

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185629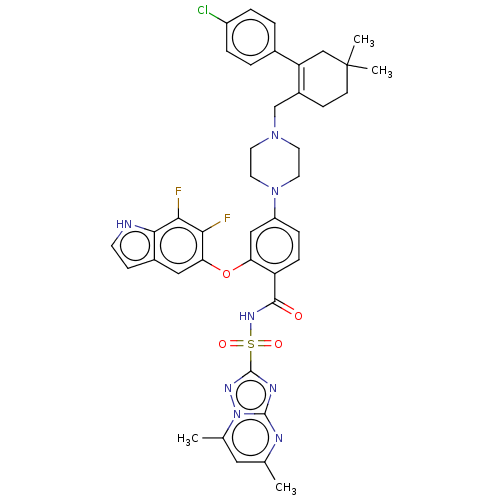 (US9156856, 42 | US9493431, 42) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0390 | -14.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185628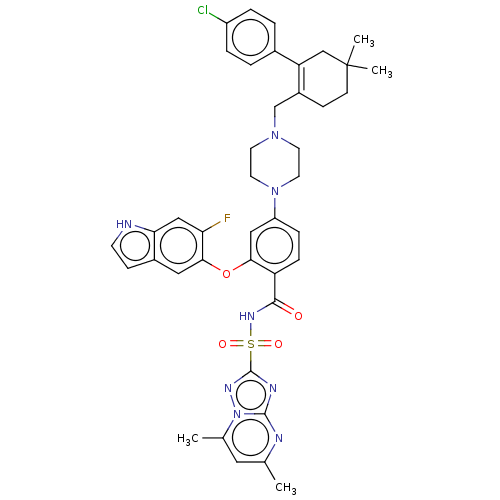 (US9156856, 41 | US9493431, 41) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0530 | -14.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185598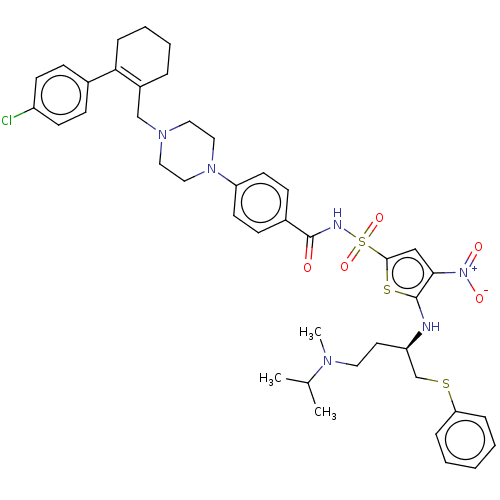 (US9156856, 8 | US9493431, 8) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0640 | -13.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185597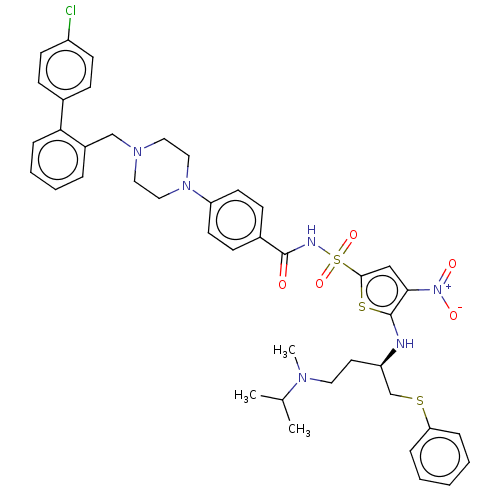 (US9156856, 7 | US9493431, 7) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0840 | -13.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185599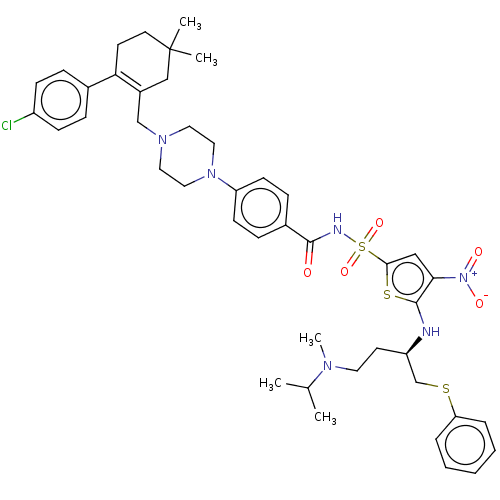 (US9156856, 9 | US9493431, 9) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.307 | -13.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185627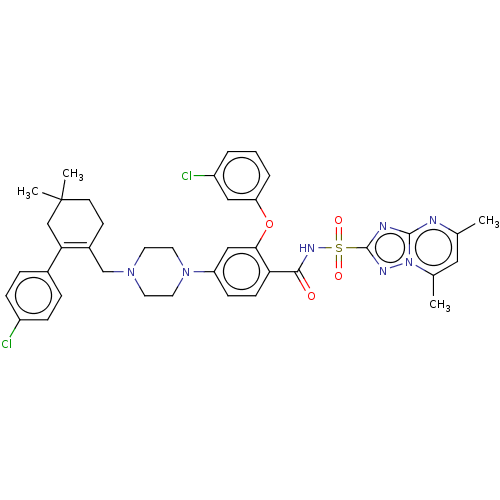 (US9156856, 40 | US9493431, 40) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.407 | -12.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185622 (US9156856, 33 | US9493431, 33) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.649 | -12.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185635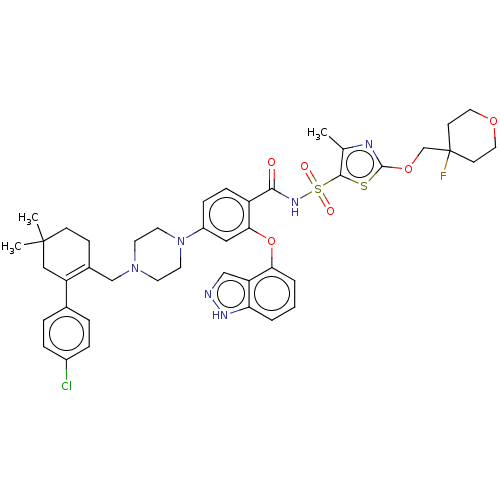 (US9156856, 48 | US9493431, 48) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.64 | -12.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185633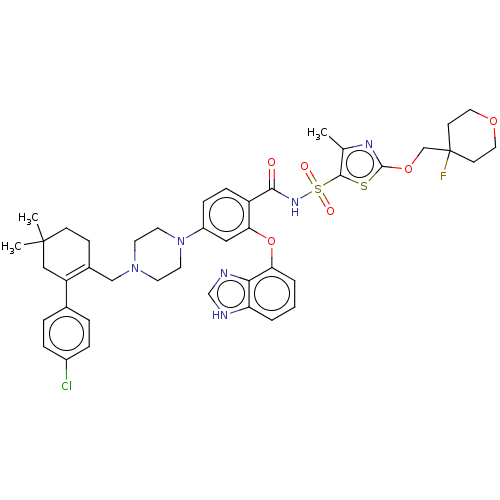 (US9156856, 46 | US9493431, 46) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.75 | -11.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185623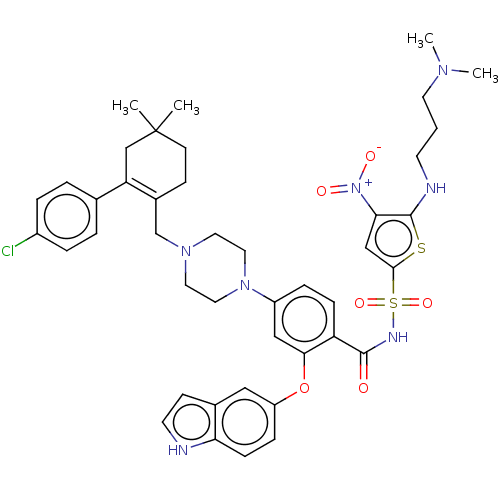 (US9156856, 34 | US9493431, 34) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.79 | -11.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185624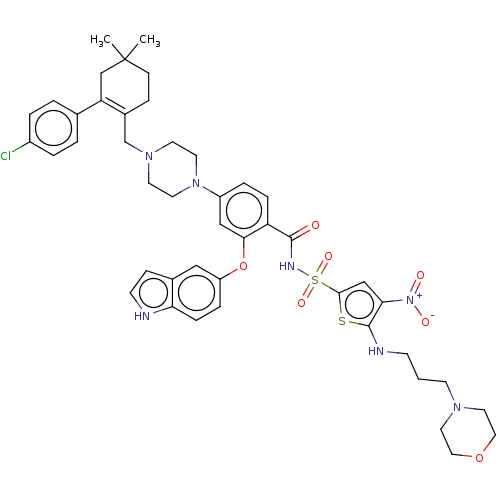 (US9156856, 35 | US9493431, 35) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.07 | -11.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185632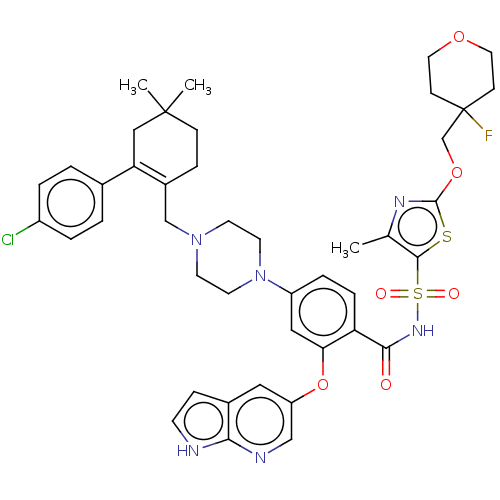 (US9156856, 45 | US9493431, 45) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.73 | -11.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185626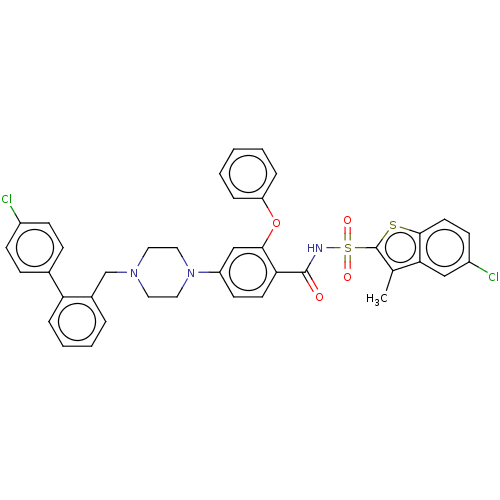 (US9156856, 39 | US9493431, 39) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20.2 | -10.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185634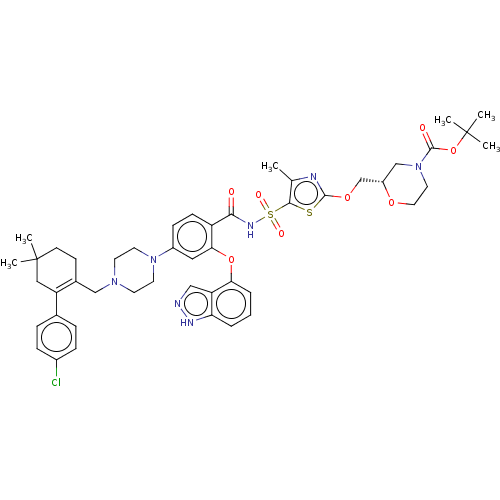 (US9156856, 47 | US9493431, 47) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 20.4 | -10.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185631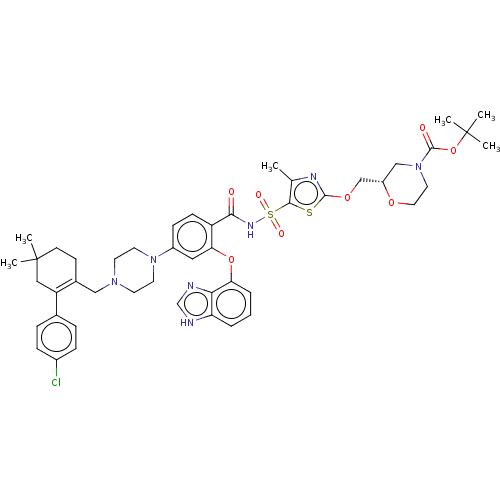 (US9156856, 44 | US9493431, 44) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 24.8 | -10.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185606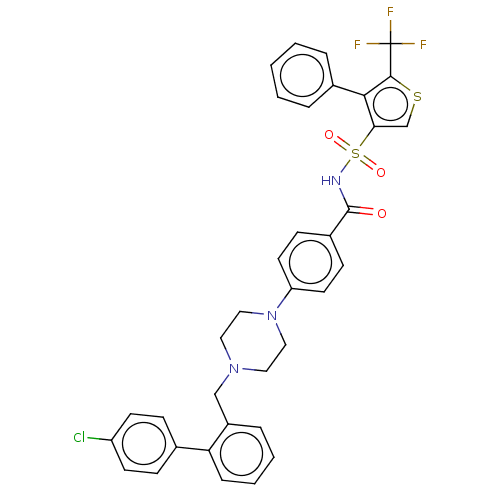 (US9156856, 16 | US9493431, 16) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.9 | -10.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185630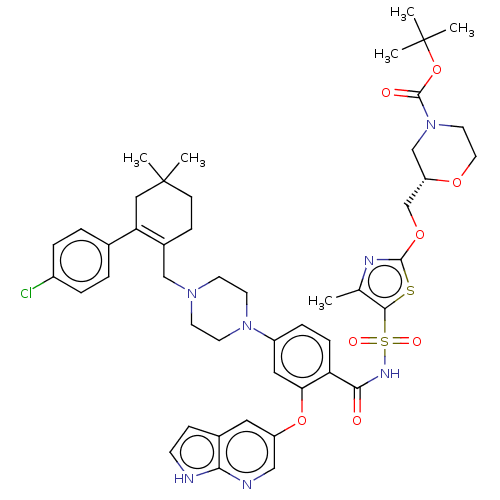 (US9156856, 43 | US9493431, 43) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 46.5 | -10.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185610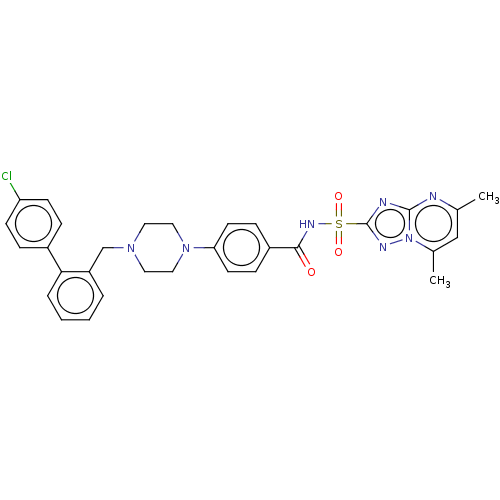 (US9156856, 20 | US9493431, 20) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 48.1 | -9.98 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876] (Homo sapiens (Human)) | BDBM256461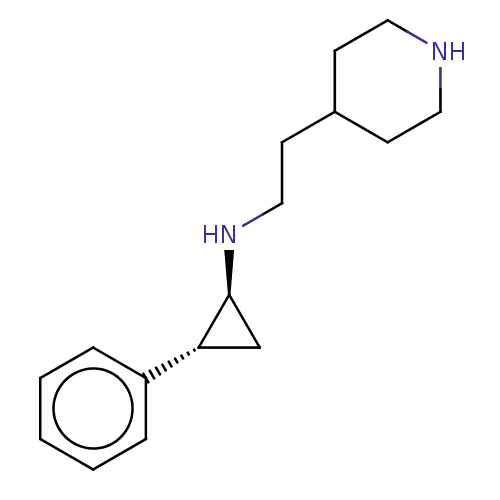 (US10329256, Example 6 | US9487512, 6 | US9944601, ...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 50 | -10.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Oryzon Genomics S.A. US Patent | Assay Description The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ... | US Patent US9487512 (2016) BindingDB Entry DOI: 10.7270/Q2765D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876] (Homo sapiens (Human)) | BDBM50142189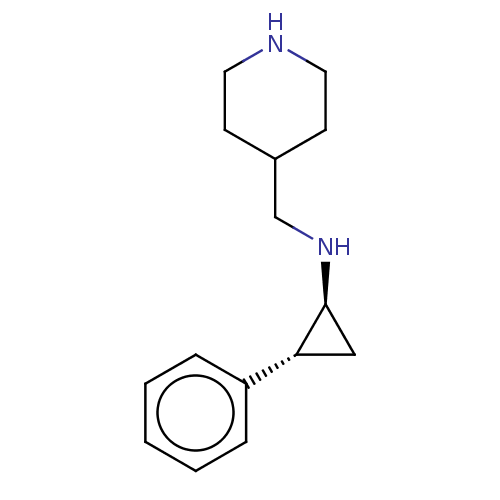 (CHEMBL3759239 | US10329256, Example 5 | US9487512,...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 50 | -10.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Oryzon Genomics S.A. US Patent | Assay Description The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ... | US Patent US9487512 (2016) BindingDB Entry DOI: 10.7270/Q2765D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876] (Homo sapiens (Human)) | BDBM256460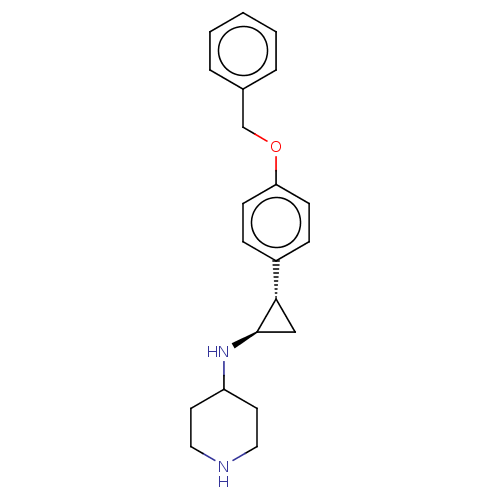 (US9487512, 4) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 50 | -10.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Oryzon Genomics S.A. US Patent | Assay Description The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ... | US Patent US9487512 (2016) BindingDB Entry DOI: 10.7270/Q2765D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876] (Homo sapiens (Human)) | BDBM256459 (US10329256, Example 3 | US9487512, 3 | US9944601, ...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 50 | -10.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Oryzon Genomics S.A. US Patent | Assay Description The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ... | US Patent US9487512 (2016) BindingDB Entry DOI: 10.7270/Q2765D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876] (Homo sapiens (Human)) | BDBM256457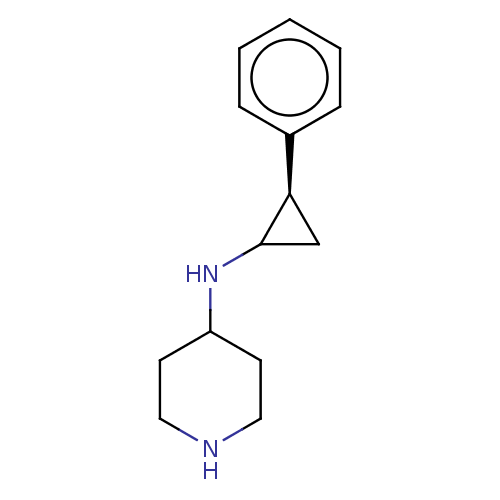 (US9487512, 1) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 50 | -10.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Oryzon Genomics S.A. US Patent | Assay Description The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ... | US Patent US9487512 (2016) BindingDB Entry DOI: 10.7270/Q2765D89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [158-852] (Homo sapiens (Human)) | BDBM254556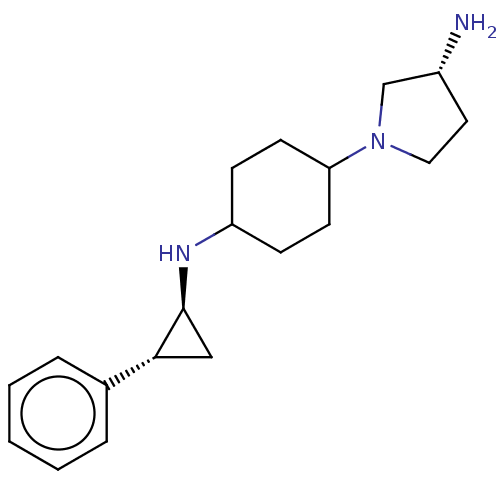 (US10214477, Example 15 | US9469597, 15 | US9670136...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 50 | -10.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Oryzon Genomics S.A. US Patent | Assay Description The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ... | US Patent US9469597 (2016) BindingDB Entry DOI: 10.7270/Q2ZW1JVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [158-852] (Homo sapiens (Human)) | BDBM254553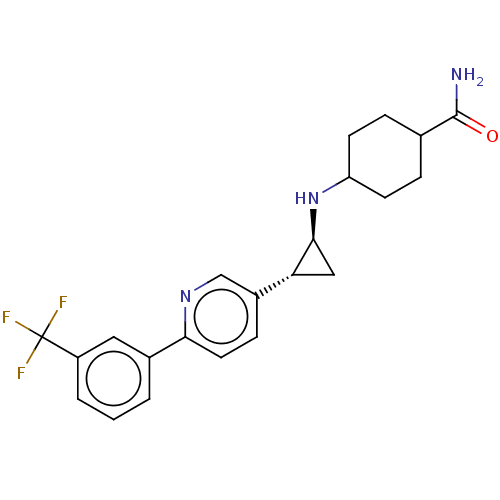 (US9469597, 12 | US9670136, 12 4-(((trans)-2-(6-(3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 50 | -10.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Oryzon Genomics S.A. US Patent | Assay Description The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ... | US Patent US9469597 (2016) BindingDB Entry DOI: 10.7270/Q2ZW1JVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [158-852] (Homo sapiens (Human)) | BDBM50155773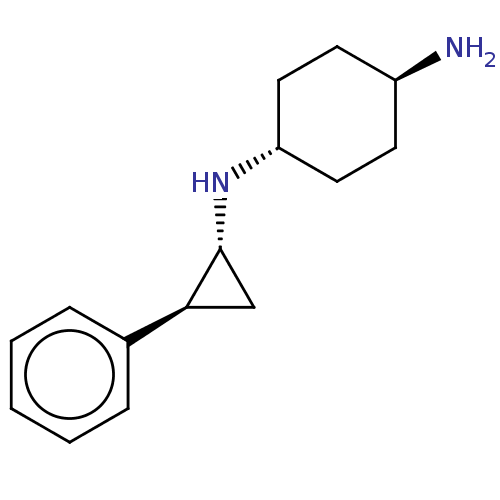 (CHEMBL3781751 | US9469597, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 50 | -10.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Oryzon Genomics S.A. US Patent | Assay Description The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ... | US Patent US9469597 (2016) BindingDB Entry DOI: 10.7270/Q2ZW1JVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [158-852] (Homo sapiens (Human)) | BDBM254546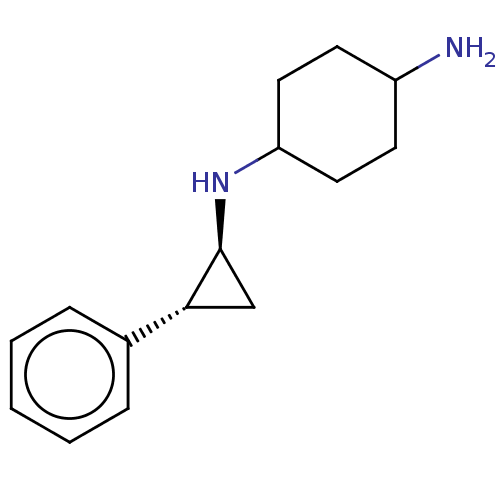 (US10214477, Example 3 | US9469597, 1 | US9670136, ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 50 | -10.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Oryzon Genomics S.A. US Patent | Assay Description The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ... | US Patent US9469597 (2016) BindingDB Entry DOI: 10.7270/Q2ZW1JVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [158-852] (Homo sapiens (Human)) | BDBM254603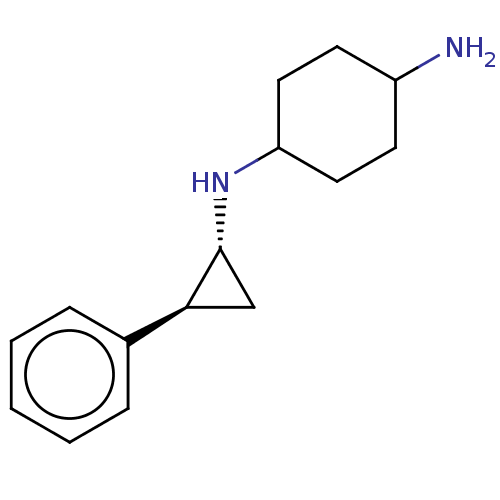 (US10214477, Example 5 | US9469597, 4 | US9670136, ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 50 | -10.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Oryzon Genomics S.A. US Patent | Assay Description The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ... | US Patent US9469597 (2016) BindingDB Entry DOI: 10.7270/Q2ZW1JVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185592 (US9156856, 1 | US9493431, 1) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 51.4 | -9.94 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185594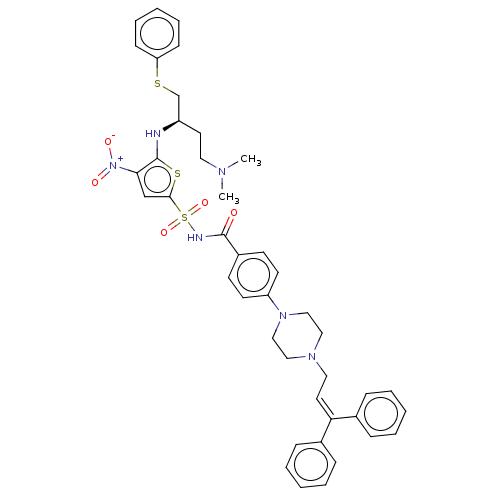 (US9156856, 3 | US9493431, 3) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 51.7 | -9.93 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185593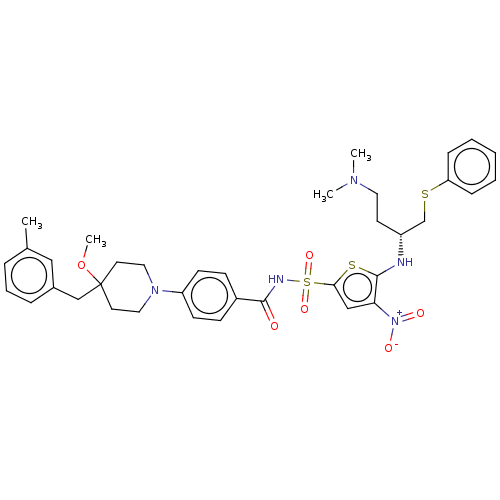 (US9156856, 2 | US9493431, 2) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 82.9 | -9.65 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50043760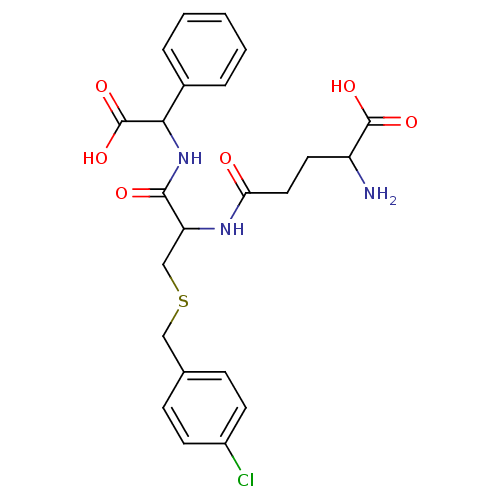 (2-Amino-4-[1-[(carboxy-phenyl-methyl)-carbamoyl]-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185608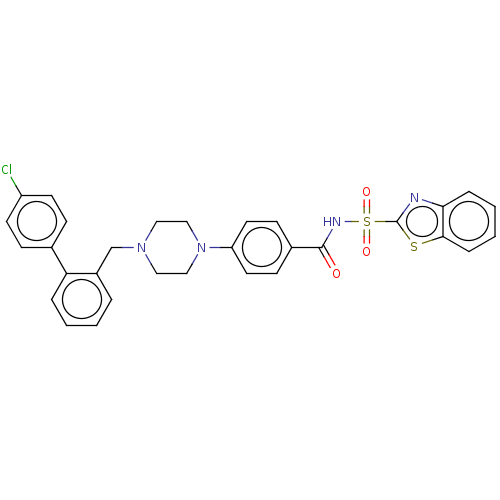 (US9156856, 18 | US9493431, 18) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 129 | -9.39 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185620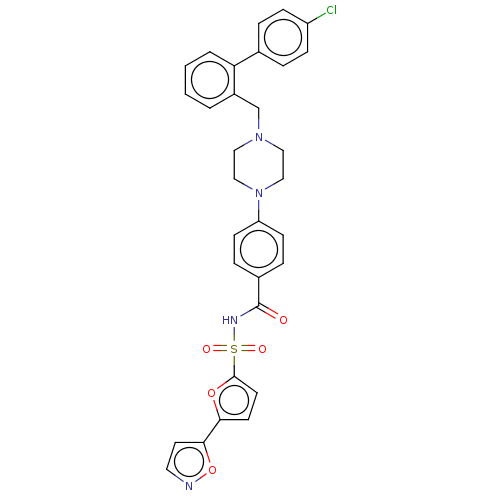 (US9156856, 31 | US9493431, 31) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 139 | -9.35 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50562986 (CHEMBL4757438) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of GSTP1-1 (unknown origin) assessed as inhibition constant using reduced GSH and CDNB as substrate by spectrophotometric m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02048 BindingDB Entry DOI: 10.7270/Q27948D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185595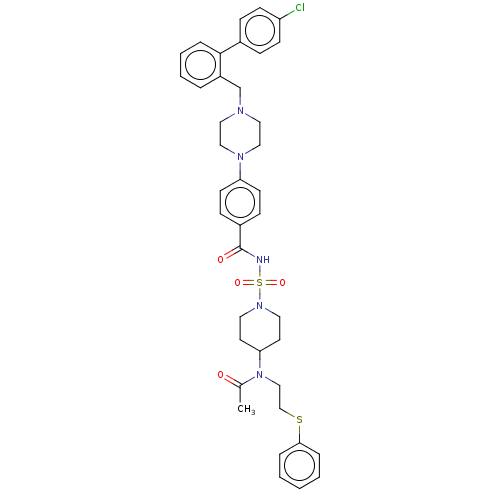 (US9156856, 5 | US9493431, 5) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 176 | -9.21 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185601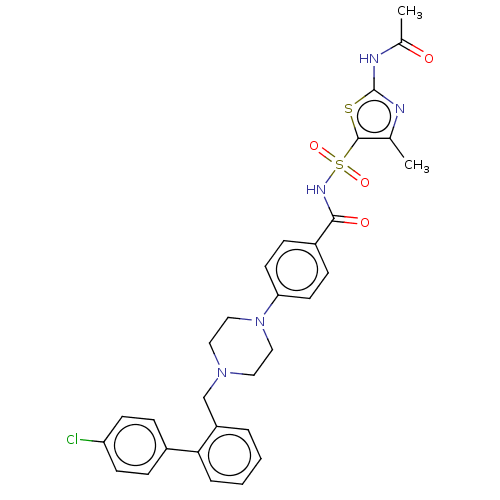 (US9156856, 11 | US9493431, 11) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 177 | -9.20 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185611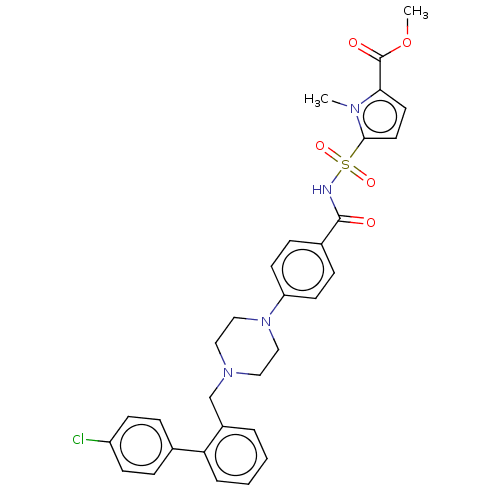 (US9156856, 22 | US9493431, 22) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 188 | -9.17 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185609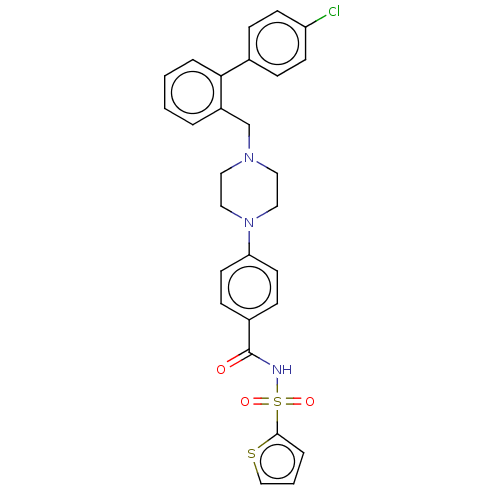 (US9156856, 19 | US9493431, 19) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 234 | -9.04 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185619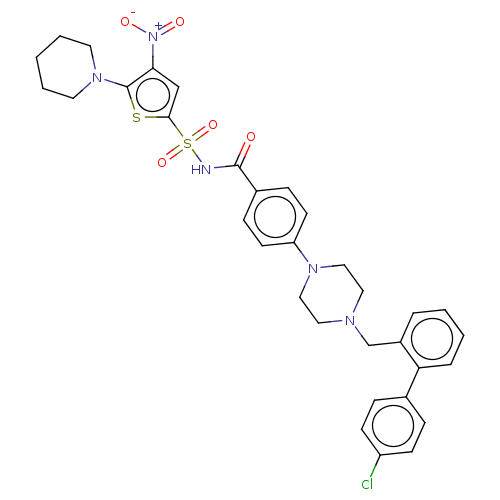 (US9156856, 30 | US9493431, 30) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 269 | -8.96 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185605 (US9156856, 15 | US9493431, 15) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 282 | -8.93 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185625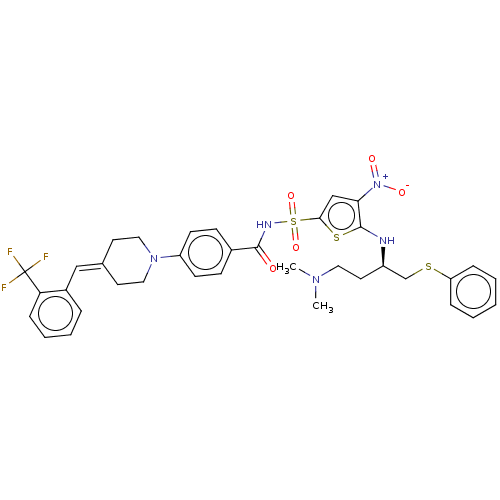 (US9156856, 36 | US9493431, 36) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 294 | -8.90 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185618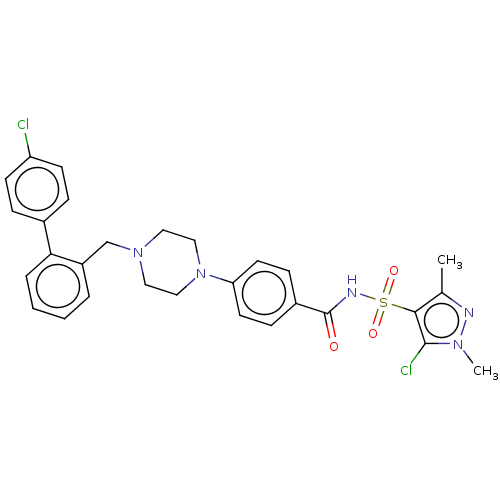 (US9156856, 29 | US9493431, 29) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 340 | -8.82 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185613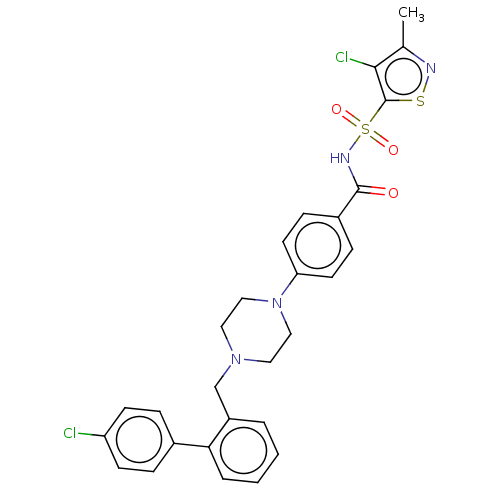 (US9156856, 24 | US9493431, 24) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 342 | -8.81 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185607 (US9156856, 17 | US9493431, 17) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 356 | -8.79 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185614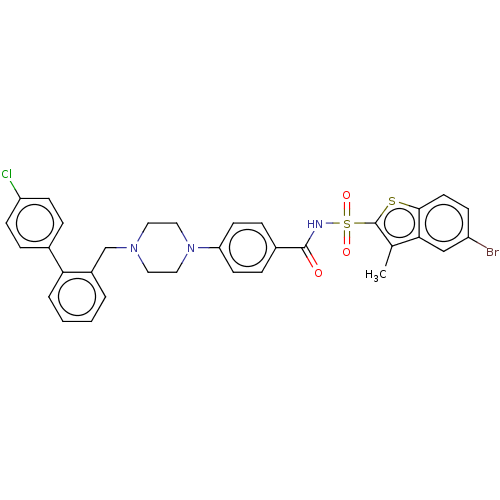 (US9156856, 25 | US9493431, 25) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 372 | -8.76 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185603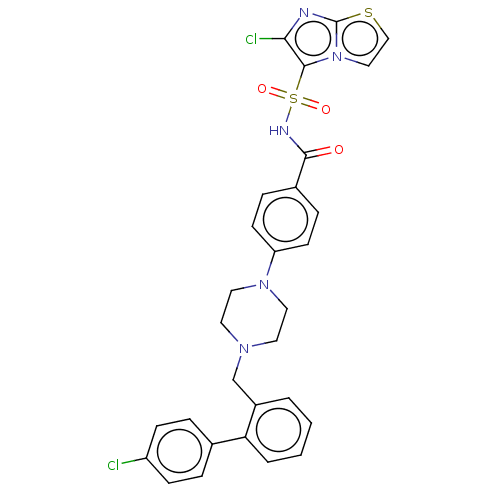 (US9156856, 13 | US9493431, 13) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 388 | -8.74 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185602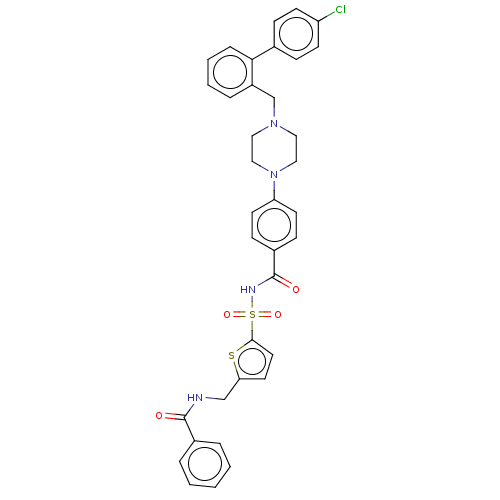 (US9156856, 12 | US9493431, 12) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 393 | -8.73 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185615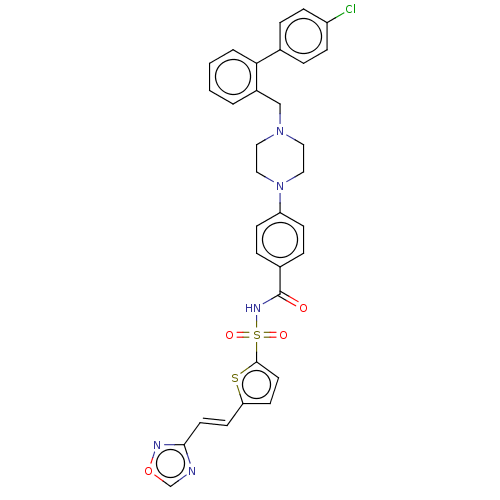 (US9156856, 26 | US9493431, 26) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 393 | -8.73 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2/Glutathione S-transferase P (Homo sapiens (Human)) | BDBM185604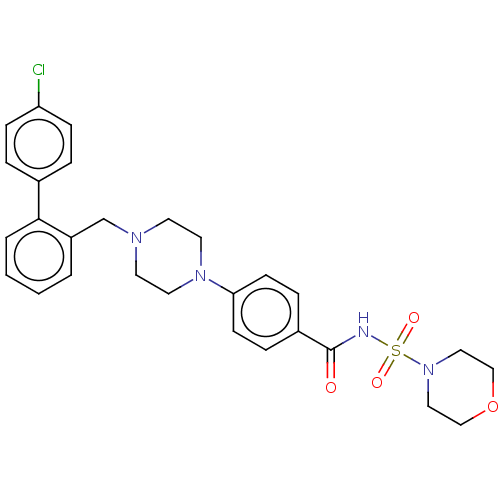 (US9156856, 14 | US9493431, 14) | PDB UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 409 | -8.71 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9493431 (2016) BindingDB Entry DOI: 10.7270/Q2RB73HH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 79 total ) | Next | Last >> |