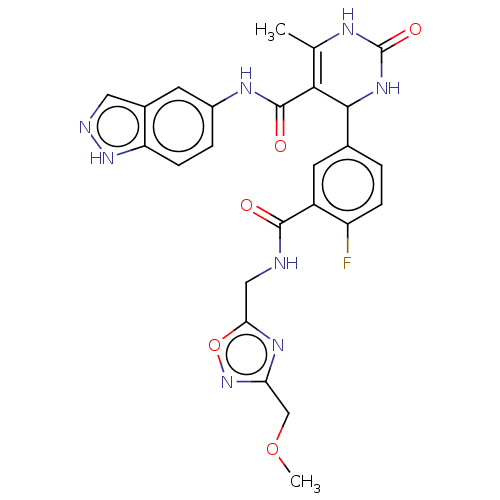 (E24 | US10023564, Example 24) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM284006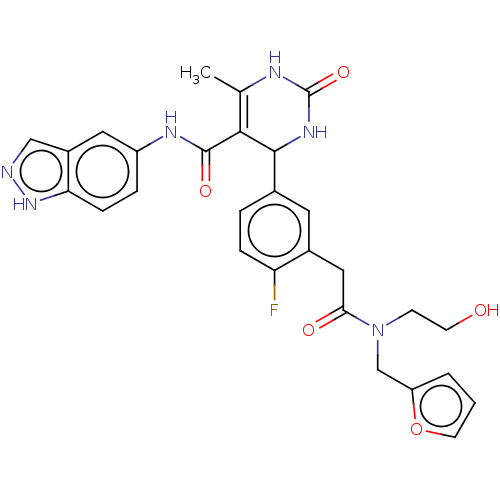 (E32 | US10023564, Example 32) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260129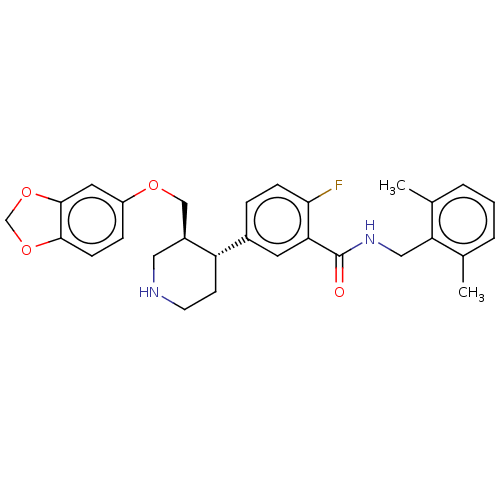 (CHEMBL4098656) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260147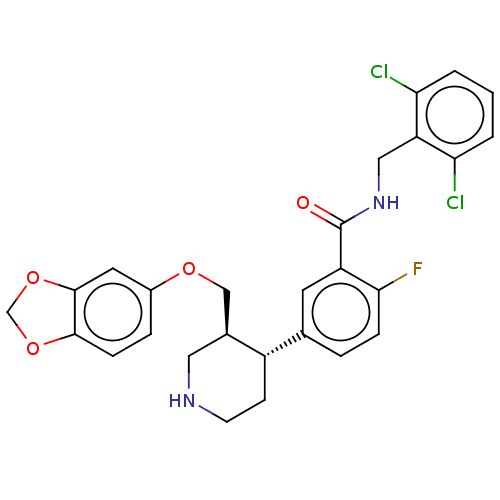 (CHEMBL4063014) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Binding affinity towards serotonin transporter determined using [3H]paroxetine as radioligand | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM284006 (E32 | US10023564, Example 32) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) preincubated for 10 mins followed by peptide substrate and ATP addition measured after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 28: 1507-1515 (2018) Article DOI: 10.1016/j.bmcl.2018.03.082 BindingDB Entry DOI: 10.7270/Q22Z1868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50257344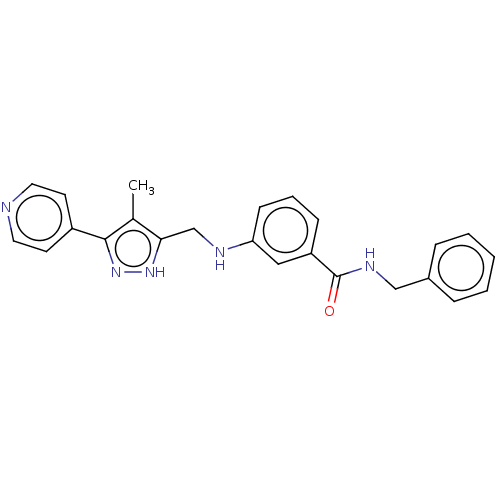 (CHEMBL4065690) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged GRK1 expressed in baculovirus infected fall armyworm Sf9 cells after 60 mins by LanthaScreen eu kinase bin... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50257365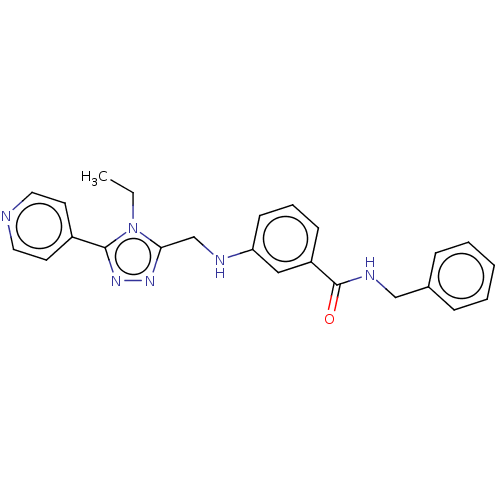 (CHEMBL4083276) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged GRK1 expressed in baculovirus infected fall armyworm Sf9 cells after 60 mins by LanthaScreen eu kinase bin... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50257351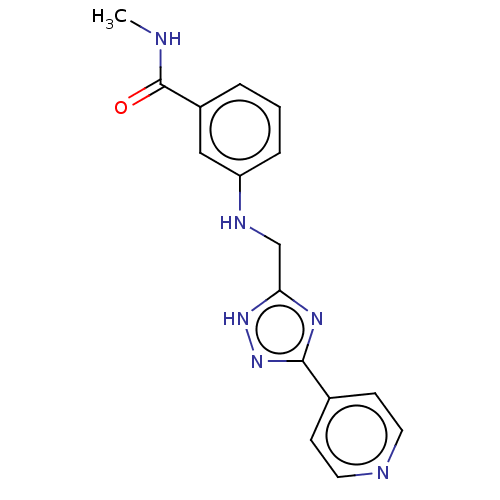 (CHEMBL4095595) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged GRK1 expressed in baculovirus infected fall armyworm Sf9 cells after 60 mins by LanthaScreen eu kinase bin... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM283998 (E24 | US10023564, Example 24) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) preincubated for 10 mins followed by peptide substrate and ATP addition measured after 1 hr by TR-FRET assay | Bioorg Med Chem Lett 28: 1507-1515 (2018) Article DOI: 10.1016/j.bmcl.2018.03.082 BindingDB Entry DOI: 10.7270/Q22Z1868 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50257328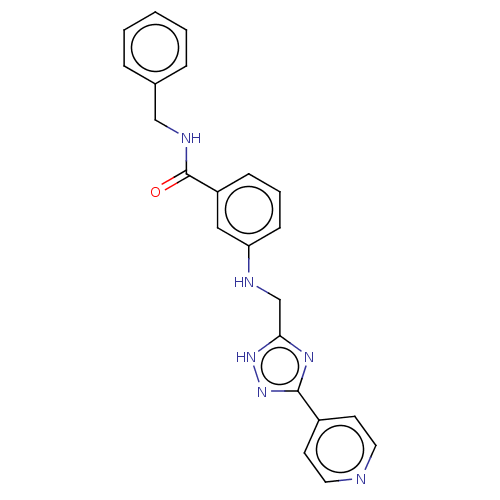 (CHEMBL4082775) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged GRK1 expressed in baculovirus infected fall armyworm Sf9 cells after 60 mins by LanthaScreen eu kinase bin... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260130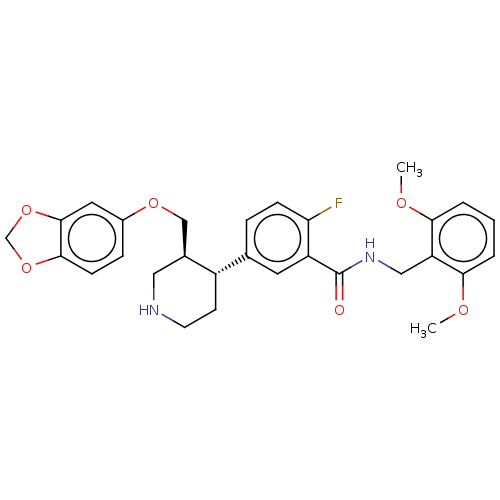 (CHEMBL4090923) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against human alpha-L-fucosidase | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50257350 (CHEMBL1738877) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 5.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibitory concentration against alpha-galactosidase of coffee bean | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Bos taurus) | BDBM50260137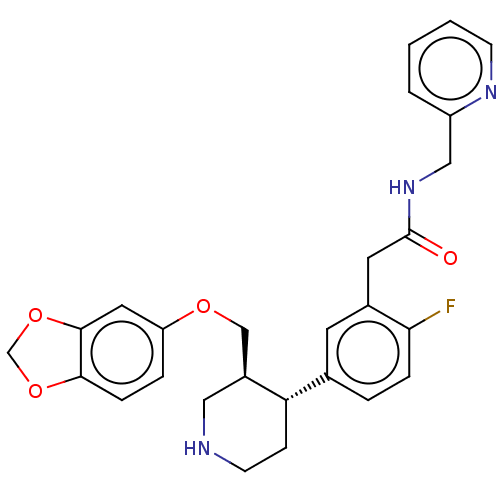 (CHEMBL4062790) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, College of Pharmacy,?Departments of Pharmacology and Biological Chemistry, Life Sciences Institute,�Ph.D. Program in Chemical Biology,?Vahlteich Medicinal Chemistry Curated by ChEMBL | Assay Description Inhibition of bovine GRK1 (1 to 535 residues) after 5 mins after 5 mins in presence of ATP by phosphorimaging assay | J Med Chem 60: 3052-3069 (2017) Article DOI: 10.1021/acs.jmedchem.7b00112 BindingDB Entry DOI: 10.7270/Q2M047XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM283997 (E23 | US10023564, Example 23) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173314 (CHEMBL3808840 | US10023564, Example 16) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN US Patent | Assay Description GRK1, 2 and 5 kinetic assays were conducted in a buffer containing 20 mM HEPES pH 7.0, 5 μM ATP, 2 mM MgCl2, and 0.025% DDM with 50 nM GRK and e... | US Patent US10023564 (2018) BindingDB Entry DOI: 10.7270/Q2V126V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173314 (CHEMBL3808840 | US10023564, Example 16) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 262 total ) | Next | Last >> |