 Found 137 hits of ki for UniProtKB: P12821
Found 137 hits of ki for UniProtKB: P12821 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50614466
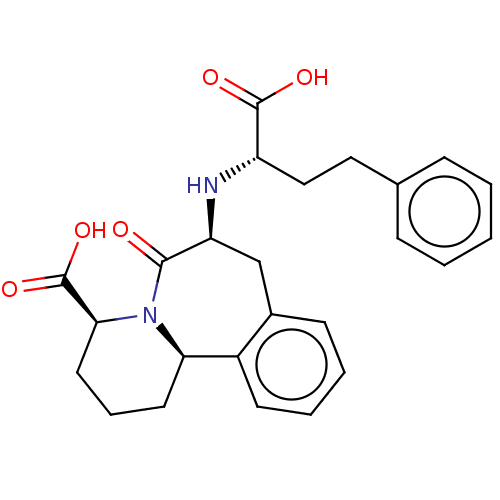
(CHEMBL5272537)Show SMILES [H][C@]12CCC[C@H](N1C(=O)[C@H](Cc1ccccc21)N[C@@H](CCc1ccccc1)C(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50073120

((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...)Show SMILES OC(=O)[C@@H]1CCC[C@@H]2SCC[C@H](NC(=O)[C@@H](S)Cc3ccccc3)C(=O)N12 |r| Show InChI InChI=1S/C19H24N2O4S2/c22-17(15(26)11-12-5-2-1-3-6-12)20-13-9-10-27-16-8-4-7-14(19(24)25)21(16)18(13)23/h1-3,5-6,13-16,26H,4,7-11H2,(H,20,22)(H,24,25)/t13-,14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of human fully glycosylated ACE N-terminal domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins follo... |
J Med Chem 61: 10141-10154 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01309
BindingDB Entry DOI: 10.7270/Q2862K4R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50073120

((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...)Show SMILES OC(=O)[C@@H]1CCC[C@@H]2SCC[C@H](NC(=O)[C@@H](S)Cc3ccccc3)C(=O)N12 |r| Show InChI InChI=1S/C19H24N2O4S2/c22-17(15(26)11-12-5-2-1-3-6-12)20-13-9-10-27-16-8-4-7-14(19(24)25)21(16)18(13)23/h1-3,5-6,13-16,26H,4,7-11H2,(H,20,22)(H,24,25)/t13-,14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of human fully glycosylated ACE C-terminal domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins follo... |
J Med Chem 61: 10141-10154 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01309
BindingDB Entry DOI: 10.7270/Q2862K4R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50369460
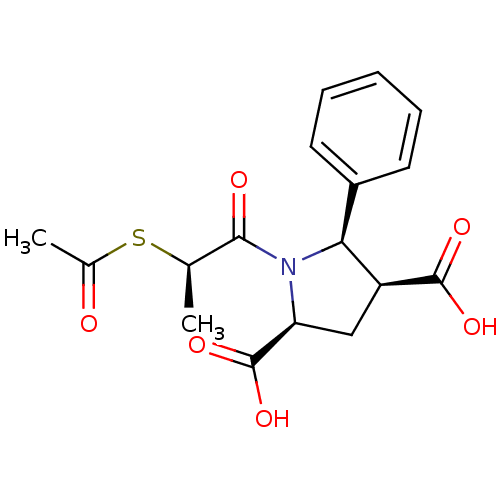
(CHEMBL1788109)Show SMILES C[C@@H](SC(C)=O)C(=O)N1[C@@H](C[C@@H]([C@@H]1c1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C17H19NO6S/c1-9(25-10(2)19)15(20)18-13(17(23)24)8-12(16(21)22)14(18)11-6-4-3-5-7-11/h3-7,9,12-14H,8H2,1-2H3,(H,21,22)(H,23,24)/t9-,12+,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Enzyme inhibitory activity towards Angiotensin I converting enzyme |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303321
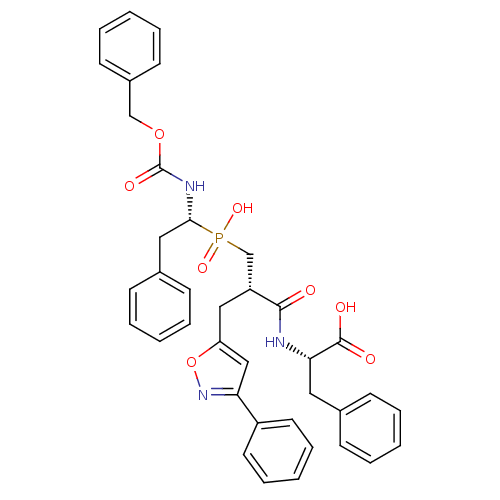
((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O8P/c42-36(39-34(37(43)44)21-27-13-5-1-6-14-27)31(23-32-24-33(41-49-32)30-19-11-4-12-20-30)26-50(46,47)35(22-28-15-7-2-8-16-28)40-38(45)48-25-29-17-9-3-10-18-29/h1-20,24,31,34-35H,21-23,25-26H2,(H,39,42)(H,40,45)(H,43,44)(H,46,47)/t31-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303319
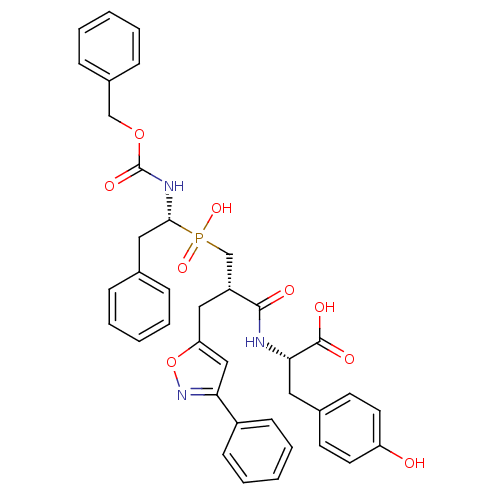
((S)-2-[(S)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367249

(CHEMBL309601)Show SMILES C[C@H](NP(O)(=O)CCc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C16H23N2O5P/c1-12(15(19)18-10-5-8-14(18)16(20)21)17-24(22,23)11-9-13-6-3-2-4-7-13/h2-4,6-7,12,14H,5,8-11H2,1H3,(H,20,21)(H2,17,22,23)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 393-9 (1985)
BindingDB Entry DOI: 10.7270/Q28W3DWT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE in presence of buffer |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471705
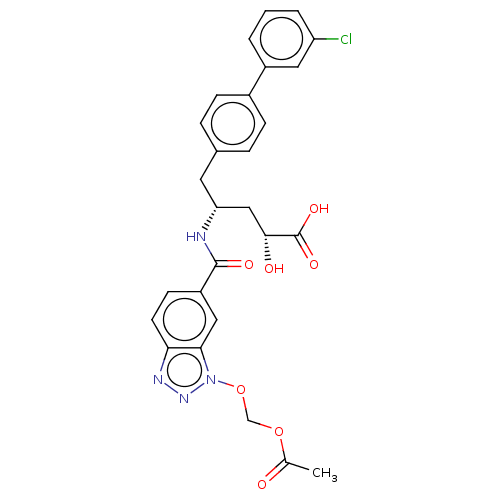
(US10829438, Example 1G | US11174219, Example 1G)Show SMILES CC(=O)OCOn1nnc2ccc(cc12)C(=O)N[C@@H](C[C@@H](O)C(O)=O)Cc1ccc(cc1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C27H25ClN4O7/c1-16(33)38-15-39-32-24-13-20(9-10-23(24)30-31-32)26(35)29-22(14-25(34)27(36)37)11-17-5-7-18(8-6-17)19-3-2-4-21(28)12-19/h2-10,12-13,22,25,34H,11,14-15H2,1H3,(H,29,35)(H,36,37)/t22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H0G |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471710

(US10829438, Example 1L | US11174219, Example 1L)Show SMILES CC(C)[C@@H](N)C(=O)OCOn1nnc2ccc(cc12)C(=O)N[C@@H](C[C@@H](O)C(O)=O)Cc1ccc(cc1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C30H32ClN5O7/c1-17(2)27(32)30(41)42-16-43-36-25-14-21(10-11-24(25)34-35-36)28(38)33-23(15-26(37)29(39)40)12-18-6-8-19(9-7-18)20-4-3-5-22(31)13-20/h3-11,13-14,17,23,26-27,37H,12,15-16,32H2,1-2H3,(H,33,38)(H,39,40)/t23-,26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC
US Patent
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
US Patent US10829438 (2020)
BindingDB Entry DOI: 10.7270/Q29C71H0 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471714

(US10829438, Example 6A | US11174219, Example 6A)Show SMILES O[C@H](C[C@@H](Cc1ccc(cc1)-c1cccc(Cl)c1)NC(=O)c1cc(no1)-c1ccccc1F)C(O)=O |r| Show InChI InChI=1S/C27H22ClFN2O5/c28-19-5-3-4-18(13-19)17-10-8-16(9-11-17)12-20(14-24(32)27(34)35)30-26(33)25-15-23(31-36-25)21-6-1-2-7-22(21)29/h1-11,13,15,20,24,32H,12,14H2,(H,30,33)(H,34,35)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H0G |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471713

(US10829438, Example 5B | US11174219, Example 5B)Show SMILES CCOC(=O)[C@H](O)C[C@@H](Cc1ccc(cc1)-c1cccc(Cl)c1)NC(=O)c1cc([nH]n1)C(C)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H0G |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471712
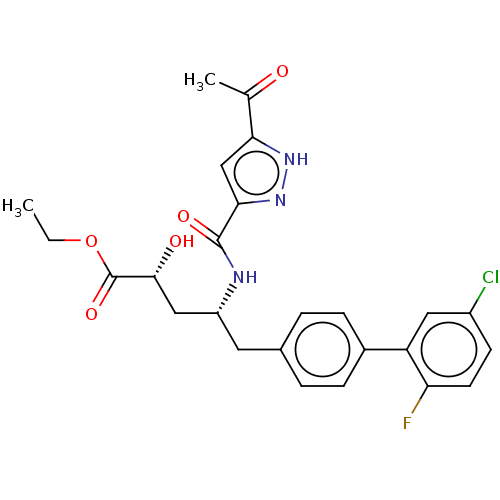
(US10829438, Example 4B | US11174219, Example 4B)Show SMILES CCOC(=O)[C@H](O)C[C@@H](Cc1ccc(cc1)-c1cc(Cl)ccc1F)NC(=O)c1cc([nH]n1)C(C)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H0G |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471710

(US10829438, Example 1L | US11174219, Example 1L)Show SMILES CC(C)[C@@H](N)C(=O)OCOn1nnc2ccc(cc12)C(=O)N[C@@H](C[C@@H](O)C(O)=O)Cc1ccc(cc1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C30H32ClN5O7/c1-17(2)27(32)30(41)42-16-43-36-25-14-21(10-11-24(25)34-35-36)28(38)33-23(15-26(37)29(39)40)12-18-6-8-19(9-7-18)20-4-3-5-22(31)13-20/h3-11,13-14,17,23,26-27,37H,12,15-16,32H2,1-2H3,(H,33,38)(H,39,40)/t23-,26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H0G |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471705

(US10829438, Example 1G | US11174219, Example 1G)Show SMILES CC(=O)OCOn1nnc2ccc(cc12)C(=O)N[C@@H](C[C@@H](O)C(O)=O)Cc1ccc(cc1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C27H25ClN4O7/c1-16(33)38-15-39-32-24-13-20(9-10-23(24)30-31-32)26(35)29-22(14-25(34)27(36)37)11-17-5-7-18(8-6-17)19-3-2-4-21(28)12-19/h2-10,12-13,22,25,34H,11,14-15H2,1H3,(H,29,35)(H,36,37)/t22-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC
US Patent
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
US Patent US10829438 (2020)
BindingDB Entry DOI: 10.7270/Q29C71H0 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471714

(US10829438, Example 6A | US11174219, Example 6A)Show SMILES O[C@H](C[C@@H](Cc1ccc(cc1)-c1cccc(Cl)c1)NC(=O)c1cc(no1)-c1ccccc1F)C(O)=O |r| Show InChI InChI=1S/C27H22ClFN2O5/c28-19-5-3-4-18(13-19)17-10-8-16(9-11-17)12-20(14-24(32)27(34)35)30-26(33)25-15-23(31-36-25)21-6-1-2-7-22(21)29/h1-11,13,15,20,24,32H,12,14H2,(H,30,33)(H,34,35)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC
US Patent
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
US Patent US10829438 (2020)
BindingDB Entry DOI: 10.7270/Q29C71H0 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471713

(US10829438, Example 5B | US11174219, Example 5B)Show SMILES CCOC(=O)[C@H](O)C[C@@H](Cc1ccc(cc1)-c1cccc(Cl)c1)NC(=O)c1cc([nH]n1)C(C)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC
US Patent
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
US Patent US10829438 (2020)
BindingDB Entry DOI: 10.7270/Q29C71H0 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471712

(US10829438, Example 4B | US11174219, Example 4B)Show SMILES CCOC(=O)[C@H](O)C[C@@H](Cc1ccc(cc1)-c1cc(Cl)ccc1F)NC(=O)c1cc([nH]n1)C(C)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC
US Patent
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
US Patent US10829438 (2020)
BindingDB Entry DOI: 10.7270/Q29C71H0 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879
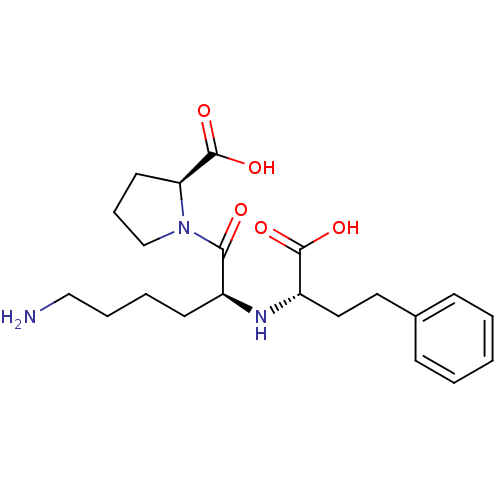
(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50011270

(2-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-1...)Show SMILES OC(=O)C(CCc1ccccc1)NC1Cc2ccccc2[C@H]2CCC[C@H](N2C1=O)C(O)=O Show InChI InChI=1S/C25H28N2O5/c28-23-20(26-19(24(29)30)14-13-16-7-2-1-3-8-16)15-17-9-4-5-10-18(17)21-11-6-12-22(25(31)32)27(21)23/h1-5,7-10,19-22,26H,6,11-15H2,(H,29,30)(H,31,32)/t19?,20?,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Calgary
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory potency against angiotensin I converting enzyme. |
J Med Chem 34: 511-7 (1991)
BindingDB Entry DOI: 10.7270/Q2JS9R1G |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50115848
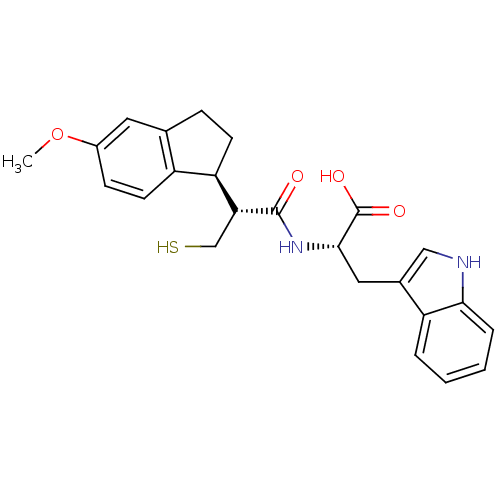
((S)-3-(1H-Indol-3-yl)-2-[(R)-3-mercapto-2-((S)-5-m...)Show SMILES COc1ccc2[C@@H](CCc2c1)[C@@H](CS)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-30-16-7-9-17-14(10-16)6-8-19(17)20(13-31)23(27)26-22(24(28)29)11-15-12-25-21-5-3-2-4-18(15)21/h2-5,7,9-10,12,19-20,22,25,31H,6,8,11,13H2,1H3,(H,26,27)(H,28,29)/t19-,20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against angiotensin I converting enzyme |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303322
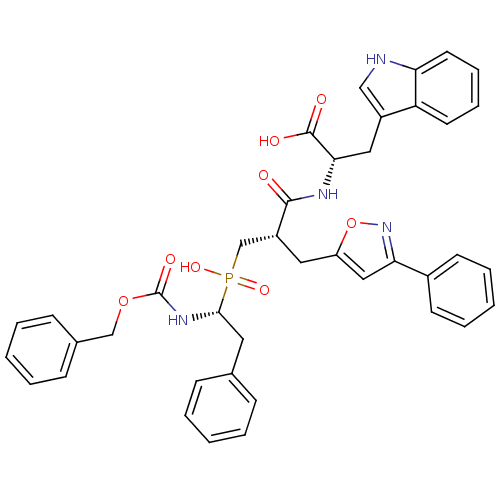
((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C40H39N4O8P/c45-38(42-36(39(46)47)22-30-24-41-34-19-11-10-18-33(30)34)31(21-32-23-35(44-52-32)29-16-8-3-9-17-29)26-53(49,50)37(20-27-12-4-1-5-13-27)43-40(48)51-25-28-14-6-2-7-15-28/h1-19,23-24,31,36-37,41H,20-22,25-26H2,(H,42,45)(H,43,48)(H,46,47)(H,49,50)/t31-,36+,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50020843

(2-[(2S)-2-carboxypyrrolidin-1-yl]-1-methyl-2-oxoet...)Show SMILES C[C@@H](NP([O-])([O-])=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C8H15N2O6P/c1-5(9-17(14,15)16)7(11)10-4-2-3-6(10)8(12)13/h5-6H,2-4H2,1H3,(H,12,13)(H3,9,14,15,16)/p-2/t5?,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Angiotensin I converting enzyme |
J Med Chem 28: 393-9 (1985)
BindingDB Entry DOI: 10.7270/Q28W3DWT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50018849

(4-Cyclohexyl-1-{2-[hydroxy-(4-phenyl-butyl)-phosph...)Show SMILES OC(=O)[C@@H]1C[C@H](CN1C(=O)CP(O)(=O)CCCCc1ccccc1)C1CCCCC1 Show InChI InChI=1S/C23H34NO5P/c25-22(17-30(28,29)14-8-7-11-18-9-3-1-4-10-18)24-16-20(15-21(24)23(26)27)19-12-5-2-6-13-19/h1,3-4,9-10,19-21H,2,5-8,11-17H2,(H,26,27)(H,28,29)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of ACE |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50281632
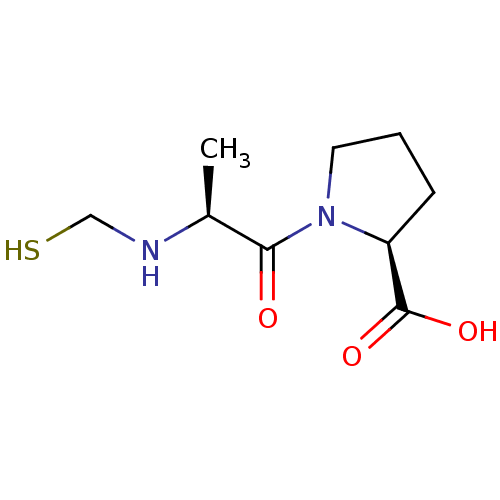
((2S)-1-{(2S)-2-[(lambda~4~-sulfanylmethyl)amino]pr...)Show InChI InChI=1S/C9H16N2O3S/c1-6(10-5-15)8(12)11-4-2-3-7(11)9(13)14/h6-7,10,15H,2-5H2,1H3,(H,13,14)/t6-,7-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Angiotensin I converting enzyme |
Bioorg Med Chem Lett 3: 799-802 (1993)
Article DOI: 10.1016/S0960-894X(00)80669-1
BindingDB Entry DOI: 10.7270/Q29S1QZZ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50614464
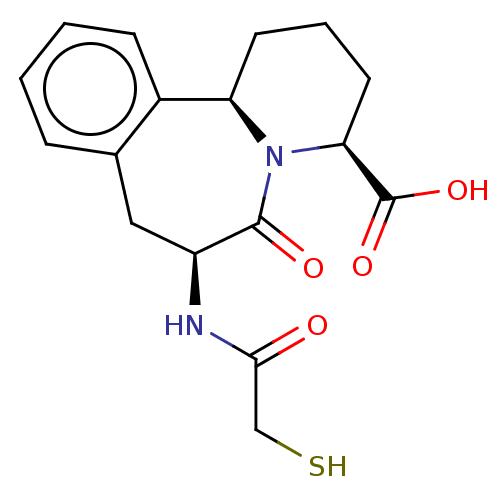
(CHEMBL5281720)Show SMILES [H][C@]12CCC[C@H](N1C(=O)[C@H](Cc1ccccc21)NC(=O)CS)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
E£tv£s Lor£nd University
Curated by ChEMBL
| Assay Description
Inhibition of ACE (unknown origin) assessed as 3-Hydroxybutyril-glycil-glycil-glycine conversion to 3-hydroxybutyric acid after 60 mins by WST assay |
J Med Chem 56: 8377-88 (2013)
Article DOI: 10.1021/jm400813y
BindingDB Entry DOI: 10.7270/Q2MG7SGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50406931
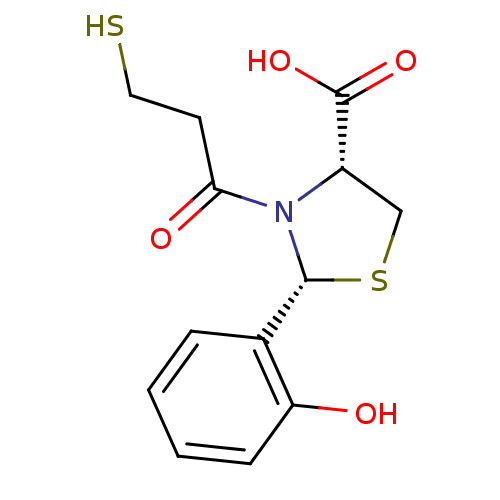
(RENTIAPRIL)Show InChI InChI=1S/C13H15NO4S2/c15-10-4-2-1-3-8(10)12-14(11(16)5-6-19)9(7-20-12)13(17)18/h1-4,9,12,15,19H,5-7H2,(H,17,18)/t9-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303327
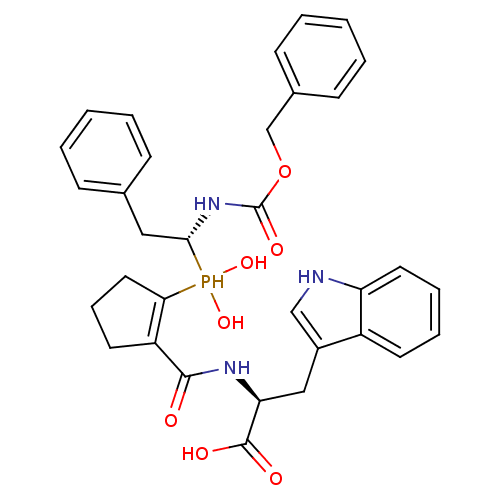
((2S)-2-((1S,2S)-2-(((R)-1-(benzyloxycarbonylamino)...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1=C(CCC1)P(O)(O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r,t:19| Show InChI InChI=1S/C33H36N3O7P/c37-31(35-28(32(38)39)19-24-20-34-27-16-8-7-14-25(24)27)26-15-9-17-29(26)44(41,42)30(18-22-10-3-1-4-11-22)36-33(40)43-21-23-12-5-2-6-13-23/h1-8,10-14,16,20,28,30,34,41-42,44H,9,15,17-19,21H2,(H,35,37)(H,36,40)(H,38,39)/t28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE C-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303327

((2S)-2-((1S,2S)-2-(((R)-1-(benzyloxycarbonylamino)...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C1=C(CCC1)P(O)(O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r,t:19| Show InChI InChI=1S/C33H36N3O7P/c37-31(35-28(32(38)39)19-24-20-34-27-16-8-7-14-25(24)27)26-15-9-17-29(26)44(41,42)30(18-22-10-3-1-4-11-22)36-33(40)43-21-23-12-5-2-6-13-23/h1-8,10-14,16,20,28,30,34,41-42,44H,9,15,17-19,21H2,(H,35,37)(H,36,40)(H,38,39)/t28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50411736
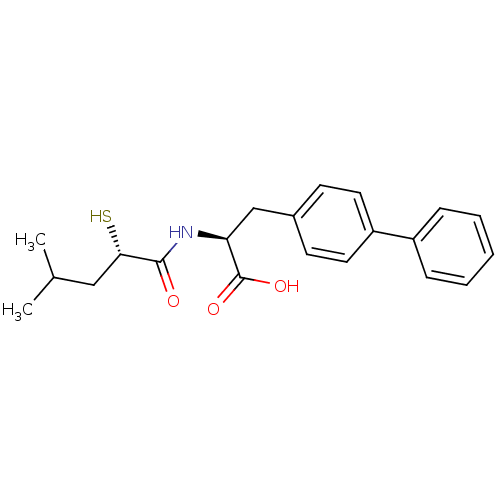
(CHEMBL271225)Show SMILES CC(C)C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-14(2)12-19(26)20(23)22-18(21(24)25)13-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-11,14,18-19,26H,12-13H2,1-2H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471711

(US10829438, Example 1M | US11174219, Example 1M)Show SMILES CCCC(=O)OCOn1nnc2ccc(cc12)C(=O)N[C@@H](C[C@@H](O)C(O)=O)Cc1ccc(cc1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H29ClN4O7/c1-2-4-27(36)40-17-41-34-25-15-21(11-12-24(25)32-33-34)28(37)31-23(16-26(35)29(38)39)13-18-7-9-19(10-8-18)20-5-3-6-22(30)14-20/h3,5-12,14-15,23,26,35H,2,4,13,16-17H2,1H3,(H,31,37)(H,38,39)/t23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC
US Patent
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
US Patent US10829438 (2020)
BindingDB Entry DOI: 10.7270/Q29C71H0 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1
(Human-Homo sapiens (Human)) | BDBM471711

(US10829438, Example 1M | US11174219, Example 1M)Show SMILES CCCC(=O)OCOn1nnc2ccc(cc12)C(=O)N[C@@H](C[C@@H](O)C(O)=O)Cc1ccc(cc1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H29ClN4O7/c1-2-4-27(36)40-17-41-34-25-15-21(11-12-24(25)32-33-34)28(37)31-23(16-26(35)29(38)39)13-18-7-9-19(10-8-18)20-5-3-6-22(30)14-20/h3,5-12,14-15,23,26,35H,2,4,13,16-17H2,1H3,(H,31,37)(H,38,39)/t23-,26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20V8H0G |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303323
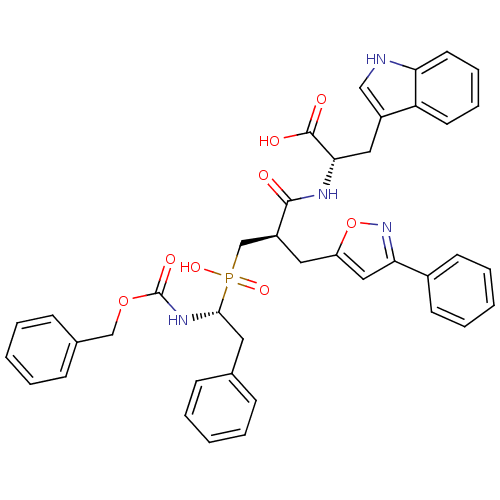
((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C40H39N4O8P/c45-38(42-36(39(46)47)22-30-24-41-34-19-11-10-18-33(30)34)31(21-32-23-35(44-52-32)29-16-8-3-9-17-29)26-53(49,50)37(20-27-12-4-1-5-13-27)43-40(48)51-25-28-14-6-2-7-15-28/h1-19,23-24,31,36-37,41H,20-22,25-26H2,(H,42,45)(H,43,48)(H,46,47)(H,49,50)/t31-,36-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303328

((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1{[(benzyloxy)c...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O8P/c42-36(39-34(37(43)44)21-27-13-5-1-6-14-27)31(23-32-24-33(41-49-32)30-19-11-4-12-20-30)26-50(46,47)35(22-28-15-7-2-8-16-28)40-38(45)48-25-29-17-9-3-10-18-29/h1-20,24,31,34-35H,21-23,25-26H2,(H,39,42)(H,40,45)(H,43,44)(H,46,47)/t31-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303328

((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1{[(benzyloxy)c...)Show SMILES OC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O8P/c42-36(39-34(37(43)44)21-27-13-5-1-6-14-27)31(23-32-24-33(41-49-32)30-19-11-4-12-20-30)26-50(46,47)35(22-28-15-7-2-8-16-28)40-38(45)48-25-29-17-9-3-10-18-29/h1-20,24,31,34-35H,21-23,25-26H2,(H,39,42)(H,40,45)(H,43,44)(H,46,47)/t31-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE in presence of buffer |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE in presence of 1:100 diluted SHR rat plasma |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50367879

(LISINOPRIL)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C21H31N3O5/c22-13-5-4-9-16(19(25)24-14-6-10-18(24)21(28)29)23-17(20(26)27)12-11-15-7-2-1-3-8-15/h1-3,7-8,16-18,23H,4-6,9-14,22H2,(H,26,27)(H,28,29)/t16-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE N-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303325

((2S)-2-{[3-(4',5'-Dihydro-3'-phenyl-5'-isoxazolyl)...)Show SMILES C[C@H](NC(=O)C(CC1CC(=NO1)c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(O)=O |r,c:9| Show InChI InChI=1S/C32H36N3O8P/c1-22(31(37)38)33-30(36)26(18-27-19-28(35-43-27)25-15-9-4-10-16-25)21-44(40,41)29(17-23-11-5-2-6-12-23)34-32(39)42-20-24-13-7-3-8-14-24/h2-16,22,26-27,29H,17-21H2,1H3,(H,33,36)(H,34,39)(H,37,38)(H,40,41)/t22-,26?,27?,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50073120

((4S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...)Show SMILES OC(=O)[C@@H]1CCC[C@@H]2SCC[C@H](NC(=O)[C@@H](S)Cc3ccccc3)C(=O)N12 |r| Show InChI InChI=1S/C19H24N2O4S2/c22-17(15(26)11-12-5-2-1-3-6-12)20-13-9-10-27-16-8-4-7-14(19(24)25)21(16)18(13)23/h1-3,5-6,13-16,26H,4,7-11H2,(H,20,22)(H,24,25)/t13-,14-,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE C-terminal domain |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50393213
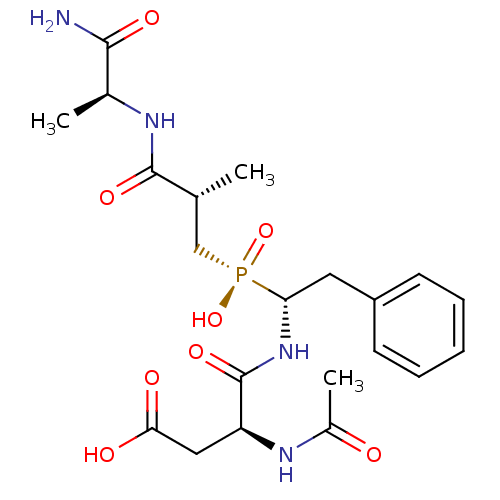
(CHEMBL1235767)Show SMILES C[C@H](C[P@](O)(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](C)C(N)=O |r| Show InChI InChI=1S/C21H31N4O8P/c1-12(20(30)23-13(2)19(22)29)11-34(32,33)17(9-15-7-5-4-6-8-15)25-21(31)16(10-18(27)28)24-14(3)26/h4-8,12-13,16-17H,9-11H2,1-3H3,(H2,22,29)(H,23,30)(H,24,26)(H,25,31)(H,27,28)(H,32,33)/t12-,13+,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human ACE N-terminal domain expressed in CHO cells after 90 mins by fluorescence assay |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50303320

((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](Cc1cc(no1)-c1ccccc1)CP(O)(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C38H38N3O9P/c42-31-18-16-27(17-19-31)20-34(37(44)45)39-36(43)30(22-32-23-33(41-50-32)29-14-8-3-9-15-29)25-51(47,48)35(21-26-10-4-1-5-11-26)40-38(46)49-24-28-12-6-2-7-13-28/h1-19,23,30,34-35,42H,20-22,24-25H2,(H,39,43)(H,40,46)(H,44,45)(H,47,48)/t30-,34-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO)
Curated by ChEMBL
| Assay Description
Inhibition of human somatic ACE in presence of 1:50 diluted SHR rat plasma |
J Med Chem 53: 208-20 (2010)
Article DOI: 10.1021/jm9010803
BindingDB Entry DOI: 10.7270/Q2736R06 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50411731
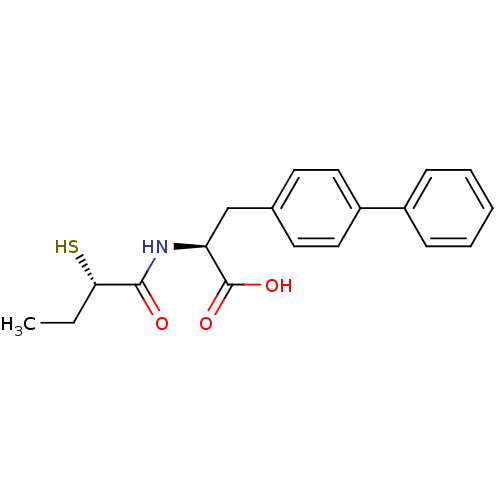
(CHEMBL257726)Show SMILES CC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C19H21NO3S/c1-2-17(24)18(21)20-16(19(22)23)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11,16-17,24H,2,12H2,1H3,(H,20,21)(H,22,23)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50189452
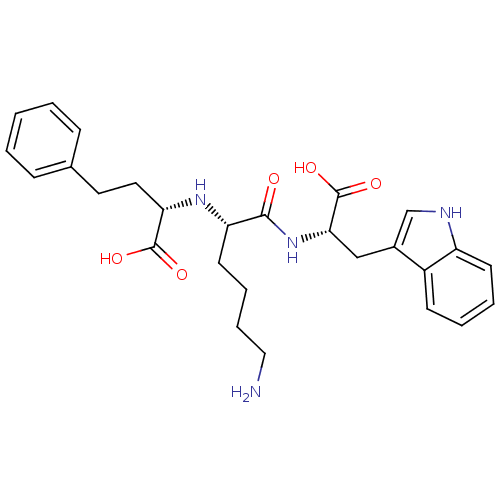
((S)-2-((S)-6-amino-1-((S)-1-carboxy-2-(1H-indol-3-...)Show SMILES NCCCC[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C27H34N4O5/c28-15-7-6-12-22(30-23(26(33)34)14-13-18-8-2-1-3-9-18)25(32)31-24(27(35)36)16-19-17-29-21-11-5-4-10-20(19)21/h1-5,8-11,17,22-24,29-30H,6-7,12-16,28H2,(H,31,32)(H,33,34)(H,35,36)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE C-domain expressed in CHO cells using Cbz-Phe-His-Leu as substrate preincubated for 15 mins followed by substrate... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01924
BindingDB Entry DOI: 10.7270/Q2FT8QZ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50411730
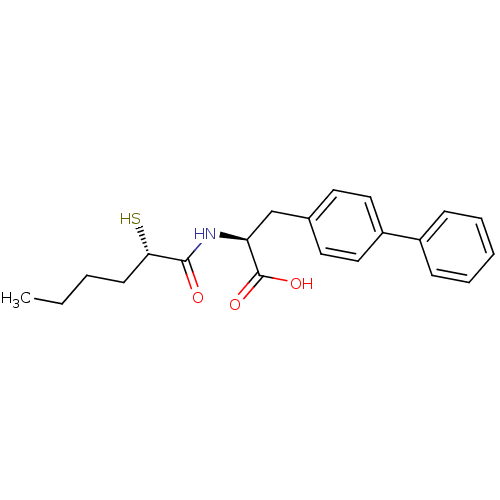
(CHEMBL257270)Show SMILES CCCC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-2-3-9-19(26)20(23)22-18(21(24)25)14-15-10-12-17(13-11-15)16-7-5-4-6-8-16/h4-8,10-13,18-19,26H,2-3,9,14H2,1H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50084628
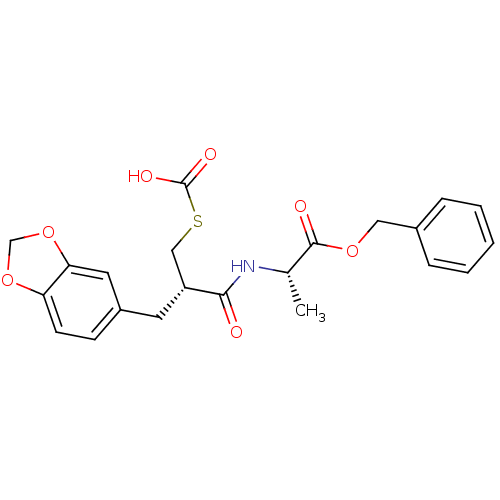
(2-(2-Benzo[1,3]dioxol-5-ylmethyl-3-carboxysulfanyl...)Show SMILES C[C@H](NC(=O)[C@@H](CSC(O)=O)Cc1ccc2OCOc2c1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C22H23NO7S/c1-14(21(25)28-11-15-5-3-2-4-6-15)23-20(24)17(12-31-22(26)27)9-16-7-8-18-19(10-16)30-13-29-18/h2-8,10,14,17H,9,11-13H2,1H3,(H,23,24)(H,26,27)/t14-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibitory activity against angiotensin I converting enzyme (ACE) |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50279944
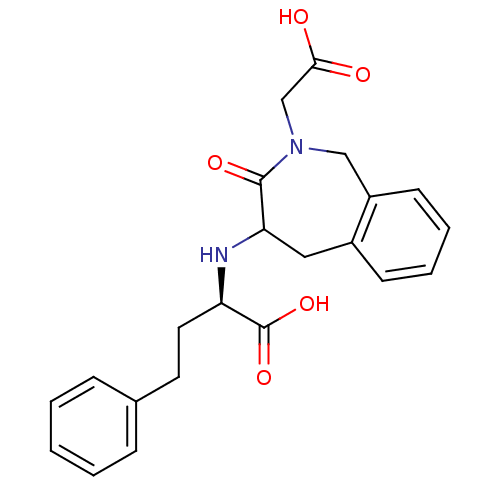
((R)-2-(2-Carboxymethyl-3-oxo-2,3,4,5-tetrahydro-1H...)Show SMILES OC(=O)CN1Cc2ccccc2CC(N[C@H](CCc2ccccc2)C(O)=O)C1=O Show InChI InChI=1S/C22H24N2O5/c25-20(26)14-24-13-17-9-5-4-8-16(17)12-19(21(24)27)23-18(22(28)29)11-10-15-6-2-1-3-7-15/h1-9,18-19,23H,10-14H2,(H,25,26)(H,28,29)/t18-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound was tested angiotensin converting enzyme |
Bioorg Med Chem Lett 1: 309-312 (1991)
Article DOI: 10.1016/S0960-894X(01)80814-3
BindingDB Entry DOI: 10.7270/Q208657V |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50614467
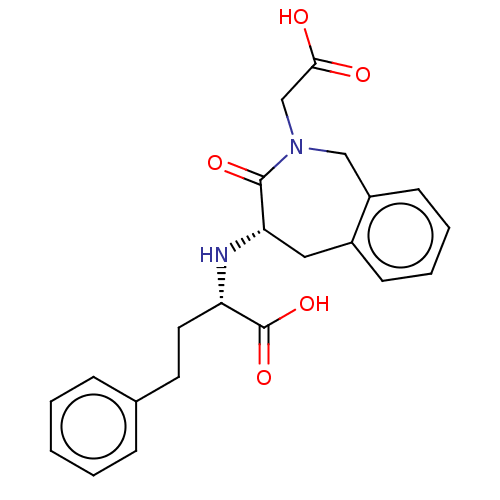
(CHEMBL5279614)Show SMILES OC(=O)CN1Cc2ccccc2C[C@H](N[C@@H](CCc2ccccc2)C(O)=O)C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data