 Found 42 hits of ic50 for UniProtKB: P14646
Found 42 hits of ic50 for UniProtKB: P14646 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50017294
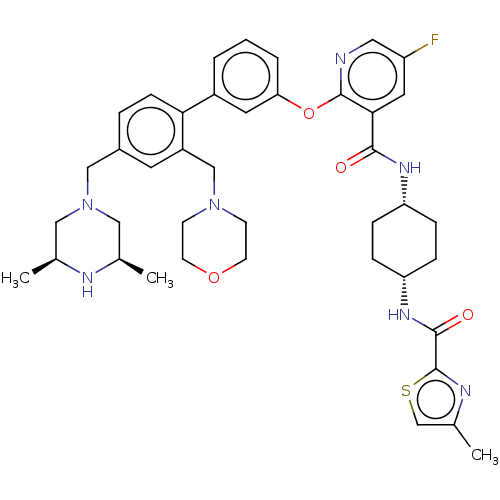
(CHEMBL3288029)Show SMILES C[C@H]1CN(Cc2ccc(c(CN3CCOCC3)c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3nc(C)cs3)c2)C[C@@H](C)N1 |r,wU:37.43,34.36,1.0,51.57,(20.55,-53.47,;19.22,-52.7,;17.9,-53.47,;16.57,-52.7,;15.24,-53.48,;13.91,-52.72,;12.58,-53.49,;11.24,-52.73,;11.24,-51.19,;12.56,-50.4,;12.55,-48.86,;13.88,-48.09,;15.21,-48.85,;16.54,-48.08,;16.53,-46.54,;15.2,-45.78,;13.86,-46.55,;13.9,-51.17,;9.9,-50.42,;8.56,-51.21,;7.23,-50.43,;7.22,-48.89,;8.56,-48.13,;8.56,-46.58,;7.22,-45.81,;5.88,-46.59,;4.54,-45.81,;4.54,-44.27,;3.21,-43.5,;5.88,-43.5,;7.22,-44.26,;8.55,-43.49,;8.54,-41.94,;9.88,-44.25,;11.22,-43.48,;12.56,-44.25,;13.89,-43.48,;13.89,-41.94,;12.55,-41.16,;11.21,-41.94,;15.23,-41.16,;16.57,-41.94,;16.57,-43.48,;17.78,-40.99,;19.26,-41.44,;20.12,-40.16,;21.66,-40.11,;19.18,-38.94,;17.73,-39.47,;9.89,-48.88,;16.55,-51.17,;17.88,-50.4,;17.88,-48.87,;19.22,-51.17,)| Show InChI InChI=1S/C41H50FN7O4S/c1-26-21-49(22-27(2)44-26)23-29-7-12-36(31(17-29)24-48-13-15-52-16-14-48)30-5-4-6-35(18-30)53-40-37(19-32(42)20-43-40)38(50)46-33-8-10-34(11-9-33)47-39(51)41-45-28(3)25-54-41/h4-7,12,17-20,25-27,33-34,44H,8-11,13-16,21-24H2,1-3H3,(H,46,50)(H,47,51)/t26-,27+,33-,34+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of rat PDE4B |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50017295
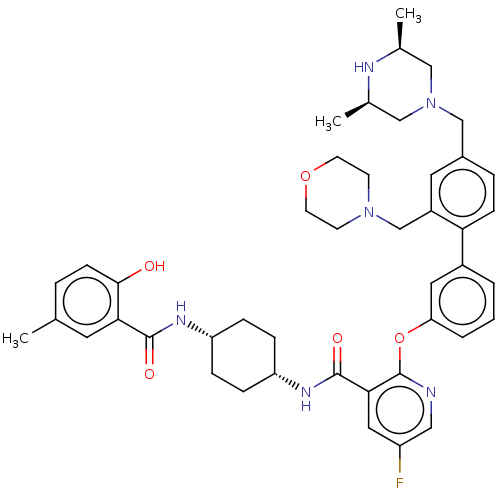
(CHEMBL3288030)Show SMILES C[C@H]1CN(Cc2ccc(c(CN3CCOCC3)c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)ccc3O)c2)C[C@@H](C)N1 |r,wU:37.43,34.36,53.59,1.0,(46.59,-30.6,;45.26,-29.83,;43.94,-30.6,;42.61,-29.83,;41.28,-30.6,;39.95,-29.84,;38.61,-30.62,;37.28,-29.86,;37.27,-28.31,;38.6,-27.53,;38.58,-25.99,;39.91,-25.21,;41.23,-25.98,;42.56,-25.21,;42.55,-23.67,;41.21,-22.91,;39.88,-23.68,;39.93,-28.29,;35.93,-27.55,;34.59,-28.33,;33.26,-27.56,;33.25,-26.02,;34.59,-25.25,;34.59,-23.7,;33.25,-22.93,;31.91,-23.71,;30.57,-22.93,;30.57,-21.38,;29.23,-20.62,;31.91,-20.61,;33.25,-21.38,;34.58,-20.6,;34.57,-19.06,;35.92,-21.37,;37.25,-20.59,;38.59,-21.37,;39.92,-20.6,;39.93,-19.05,;38.59,-18.28,;37.25,-19.06,;41.27,-18.28,;42.61,-19.05,;42.61,-20.6,;43.94,-18.29,;43.93,-16.76,;45.26,-15.99,;45.25,-14.45,;46.6,-16.76,;46.59,-18.29,;45.26,-19.06,;45.26,-20.6,;35.92,-26.01,;42.59,-28.3,;43.93,-27.53,;43.92,-25.99,;45.26,-28.29,)| Show InChI InChI=1S/C44H53FN6O5/c1-28-7-14-41(52)39(19-28)42(53)48-35-9-11-36(12-10-35)49-43(54)40-22-34(45)23-46-44(40)56-37-6-4-5-32(21-37)38-13-8-31(26-51-24-29(2)47-30(3)25-51)20-33(38)27-50-15-17-55-18-16-50/h4-8,13-14,19-23,29-30,35-36,47,52H,9-12,15-18,24-27H2,1-3H3,(H,48,53)(H,49,54)/t29-,30+,35-,36+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of rat PDE4B |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM14361
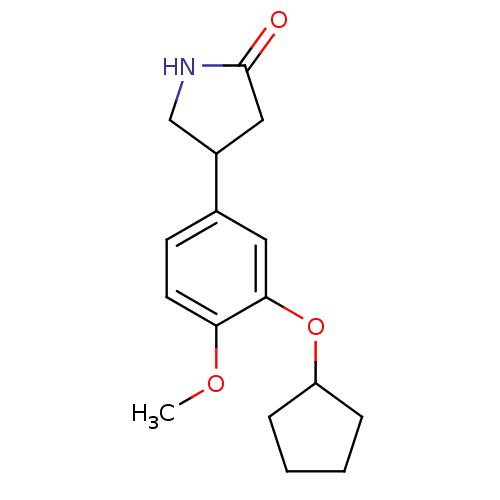
((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase (PDE) 4B |
Bioorg Med Chem Lett 8: 3229-34 (1999)
BindingDB Entry DOI: 10.7270/Q2ZS2Z11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50450772

(CHEMBL322092)Show SMILES COc1cccc(COc2cc(cc(c2)C(O)=O)N2CC(CC2=O)c2ccc(OC)c(OC3CCCC3)c2)c1 Show InChI InChI=1S/C31H33NO7/c1-36-26-9-5-6-20(12-26)19-38-27-14-22(31(34)35)13-24(17-27)32-18-23(16-30(32)33)21-10-11-28(37-2)29(15-21)39-25-7-3-4-8-25/h5-6,9-15,17,23,25H,3-4,7-8,16,18-19H2,1-2H3,(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase (PDE) 4B |
Bioorg Med Chem Lett 8: 3229-34 (1999)
BindingDB Entry DOI: 10.7270/Q2ZS2Z11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50450771
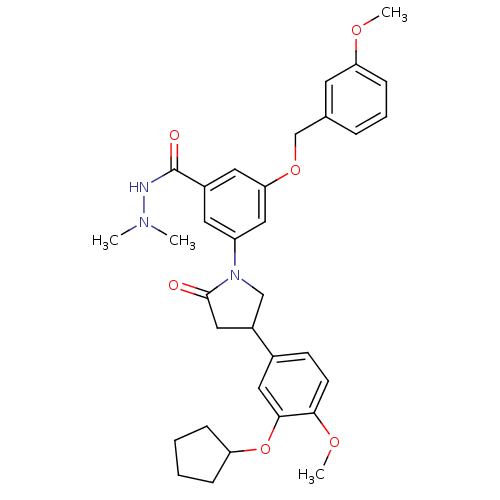
(CHEMBL326655)Show SMILES COc1cccc(COc2cc(cc(c2)C(=O)NN(C)C)N2CC(CC2=O)c2ccc(OC)c(OC3CCCC3)c2)c1 Show InChI InChI=1S/C33H39N3O6/c1-35(2)34-33(38)24-15-26(19-29(16-24)41-21-22-8-7-11-28(14-22)39-3)36-20-25(18-32(36)37)23-12-13-30(40-4)31(17-23)42-27-9-5-6-10-27/h7-8,11-17,19,25,27H,5-6,9-10,18,20-21H2,1-4H3,(H,34,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase (PDE) 4B |
Bioorg Med Chem Lett 8: 3229-34 (1999)
BindingDB Entry DOI: 10.7270/Q2ZS2Z11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50450770
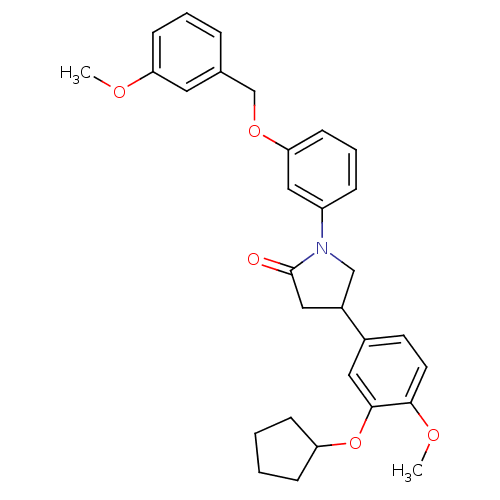
(CHEMBL320307)Show SMILES COc1cccc(COc2cccc(c2)N2CC(CC2=O)c2ccc(OC)c(OC3CCCC3)c2)c1 Show InChI InChI=1S/C30H33NO5/c1-33-26-11-5-7-21(15-26)20-35-27-12-6-8-24(18-27)31-19-23(17-30(31)32)22-13-14-28(34-2)29(16-22)36-25-9-3-4-10-25/h5-8,11-16,18,23,25H,3-4,9-10,17,19-20H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Center
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase (PDE) 4B |
Bioorg Med Chem Lett 8: 3229-34 (1999)
BindingDB Entry DOI: 10.7270/Q2ZS2Z11 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072197
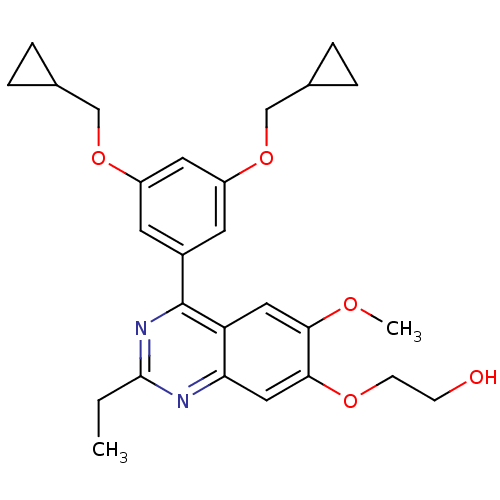
(2-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-2-ethyl-6...)Show SMILES CCc1nc(-c2cc(OCC3CC3)cc(OCC3CC3)c2)c2cc(OC)c(OCCO)cc2n1 Show InChI InChI=1S/C27H32N2O5/c1-3-26-28-23-14-25(32-9-8-30)24(31-2)13-22(23)27(29-26)19-10-20(33-15-17-4-5-17)12-21(11-19)34-16-18-6-7-18/h10-14,17-18,30H,3-9,15-16H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085138
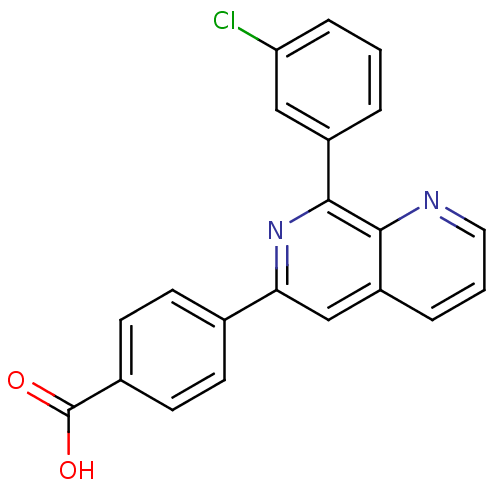
(4-[8-(3-Chloro-phenyl)-[1,7]naphthyridin-6-yl]-ben...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C21H13ClN2O2/c22-17-5-1-3-15(11-17)20-19-16(4-2-10-23-19)12-18(24-20)13-6-8-14(9-7-13)21(25)26/h1-12H,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085135
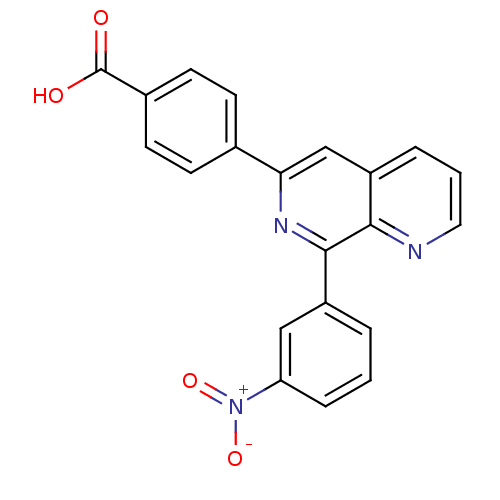
(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072195
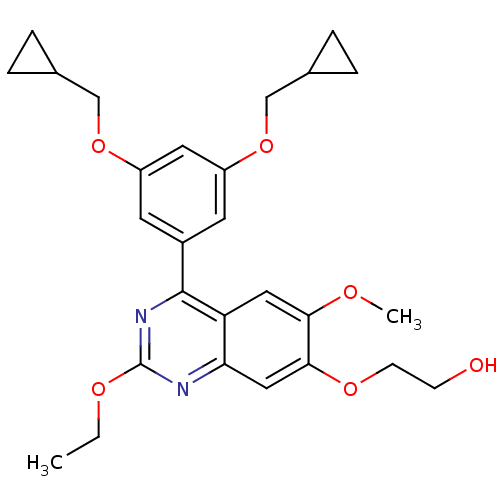
(2-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-2-ethoxy-...)Show SMILES CCOc1nc(-c2cc(OCC3CC3)cc(OCC3CC3)c2)c2cc(OC)c(OCCO)cc2n1 Show InChI InChI=1S/C27H32N2O6/c1-3-32-27-28-23-14-25(33-9-8-30)24(31-2)13-22(23)26(29-27)19-10-20(34-15-17-4-5-17)12-21(11-19)35-16-18-6-7-18/h10-14,17-18,30H,3-9,15-16H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072211
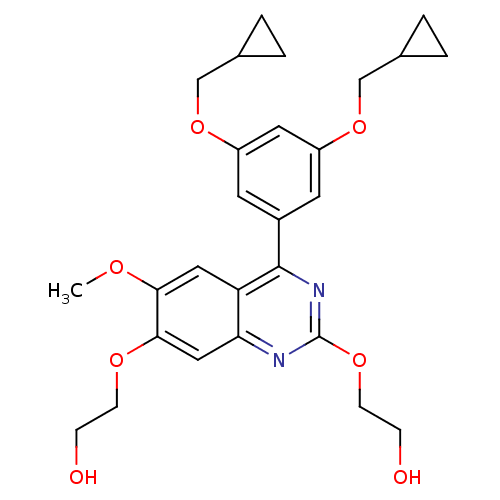
(2-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-7-(2-hydr...)Show SMILES COc1cc2c(nc(OCCO)nc2cc1OCCO)-c1cc(OCC2CC2)cc(OCC2CC2)c1 Show InChI InChI=1S/C27H32N2O7/c1-32-24-13-22-23(14-25(24)33-8-6-30)28-27(34-9-7-31)29-26(22)19-10-20(35-15-17-2-3-17)12-21(11-19)36-16-18-4-5-18/h10-14,17-18,30-31H,2-9,15-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085146
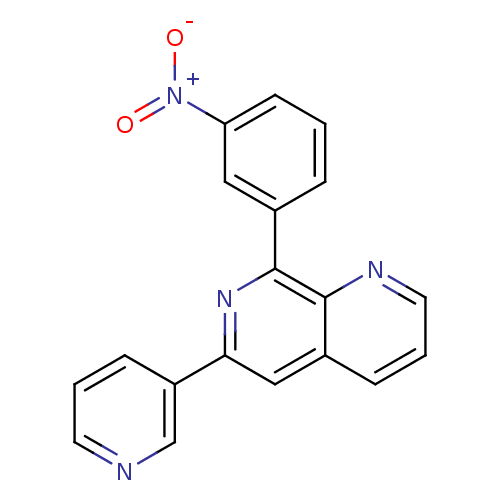
(8-(3-Nitro-phenyl)-6-pyridin-3-yl-[1,7]naphthyridi...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1nc(cc2cccnc12)-c1cccnc1 Show InChI InChI=1S/C19H12N4O2/c24-23(25)16-7-1-4-13(10-16)19-18-14(5-3-9-21-18)11-17(22-19)15-6-2-8-20-12-15/h1-12H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085139
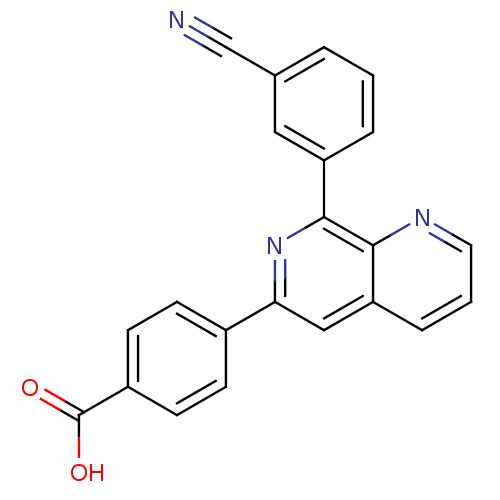
(4-[8-(3-Cyano-phenyl)-[1,7]naphthyridin-6-yl]-benz...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)C#N Show InChI InChI=1S/C22H13N3O2/c23-13-14-3-1-4-17(11-14)21-20-18(5-2-10-24-20)12-19(25-21)15-6-8-16(9-7-15)22(26)27/h1-12H,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085136
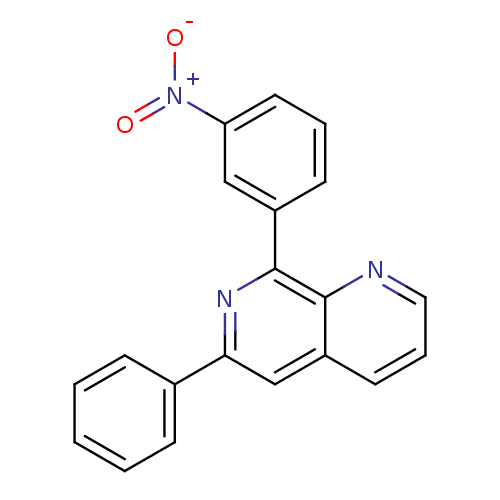
(8-(3-Nitro-phenyl)-6-phenyl-[1,7]naphthyridine | C...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1nc(cc2cccnc12)-c1ccccc1 Show InChI InChI=1S/C20H13N3O2/c24-23(25)17-10-4-8-15(12-17)20-19-16(9-5-11-21-19)13-18(22-20)14-6-2-1-3-7-14/h1-13H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072199
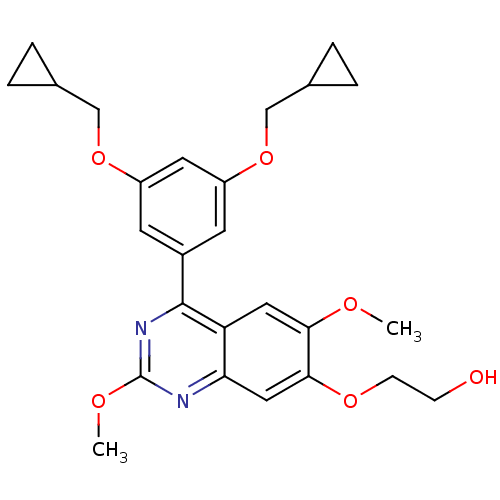
(2-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-2,6-dimet...)Show SMILES COc1nc(-c2cc(OCC3CC3)cc(OCC3CC3)c2)c2cc(OC)c(OCCO)cc2n1 Show InChI InChI=1S/C26H30N2O6/c1-30-23-12-21-22(13-24(23)32-8-7-29)27-26(31-2)28-25(21)18-9-19(33-14-16-3-4-16)11-20(10-18)34-15-17-5-6-17/h9-13,16-17,29H,3-8,14-15H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085140
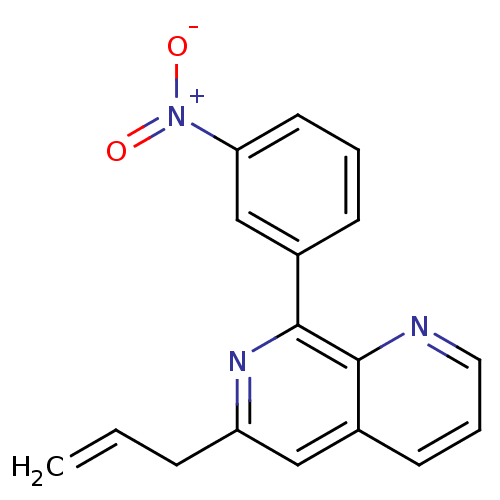
(6-Allyl-8-(3-nitro-phenyl)-[1,7]naphthyridine | CH...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1nc(CC=C)cc2cccnc12 Show InChI InChI=1S/C17H13N3O2/c1-2-5-14-10-12-7-4-9-18-16(12)17(19-14)13-6-3-8-15(11-13)20(21)22/h2-4,6-11H,1,5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072200
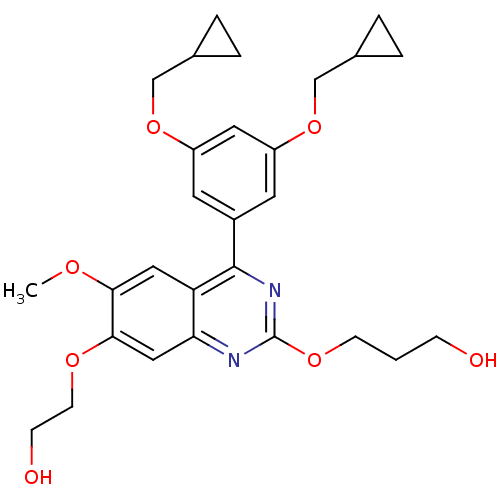
(3-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-7-(2-hydr...)Show SMILES COc1cc2c(nc(OCCCO)nc2cc1OCCO)-c1cc(OCC2CC2)cc(OCC2CC2)c1 Show InChI InChI=1S/C28H34N2O7/c1-33-25-14-23-24(15-26(25)34-10-8-32)29-28(35-9-2-7-31)30-27(23)20-11-21(36-16-18-3-4-18)13-22(12-20)37-17-19-5-6-19/h11-15,18-19,31-32H,2-10,16-17H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072194
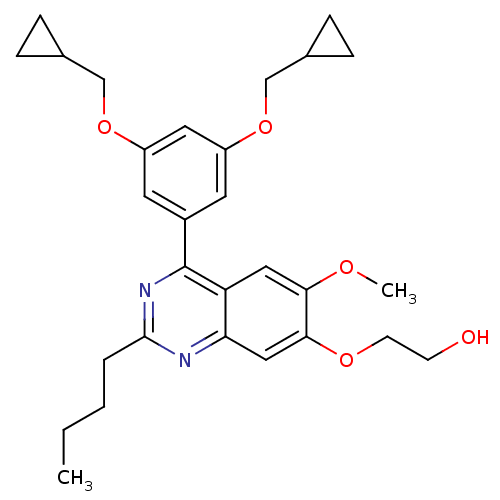
(2-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-2-butyl-6...)Show SMILES CCCCc1nc(-c2cc(OCC3CC3)cc(OCC3CC3)c2)c2cc(OC)c(OCCO)cc2n1 Show InChI InChI=1S/C29H36N2O5/c1-3-4-5-28-30-25-16-27(34-11-10-32)26(33-2)15-24(25)29(31-28)21-12-22(35-17-19-6-7-19)14-23(13-21)36-18-20-8-9-20/h12-16,19-20,32H,3-11,17-18H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085141
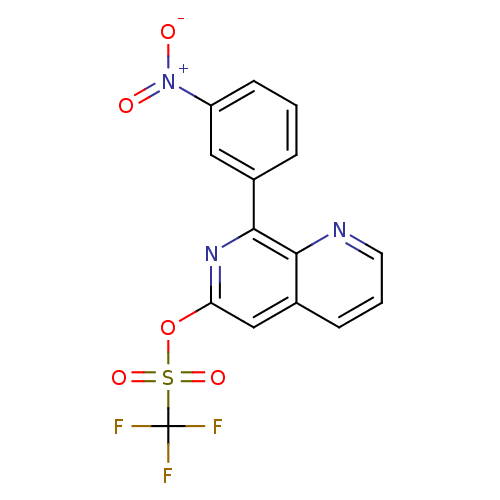
(CHEMBL351900 | Trifluoro-methanesulfonic acid 8-(3...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1nc(OS(=O)(=O)C(F)(F)F)cc2cccnc12 Show InChI InChI=1S/C15H8F3N3O5S/c16-15(17,18)27(24,25)26-12-8-10-4-2-6-19-13(10)14(20-12)9-3-1-5-11(7-9)21(22)23/h1-8H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50346088

((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@]1(CC[C@@H](CC1)C(O)=O)C#N |r,wU:17.22,14.25,(4.79,2.97,;3.46,3.74,;2.12,2.97,;.79,3.74,;-.54,2.97,;-.54,1.43,;.79,.66,;2.12,1.43,;3.46,.66,;3.46,-.88,;4.7,-1.79,;4.23,-3.25,;2.69,-3.25,;2.21,-1.79,;-1.88,.66,;-3.42,.71,;-4.23,-.6,;-3.51,-1.96,;-1.97,-2.01,;-1.16,-.7,;-4.33,-3.26,;-3.6,-4.62,;-5.87,-3.21,;-1.77,2.19,;-1.66,3.73,)| Show InChI InChI=1S/C20H25NO4/c1-24-17-7-6-15(12-18(17)25-16-4-2-3-5-16)20(13-21)10-8-14(9-11-20)19(22)23/h6-7,12,14,16H,2-5,8-11H2,1H3,(H,22,23)/t14-,20- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072209
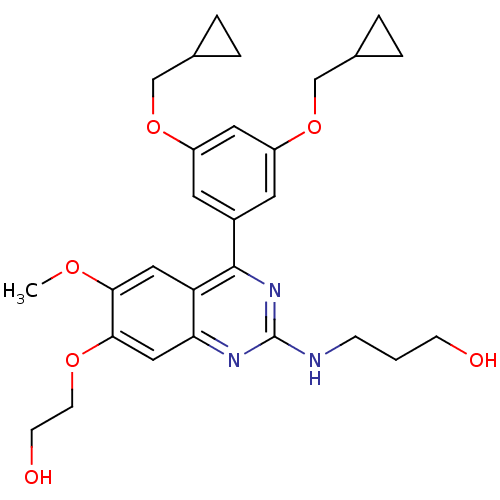
(3-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-7-(2-hydr...)Show SMILES COc1cc2c(nc(NCCCO)nc2cc1OCCO)-c1cc(OCC2CC2)cc(OCC2CC2)c1 Show InChI InChI=1S/C28H35N3O6/c1-34-25-14-23-24(15-26(25)35-10-9-33)30-28(29-7-2-8-32)31-27(23)20-11-21(36-16-18-3-4-18)13-22(12-20)37-17-19-5-6-19/h11-15,18-19,32-33H,2-10,16-17H2,1H3,(H,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085147
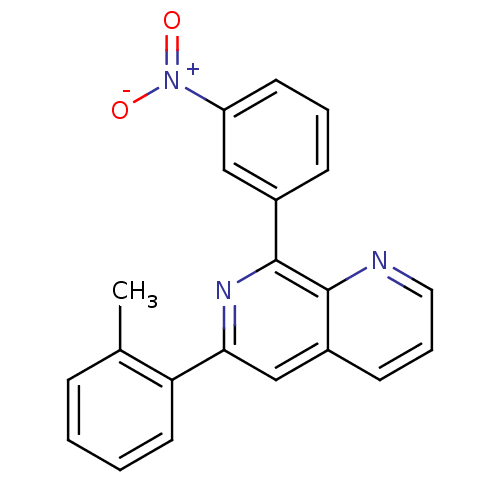
(8-(3-Nitro-phenyl)-6-o-tolyl-[1,7]naphthyridine | ...)Show SMILES Cc1ccccc1-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H15N3O2/c1-14-6-2-3-10-18(14)19-13-16-8-5-11-22-20(16)21(23-19)15-7-4-9-17(12-15)24(25)26/h2-13H,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085143
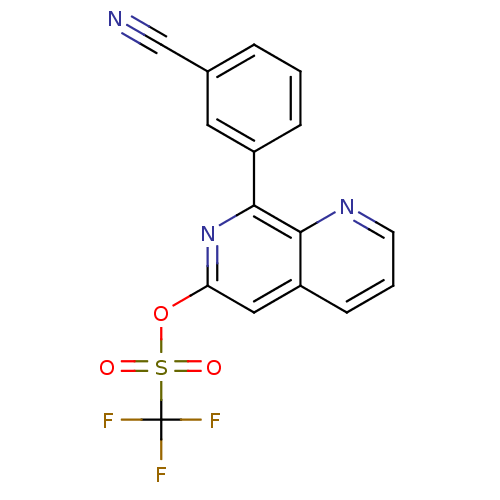
(CHEMBL158286 | Trifluoro-methanesulfonic acid 8-(3...)Show SMILES FC(F)(F)S(=O)(=O)Oc1cc2cccnc2c(n1)-c1cccc(c1)C#N Show InChI InChI=1S/C16H8F3N3O3S/c17-16(18,19)26(23,24)25-13-8-12-5-2-6-21-14(12)15(22-13)11-4-1-3-10(7-11)9-20/h1-8H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072204

(2-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-2-ethylsu...)Show SMILES CCSc1nc(-c2cc(OCC3CC3)cc(OCC3CC3)c2)c2cc(OC)c(OCCO)cc2n1 Show InChI InChI=1S/C27H32N2O5S/c1-3-35-27-28-23-14-25(32-9-8-30)24(31-2)13-22(23)26(29-27)19-10-20(33-15-17-4-5-17)12-21(11-19)34-16-18-6-7-18/h10-14,17-18,30H,3-9,15-16H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085145
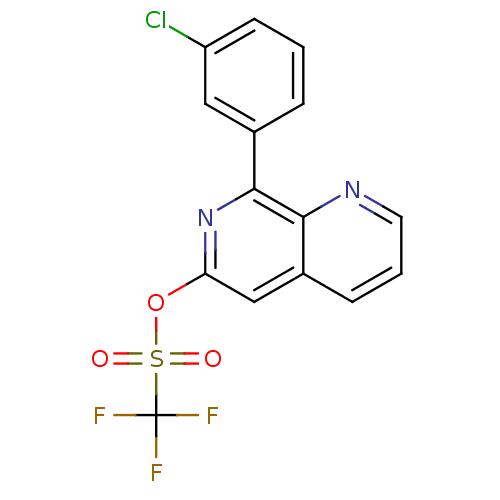
(CHEMBL161714 | Trifluoro-methanesulfonic acid 8-(3...)Show SMILES FC(F)(F)S(=O)(=O)Oc1cc2cccnc2c(n1)-c1cccc(Cl)c1 Show InChI InChI=1S/C15H8ClF3N2O3S/c16-11-5-1-3-9(7-11)14-13-10(4-2-6-20-13)8-12(21-14)24-25(22,23)15(17,18)19/h1-8H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085137
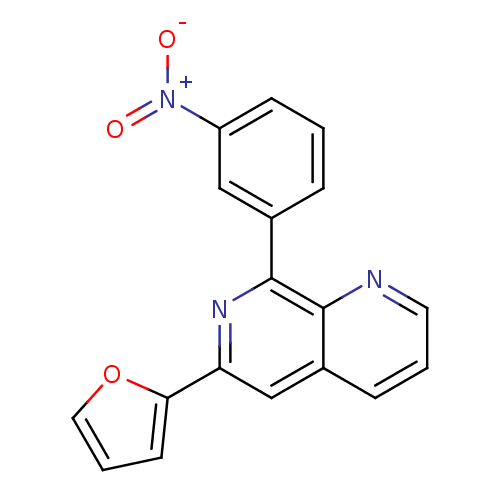
(6-Furan-2-yl-8-(3-nitro-phenyl)-[1,7]naphthyridine...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1nc(cc2cccnc12)-c1ccco1 Show InChI InChI=1S/C18H11N3O3/c22-21(23)14-6-1-4-12(10-14)18-17-13(5-2-8-19-17)11-15(20-18)16-7-3-9-24-16/h1-11H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072205
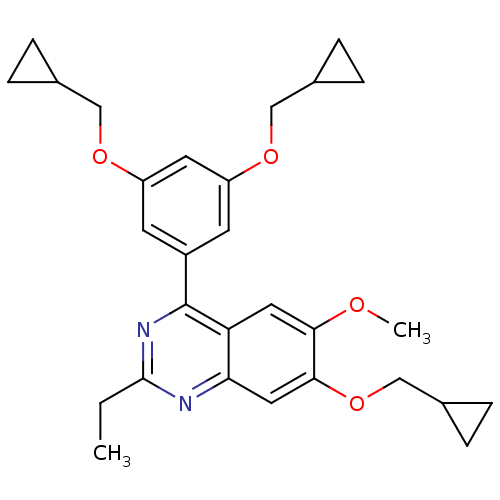
(4-(3,5-Bis-cyclopropylmethoxy-phenyl)-7-cyclopropy...)Show SMILES CCc1nc(-c2cc(OCC3CC3)cc(OCC3CC3)c2)c2cc(OC)c(OCC3CC3)cc2n1 Show InChI InChI=1S/C29H34N2O4/c1-3-28-30-25-14-27(35-17-20-8-9-20)26(32-2)13-24(25)29(31-28)21-10-22(33-15-18-4-5-18)12-23(11-21)34-16-19-6-7-19/h10-14,18-20H,3-9,15-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072206
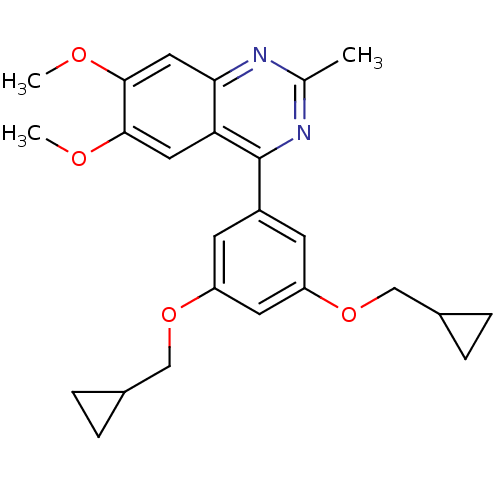
(4-(3,5-Bis-cyclopropylmethoxy-phenyl)-6,7-dimethox...)Show SMILES COc1cc2nc(C)nc(-c3cc(OCC4CC4)cc(OCC4CC4)c3)c2cc1OC Show InChI InChI=1S/C25H28N2O4/c1-15-26-22-12-24(29-3)23(28-2)11-21(22)25(27-15)18-8-19(30-13-16-4-5-16)10-20(9-18)31-14-17-6-7-17/h8-12,16-17H,4-7,13-14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072212

(2-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-2-ethyl-q...)Show SMILES CCc1nc(-c2cc(OCC3CC3)cc(OCC3CC3)c2)c2ccc(OCCO)cc2n1 Show InChI InChI=1S/C26H30N2O4/c1-2-25-27-24-14-20(30-10-9-29)7-8-23(24)26(28-25)19-11-21(31-15-17-3-4-17)13-22(12-19)32-16-18-5-6-18/h7-8,11-14,17-18,29H,2-6,9-10,15-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072198
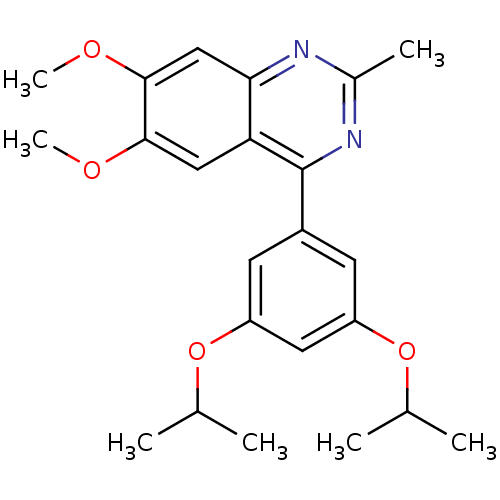
(4-(3,5-Diisopropoxy-phenyl)-6,7-dimethoxy-2-methyl...)Show SMILES COc1cc2nc(C)nc(-c3cc(OC(C)C)cc(OC(C)C)c3)c2cc1OC Show InChI InChI=1S/C23H28N2O4/c1-13(2)28-17-8-16(9-18(10-17)29-14(3)4)23-19-11-21(26-6)22(27-7)12-20(19)24-15(5)25-23/h8-14H,1-7H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072203

(2-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-6-methoxy...)Show SMILES COc1cc2c(nc(nc2cc1OCCO)-c1cccs1)-c1cc(OCC2CC2)cc(OCC2CC2)c1 Show InChI InChI=1S/C29H30N2O5S/c1-33-25-14-23-24(15-26(25)34-9-8-32)30-29(27-3-2-10-37-27)31-28(23)20-11-21(35-16-18-4-5-18)13-22(12-20)36-17-19-6-7-19/h2-3,10-15,18-19,32H,4-9,16-17H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072210
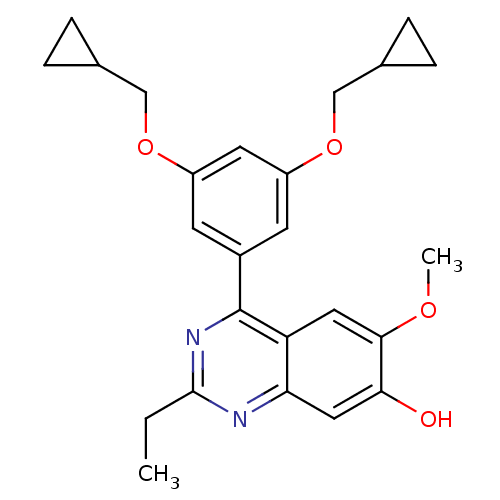
(4-(3,5-Bis-cyclopropylmethoxy-phenyl)-2-ethyl-6-me...)Show SMILES CCc1nc(-c2cc(OCC3CC3)cc(OCC3CC3)c2)c2cc(OC)c(O)cc2n1 Show InChI InChI=1S/C25H28N2O4/c1-3-24-26-21-12-22(28)23(29-2)11-20(21)25(27-24)17-8-18(30-13-15-4-5-15)10-19(9-17)31-14-16-6-7-16/h8-12,15-16,28H,3-7,13-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072208
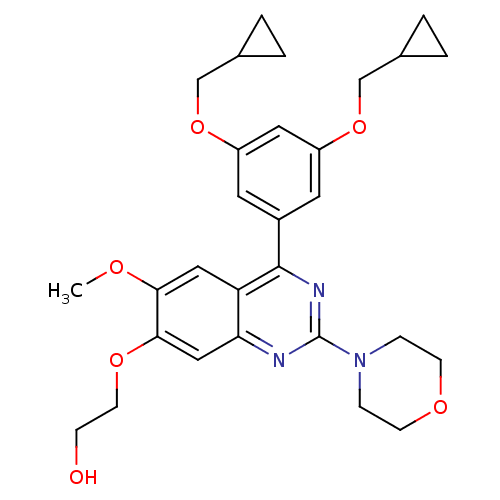
(2-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-6-methoxy...)Show SMILES COc1cc2c(nc(nc2cc1OCCO)N1CCOCC1)-c1cc(OCC2CC2)cc(OCC2CC2)c1 Show InChI InChI=1S/C29H35N3O6/c1-34-26-15-24-25(16-27(26)36-11-8-33)30-29(32-6-9-35-10-7-32)31-28(24)21-12-22(37-17-19-2-3-19)14-23(13-21)38-18-20-4-5-20/h12-16,19-20,33H,2-11,17-18H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM14361

((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085142
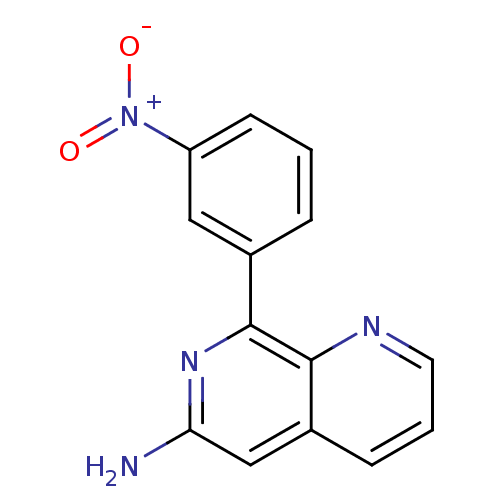
(8-(3-Nitro-phenyl)-[1,7]naphthyridin-6-ylamine | C...)Show InChI InChI=1S/C14H10N4O2/c15-12-8-10-4-2-6-16-13(10)14(17-12)9-3-1-5-11(7-9)18(19)20/h1-8H,(H2,15,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085144
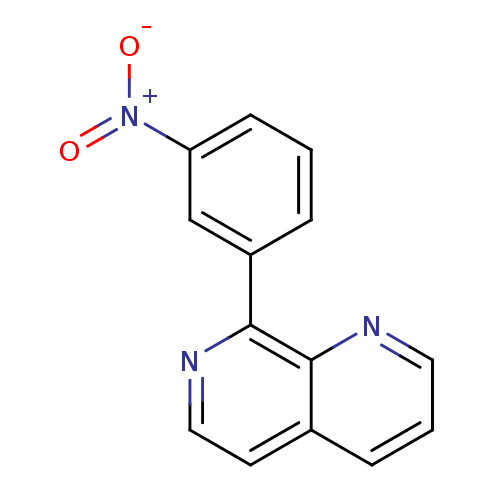
(8-(3-Nitro-phenyl)-[1,7]naphthyridine | CHEMBL3519...)Show InChI InChI=1S/C14H9N3O2/c18-17(19)12-5-1-3-11(9-12)14-13-10(6-8-16-14)4-2-7-15-13/h1-9H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50085134
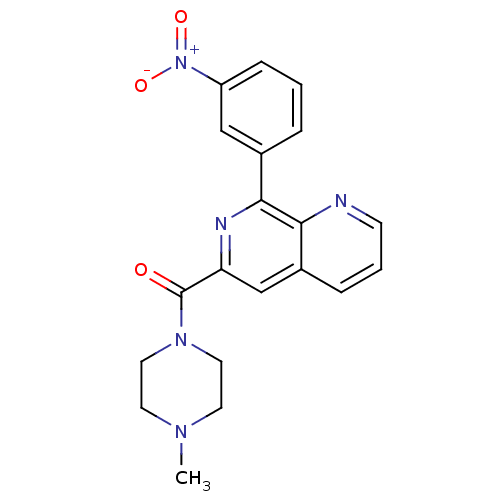
((4-Methyl-piperazin-1-yl)-[8-(3-nitro-phenyl)-[1,7...)Show SMILES CN1CCN(CC1)C(=O)c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C20H19N5O3/c1-23-8-10-24(11-9-23)20(26)17-13-15-5-3-7-21-18(15)19(22-17)14-4-2-6-16(12-14)25(27)28/h2-7,12-13H,8-11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4B (PDE4B) from rat source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072201
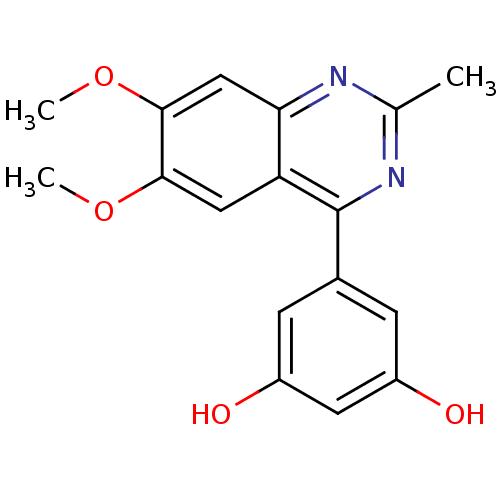
(5-(6,7-Dimethoxy-2-methyl-quinazolin-4-yl)-benzene...)Show InChI InChI=1S/C17H16N2O4/c1-9-18-14-8-16(23-3)15(22-2)7-13(14)17(19-9)10-4-11(20)6-12(21)5-10/h4-8,20-21H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072196
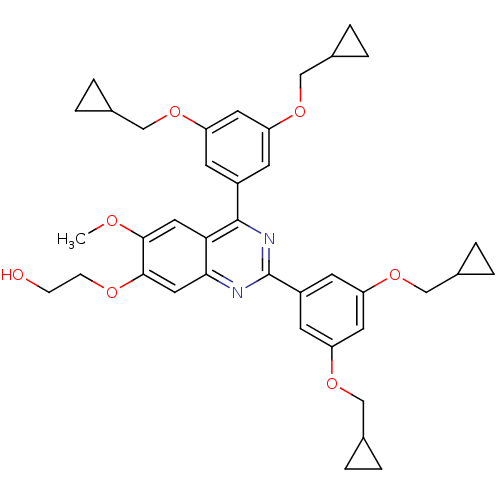
(2-[2,4-Bis-(3,5-bis-cyclopropylmethoxy-phenyl)-6-m...)Show SMILES COc1cc2c(nc(nc2cc1OCCO)-c1cc(OCC2CC2)cc(OCC2CC2)c1)-c1cc(OCC2CC2)cc(OCC2CC2)c1 Show InChI InChI=1S/C39H44N2O7/c1-43-36-18-34-35(19-37(36)44-11-10-42)40-39(29-14-32(47-22-26-6-7-26)17-33(15-29)48-23-27-8-9-27)41-38(34)28-12-30(45-20-24-2-3-24)16-31(13-28)46-21-25-4-5-25/h12-19,24-27,42H,2-11,20-23H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
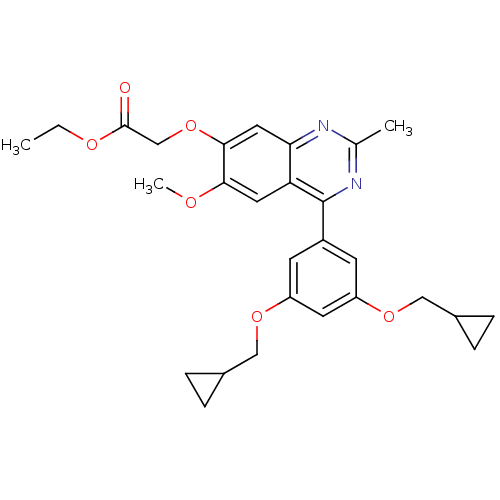
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072207
(CHEMBL97213 | [4-(3,5-Bis-cyclopropylmethoxy-pheny...)Show SMILES CCOC(=O)COc1cc2nc(C)nc(-c3cc(OCC4CC4)cc(OCC4CC4)c3)c2cc1OC Show InChI InChI=1S/C28H32N2O6/c1-4-33-27(31)16-36-26-13-24-23(12-25(26)32-3)28(30-17(2)29-24)20-9-21(34-14-18-5-6-18)11-22(10-20)35-15-19-7-8-19/h9-13,18-19H,4-8,14-16H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
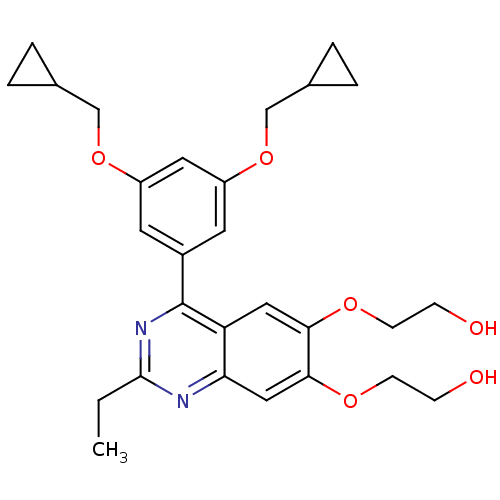
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072193
(2-[4-(3,5-Bis-cyclopropylmethoxy-phenyl)-2-ethyl-7...)Show SMILES CCc1nc(-c2cc(OCC3CC3)cc(OCC3CC3)c2)c2cc(OCCO)c(OCCO)cc2n1 Show InChI InChI=1S/C28H34N2O6/c1-2-27-29-24-15-26(34-10-8-32)25(33-9-7-31)14-23(24)28(30-27)20-11-21(35-16-18-3-4-18)13-22(12-20)36-17-19-5-6-19/h11-15,18-19,31-32H,2-10,16-17H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |
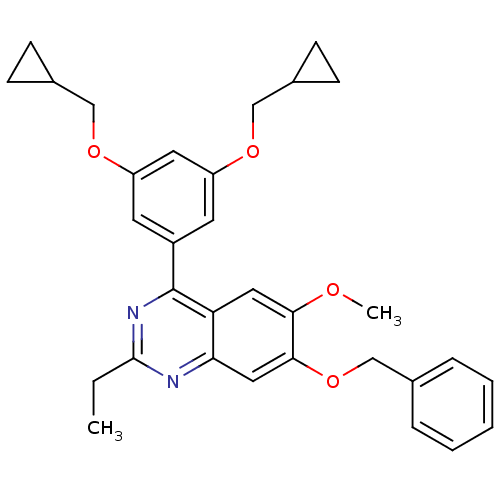
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(RAT) | BDBM50072202
(7-Benzyloxy-4-(3,5-bis-cyclopropylmethoxy-phenyl)-...)Show SMILES CCc1nc(-c2cc(OCC3CC3)cc(OCC3CC3)c2)c2cc(OC)c(OCc3ccccc3)cc2n1 Show InChI InChI=1S/C32H34N2O4/c1-3-31-33-28-17-30(38-20-21-7-5-4-6-8-21)29(35-2)16-27(28)32(34-31)24-13-25(36-18-22-9-10-22)15-26(14-24)37-19-23-11-12-23/h4-8,13-17,22-23H,3,9-12,18-20H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Evaluated for its ability to inhibit PDE4B. |
Bioorg Med Chem Lett 8: 2891-6 (1999)
BindingDB Entry DOI: 10.7270/Q2PG1QVX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data