

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50188776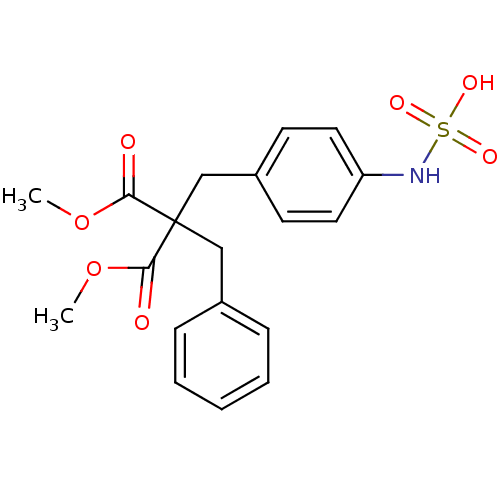 (4-(2-benzyl-3-methoxy-2-(methoxycarbonyl)-3-oxopro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition HPTPA | Bioorg Med Chem Lett 16: 4252-6 (2006) Article DOI: 10.1016/j.bmcl.2006.05.074 BindingDB Entry DOI: 10.7270/Q2D50MK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326559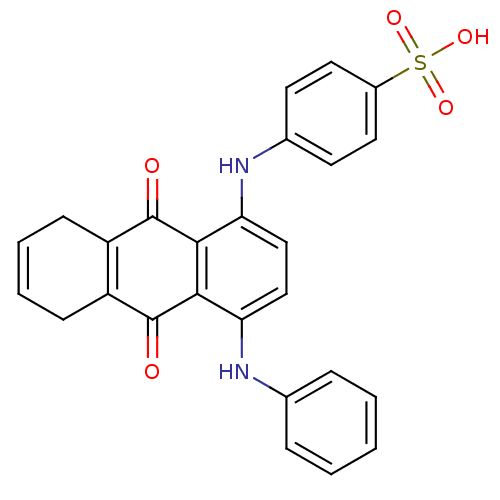 (4-(9,10-dioxo-4-(phenylamino)-5,8,9,10-tetrahydroa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326562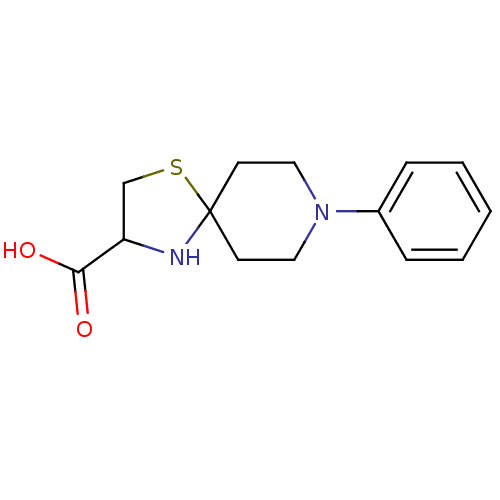 (8-phenyl-1-thia-4,8-diazaspiro[4.5]decane-3-carbox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326563 (2-amino-3-(3,5-dibromo-4-hydroxyphenyl)propanoic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326561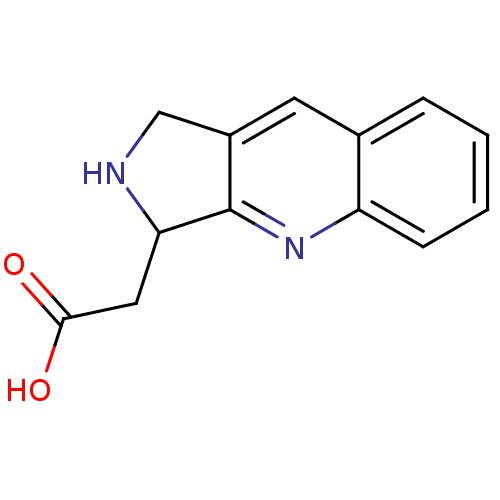 (2-(2,3-dihydro-1H-pyrrolo[3,4-b]quinolin-3-yl)acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326559 (4-(9,10-dioxo-4-(phenylamino)-5,8,9,10-tetrahydroa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326557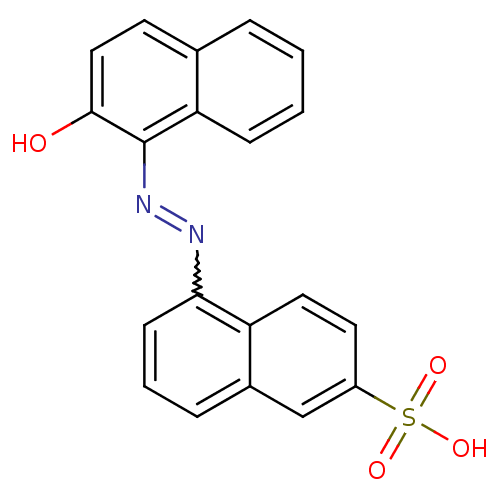 ((E)-5-((2-hydroxynaphthalen-1-yl)diazenyl)naphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326557 ((E)-5-((2-hydroxynaphthalen-1-yl)diazenyl)naphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326561 (2-(2,3-dihydro-1H-pyrrolo[3,4-b]quinolin-3-yl)acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50607111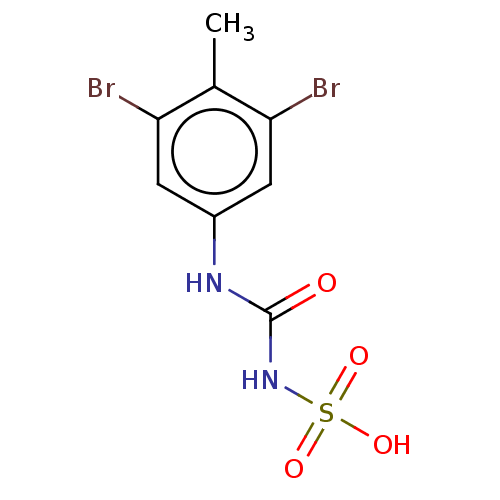 (CHEMBL5218807) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01143 BindingDB Entry DOI: 10.7270/Q2BK1HGF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50425808 (CHEMBL2316906) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPalpha (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50425807 (CHEMBL2316902) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPalpha (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50425806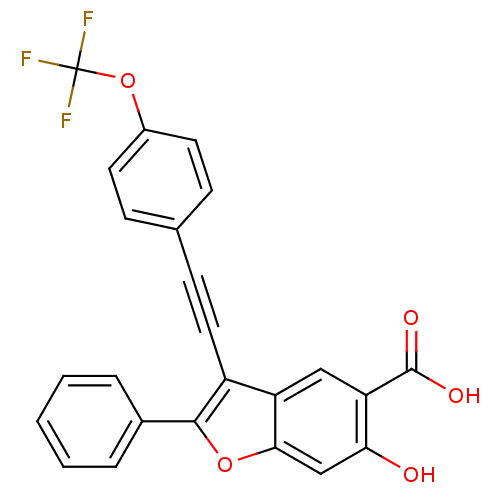 (CHEMBL2316907) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPalpha (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326560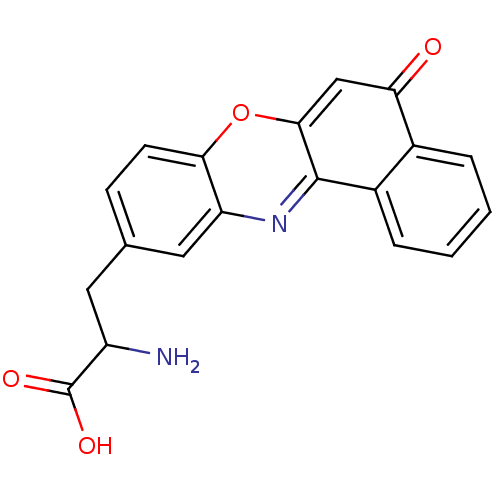 (2-amino-3-(5-oxo-5H-benzo[a]phenoxazin-10-yl)propa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50079855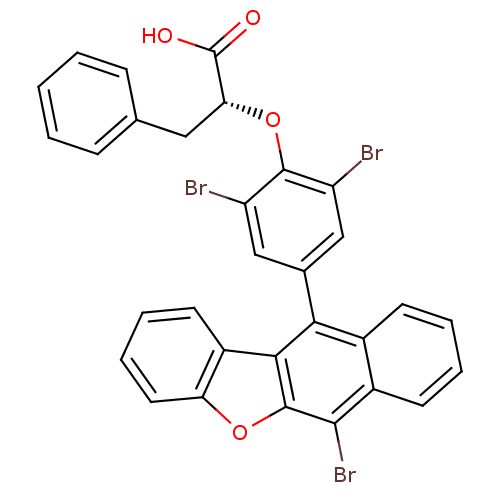 ((R)-2-[2,6-Dibromo-4-(6-bromo-benzo[b]naphtho[2,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1 alpha (LRP) (human PTPases.) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50135663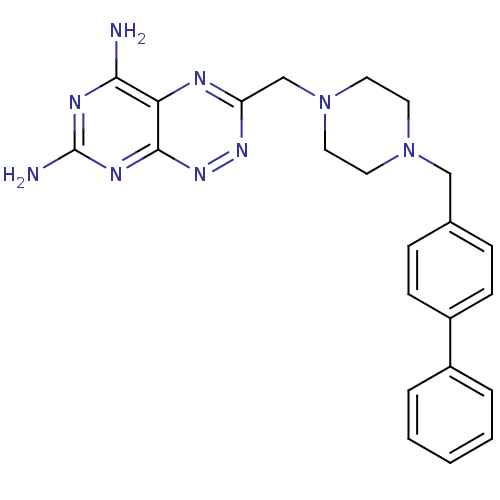 (3-(4-Biphenyl-4-ylmethyl-piperazin-1-ylmethyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory concentration required against Protein-tyrosine phosphatase alpha in the presence of 300 nM DTT | Bioorg Med Chem Lett 13: 2895-8 (2003) BindingDB Entry DOI: 10.7270/Q21V5DDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50079856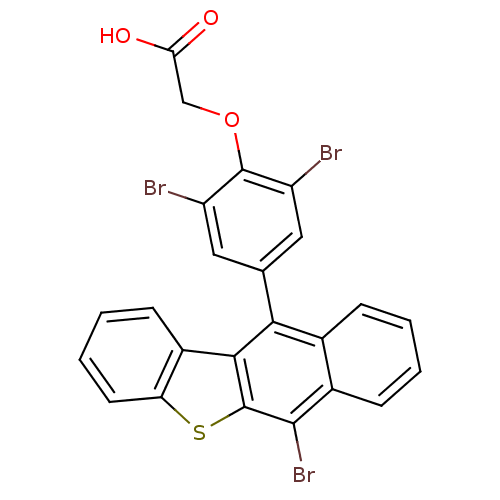 (CHEMBL108721 | [2,6-Dibromo-4-(6-bromo-benzo[b]nap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description The compound was tested in vitro for the inhibitory activity against Protein-tyrosine phosphatase 1 alpha (LRP) (human PTPases.) | J Med Chem 42: 3199-202 (1999) Article DOI: 10.1021/jm990260v BindingDB Entry DOI: 10.7270/Q2DF6QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50135668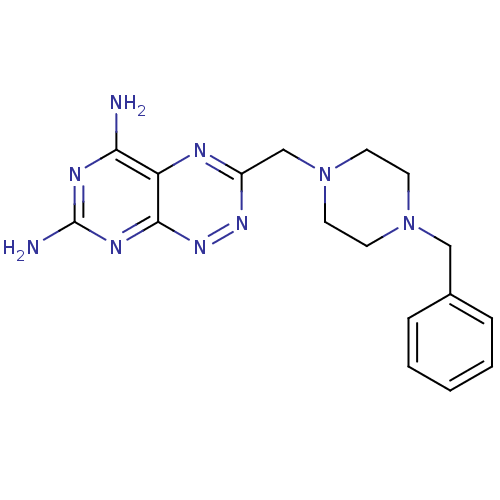 (3-(4-Benzyl-piperazin-1-ylmethyl)-pyrimido[5,4-e][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory concentration required against Protein-tyrosine phosphatase alpha in the presence of 300 nM DTT | Bioorg Med Chem Lett 13: 2895-8 (2003) BindingDB Entry DOI: 10.7270/Q21V5DDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50135673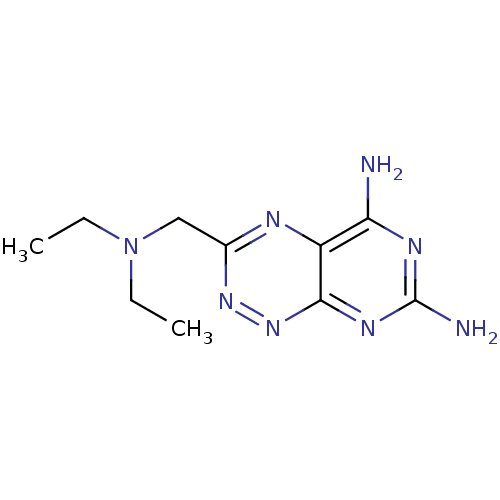 (3-Diethylaminomethyl-pyrimido[5,4-e][1,2,4]triazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory concentration required against Protein-tyrosine phosphatase alpha in the presence of 300 nM DTT | Bioorg Med Chem Lett 13: 2895-8 (2003) BindingDB Entry DOI: 10.7270/Q21V5DDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50409191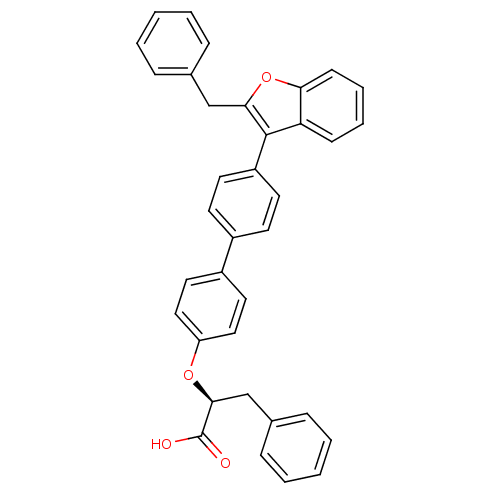 (CHEMBL365490) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326558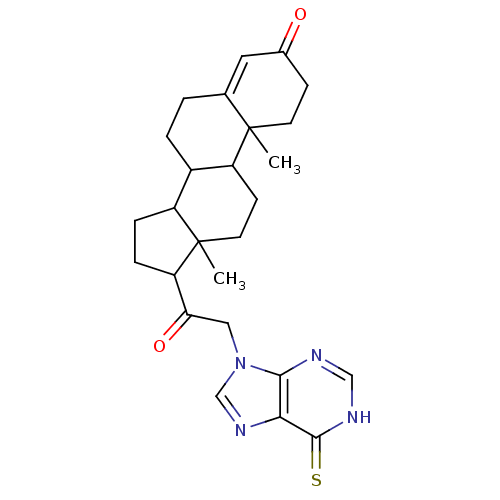 (10,13-dimethyl-17-(2-(6-thioxo-3H-purin-9(6H)-yl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50246745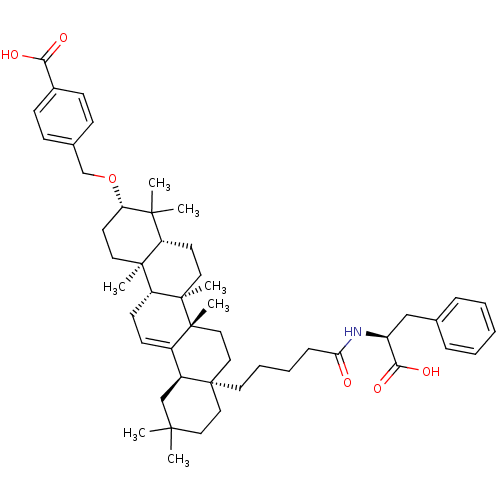 (3-(4-Carboxy-benzyloxy)-28-[4-butyric ((S)-1-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPalpha | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50079577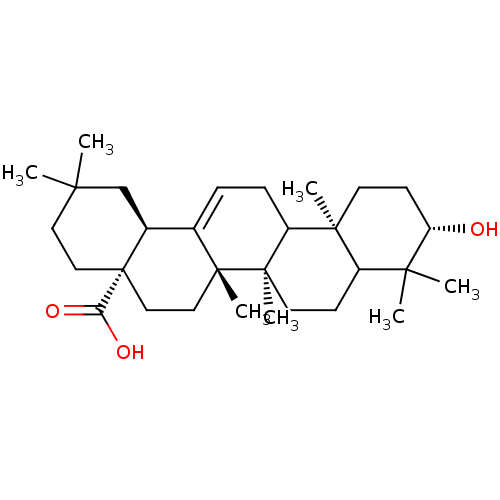 ((4aS,6aS,6bR,10S,12aR,14bS)-10-Hydroxy-2,2,6a,6b,9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPalpha | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50246744 ((4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-(4-carboxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPalpha | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50194130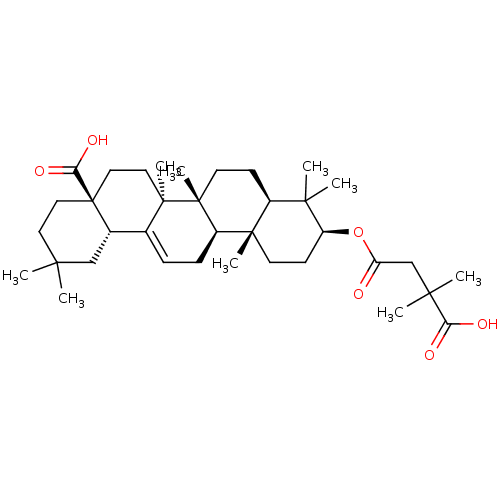 ((4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-(3-carboxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPalpha | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50246668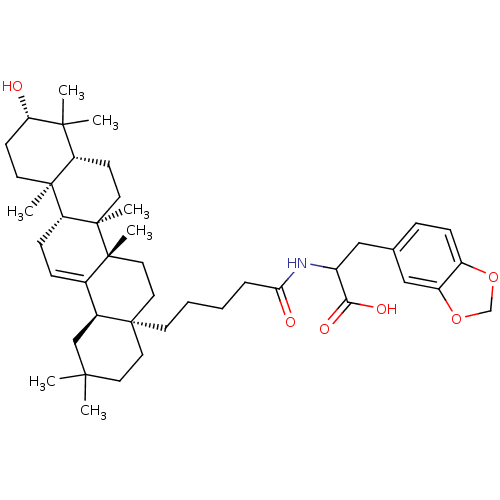 (3-(benzo[d][1,3]dioxol-5-yl)-2-(5-((4aR,6aS,6bR,8a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPalpha | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50246653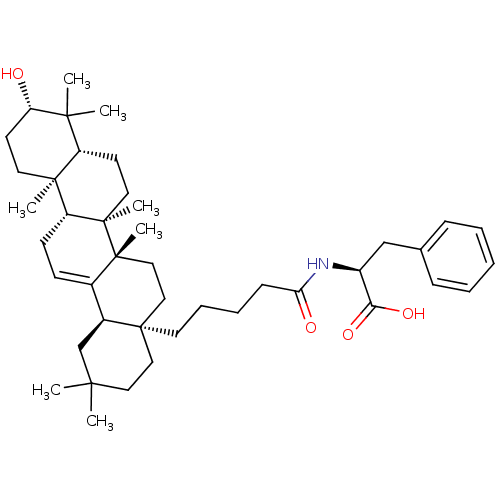 ((S)-2-(5-((4aR,6aS,6bR,8aR,10S,12aR,12bR,14bR)-10-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPalpha | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50246621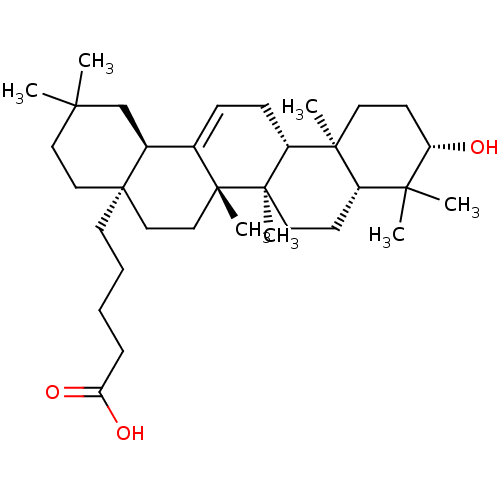 (5-((4aR,6aS,6bR,8aR,10S,12aR,12bR,14bR)-10-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPalpha | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50246620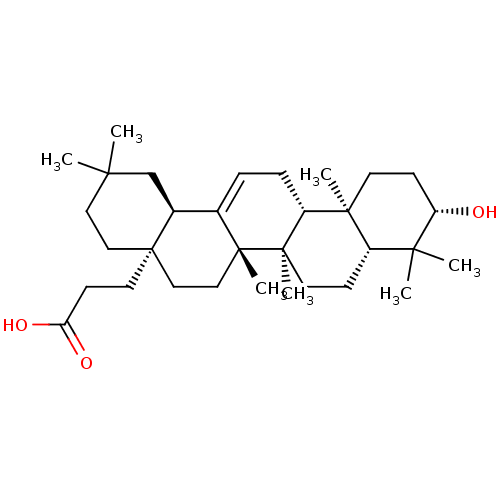 (3-((4aR,6aS,6bR,8aR,10S,12aR,12bR,14bR)-10-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPalpha | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50246580 ((S)-2-((4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-10-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPalpha | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPalpha | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50222205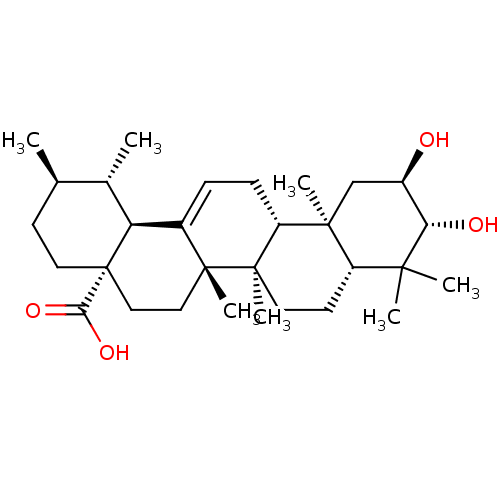 ((1S,2R,4aS,6aS,6bR,8aR,10R,11R,12aR,12bR,14bS)-10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTPalpha | Bioorg Med Chem 16: 8697-705 (2008) Article DOI: 10.1016/j.bmc.2008.07.080 BindingDB Entry DOI: 10.7270/Q2HX1CH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50343147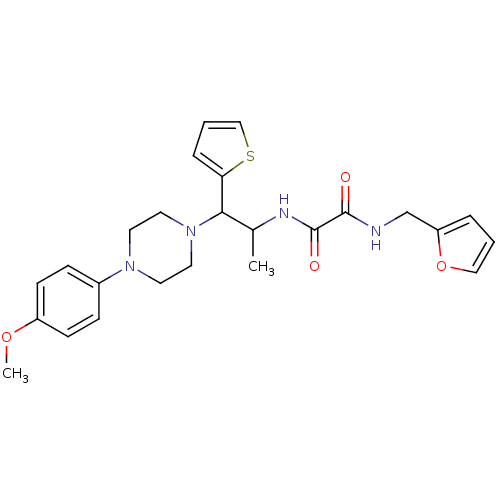 (CHEMBL1773180 | N1-(furan-2-ylmethyl)-N2-(1-(4-(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human PTPalpha expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50343142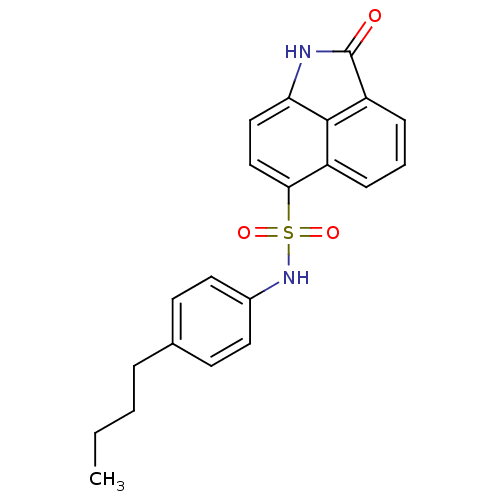 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human PTPalpha expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50308158 (3-(1-(3-(Biphenyl-4-ylamino)-3-oxopropyl)-1H-1,2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPalpha expressed in Escherichia coli assessed as inhibition of p-nitrophenyl phosphate hydrolysis at pH 7 by spectrophotometry | J Med Chem 53: 2482-93 (2010) Article DOI: 10.1021/jm901645u BindingDB Entry DOI: 10.7270/Q2639PVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50112356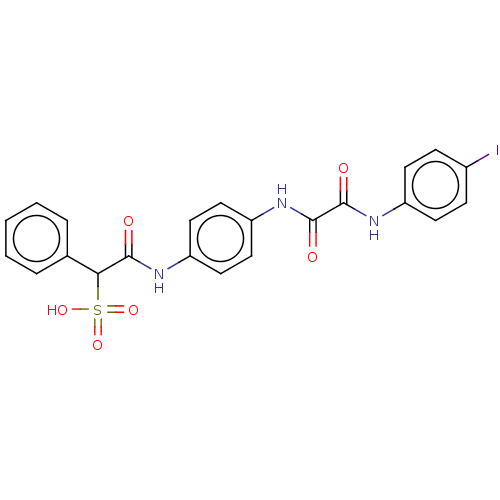 (CHEMBL3609373) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPalpha using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50112358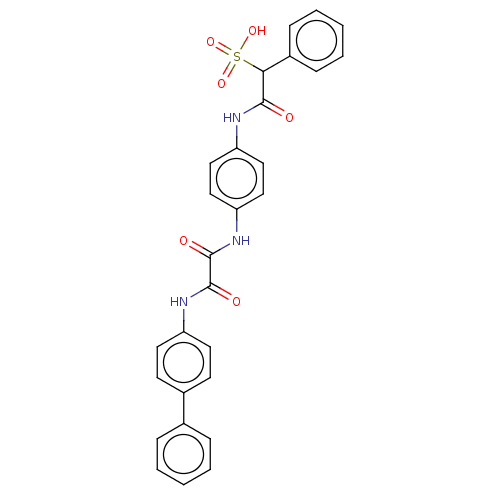 (CHEMBL3609374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPalpha using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50112357 (CHEMBL3609375) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human PTPalpha using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50343148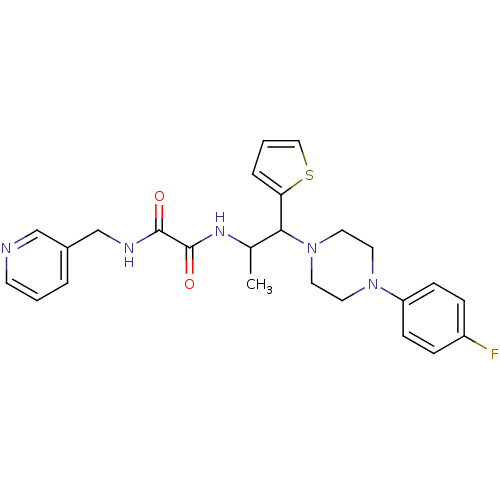 (CHEMBL1773181 | N1-(1-(4-(4-fluorophenyl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human PTPalpha expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326564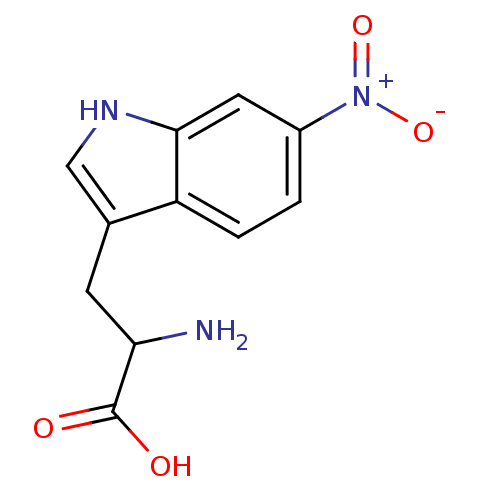 (2-amino-3-(6-nitro-1H-indol-3-yl)propanoic acid | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326565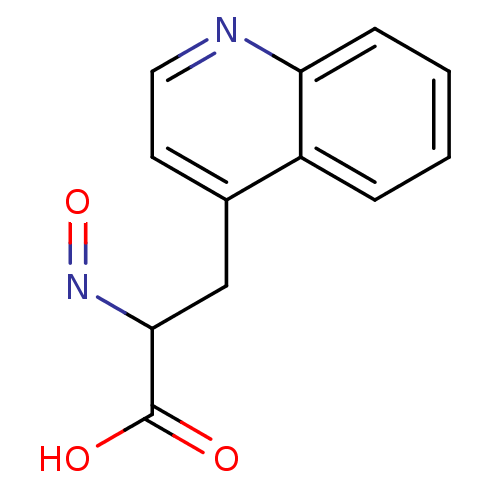 (2-(hydroxyimino)-3-(quinolin-4-yl)propanoic acid |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326566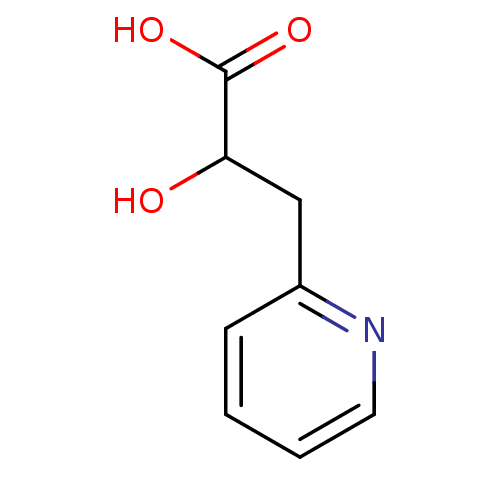 (2-hydroxy-3-(pyridin-2-yl)propanoic acid | CHEMBL1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326558 (10,13-dimethyl-17-(2-(6-thioxo-3H-purin-9(6H)-yl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30E+4 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50558461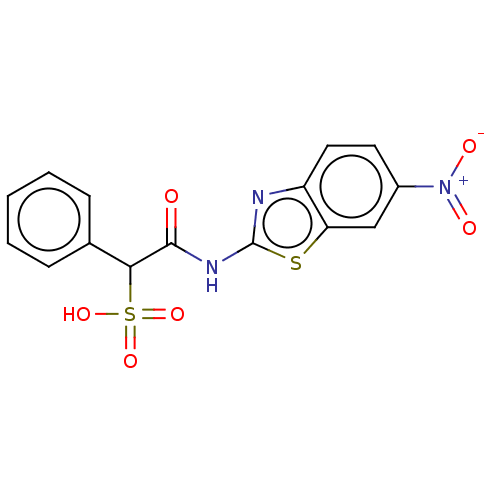 (CHEMBL4760367) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPalpha (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50054344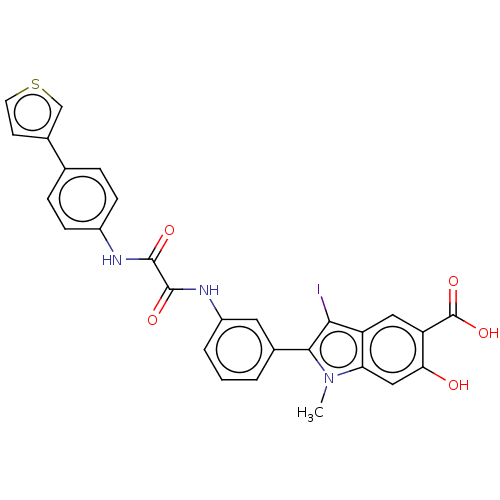 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description For selectivity studies, the PTPs, including LYP, mPTPA, SHP1-D1C, PTP1B, LMPTP, VHR, Laforin and PTPα-D1D2 were expressed and purified from E. ... | US Patent US9522881 (2016) BindingDB Entry DOI: 10.7270/Q2DN4402 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50054344 (CHEMBL3319356 | US9522881, 11a-1 L97M74 | US984453...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPalpha (unknown origin) | J Med Chem 57: 6594-609 (2014) Article DOI: 10.1021/jm5006176 BindingDB Entry DOI: 10.7270/Q24X59FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50607110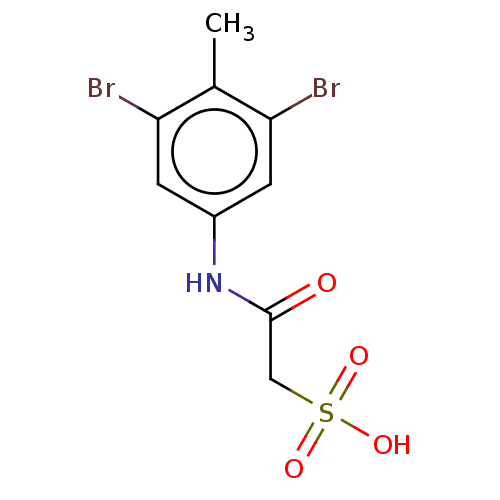 (CHEMBL5219519) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01143 BindingDB Entry DOI: 10.7270/Q2BK1HGF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50558485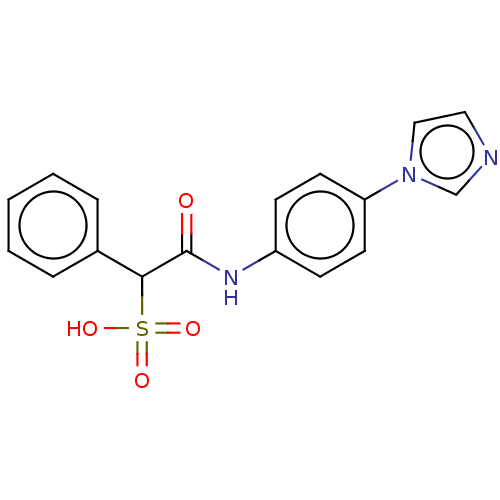 (CHEMBL4800195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPalpha (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326560 (2-amino-3-(5-oxo-5H-benzo[a]phenoxazin-10-yl)propa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+5 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50558488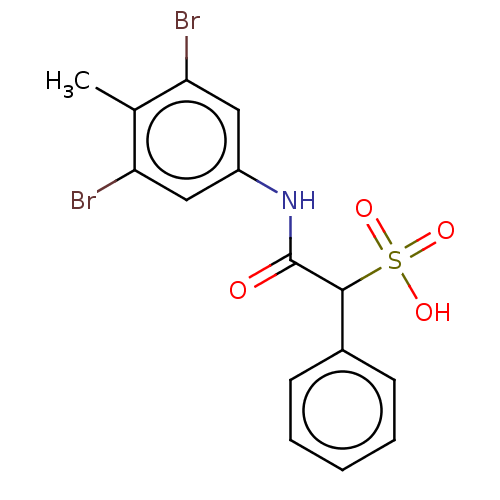 (CHEMBL4794972) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTPalpha (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 59 total ) | Next | Last >> |