

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020840 (CHEMBL113276 | [Cyclopentyl-(2-mercapto-3-methoxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020833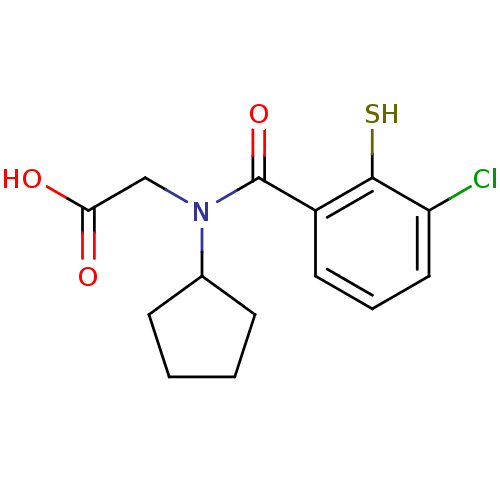 (CHEMBL326137 | [(3-Chloro-2-mercapto-benzoyl)-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020829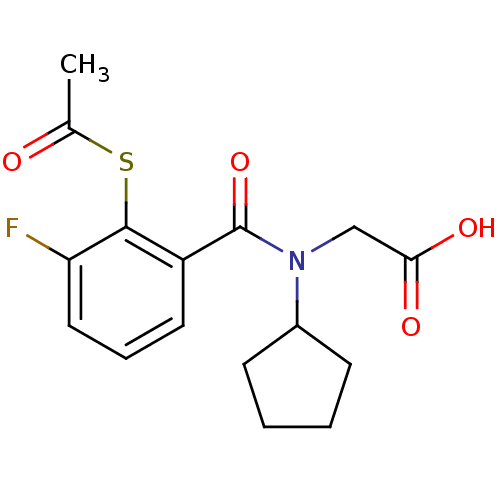 (CHEMBL114242 | [(2-Acetylsulfanyl-3-fluoro-benzoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020830 (CHEMBL263056 | [Cyclopentyl-(2-mercapto-3-trifluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020824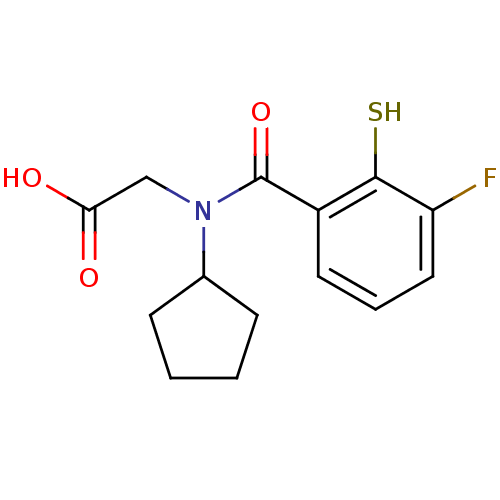 (CHEMBL325659 | [Cyclopentyl-(3-fluoro-2-mercapto-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020831 (CHEMBL113315 | [(2-Acetylsulfanyl-3-methyl-benzoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020842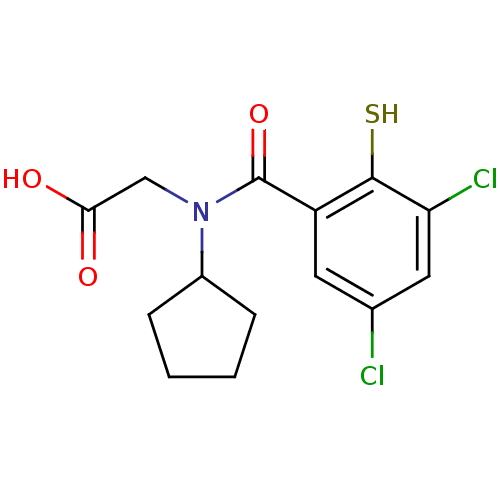 (CHEMBL114294 | [Cyclopentyl-(3,5-dichloro-2-mercap...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020841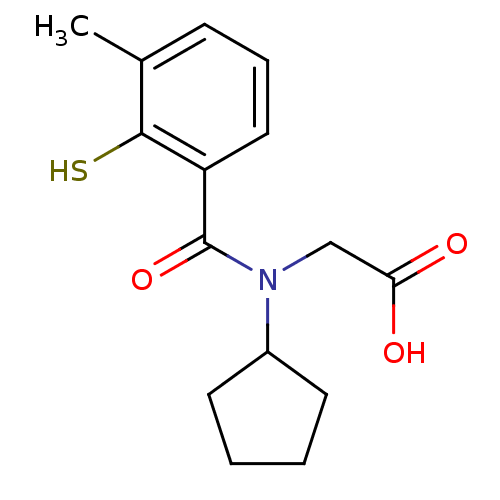 (CHEMBL324242 | [Cyclopentyl-(2-mercapto-3-methyl-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020825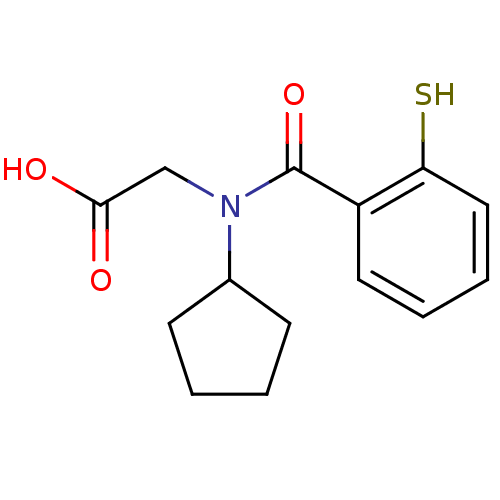 (CHEMBL112168 | [Cyclopentyl-(2-mercapto-benzoyl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020823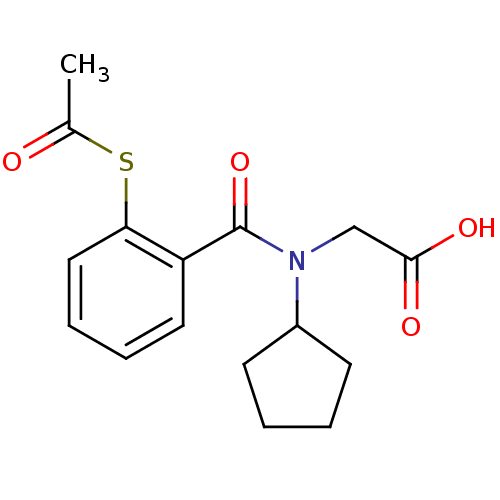 (CHEMBL112477 | [(2-Acetylsulfanyl-benzoyl)-cyclope...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020832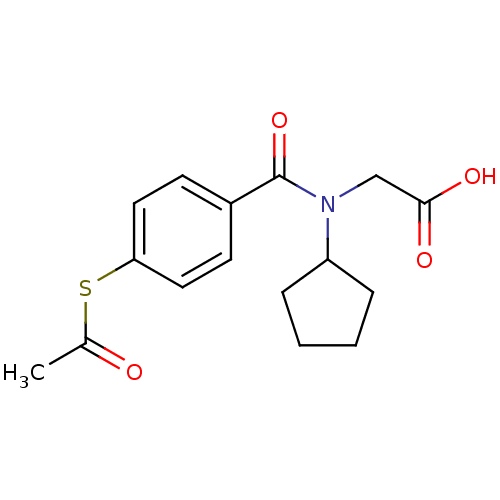 (CHEMBL112589 | [(4-Acetylsulfanyl-benzoyl)-cyclope...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020821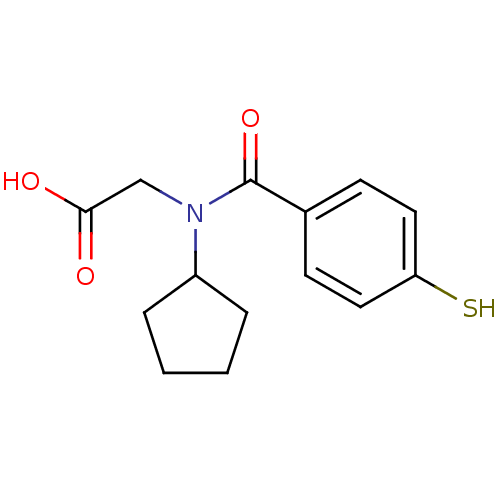 (CHEMBL113612 | [Cyclopentyl-(4-mercapto-benzoyl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020827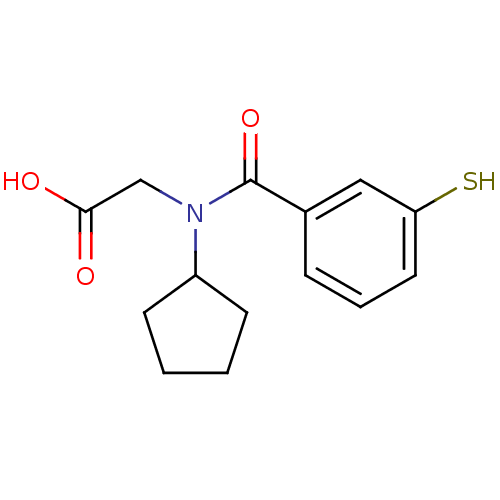 (CHEMBL324898 | [Cyclopentyl-(3-mercapto-benzoyl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020838 (CHEMBL323879 | [(3-Acetylsulfanyl-benzoyl)-cyclope...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020820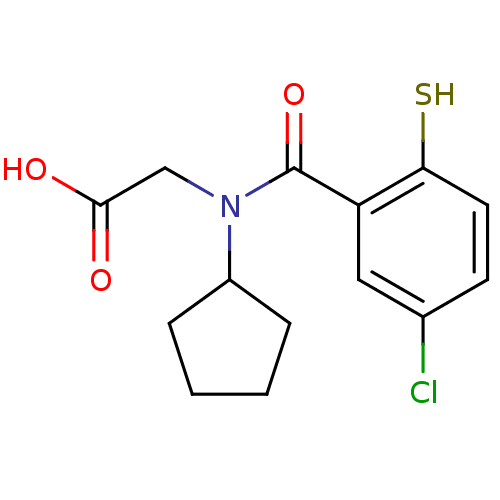 (CHEMBL112922 | [(5-Chloro-2-mercapto-benzoyl)-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50280471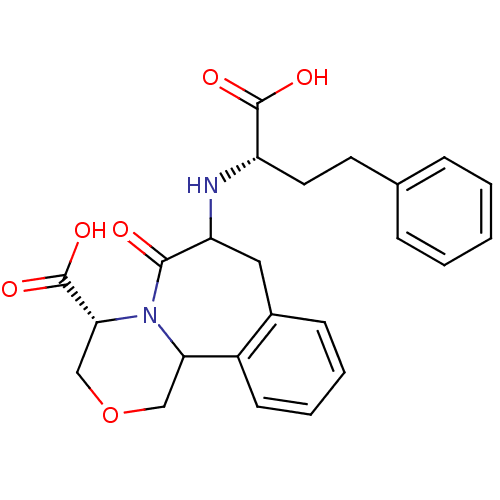 ((R)-6-((R)-(S)-1-Carboxy-3-phenyl-propylamino)-5-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against rabbit lung Angiotensin I converting enzyme (ACE) | Bioorg Med Chem Lett 2: 579-582 (1992) Article DOI: 10.1016/S0960-894X(01)81201-4 BindingDB Entry DOI: 10.7270/Q200021W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020839 (CHEMBL324703 | [Cyclopentyl-(2-nitro-benzoyl)-amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020828 (CHEMBL324676 | [Cyclopentyl-(3,5-dichloro-2-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020837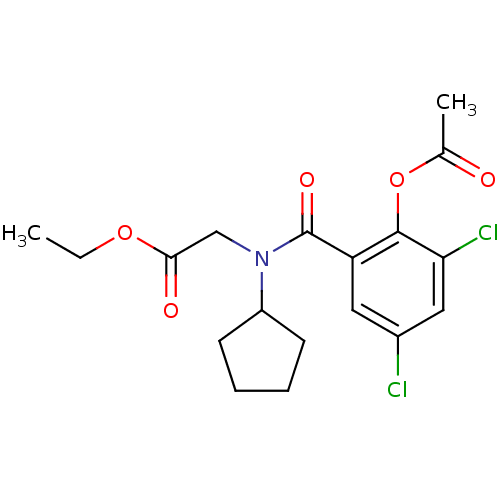 (CHEMBL114018 | [(2-Acetoxy-3,5-dichloro-benzoyl)-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020836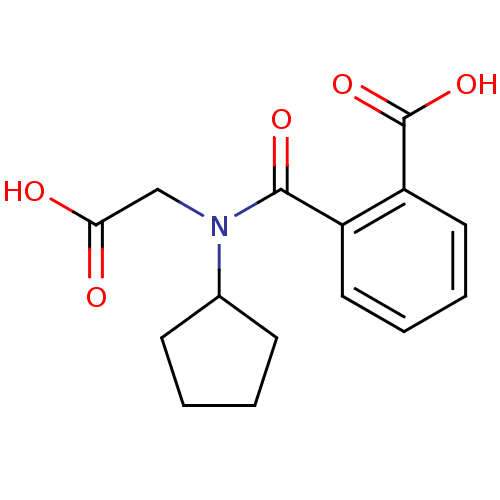 (CHEMBL115153 | N-Carboxymethyl-N-cyclopentyl-phtha...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50020834 (CHEMBL325305 | [Cyclopentyl-(3-isopropyl-2-mercapt...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against Angiotensin I converting enzyme | J Med Chem 28: 328-32 (1985) BindingDB Entry DOI: 10.7270/Q2DN45MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50279824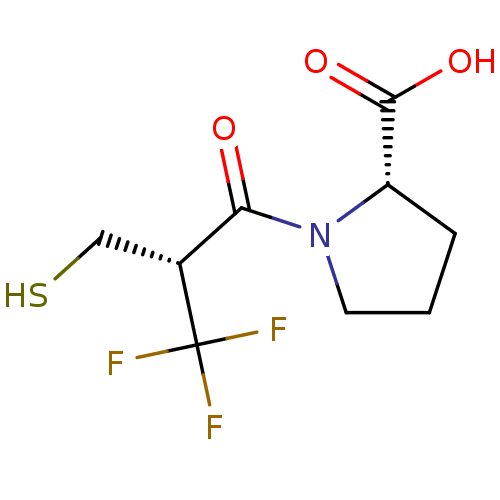 ((S)-1-((R)-3,3,3-Trifluoro-2-mercaptomethyl-propio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered base | Bioorg Med Chem Lett 1: 581-584 (1991) Article DOI: 10.1016/S0960-894X(01)81155-0 BindingDB Entry DOI: 10.7270/Q2GF0TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50146428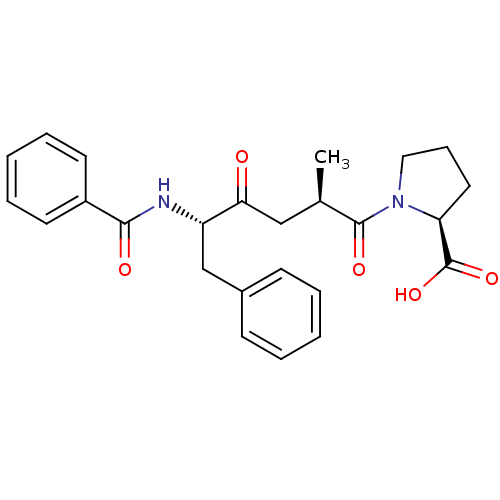 ((S)-1-((1R,5S)-5-Benzoylamino-2-methyl-4-oxo-6-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of New York Curated by ChEMBL | Assay Description Inhibitory activity of compound against angiotensin I converting enzyme was determined | Bioorg Med Chem Lett 14: 2853-6 (2004) Article DOI: 10.1016/j.bmcl.2004.03.042 BindingDB Entry DOI: 10.7270/Q2CJ8CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367254 (ENALAPRILAT) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011362 (1-(3-Mercapto-2-methyl-propionyl)-pyrrolidine-2-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011365 (1-[2-(1-Carboxy-butylamino)-propionyl]-octahydro-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021127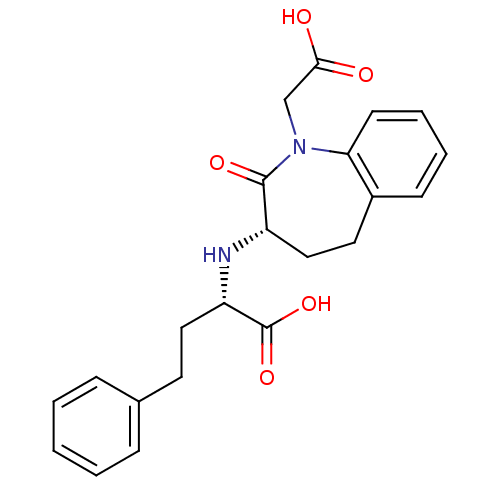 (2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1511-6 (1985) BindingDB Entry DOI: 10.7270/Q2BR8SRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021127 (2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1511-6 (1985) BindingDB Entry DOI: 10.7270/Q2BR8SRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50011361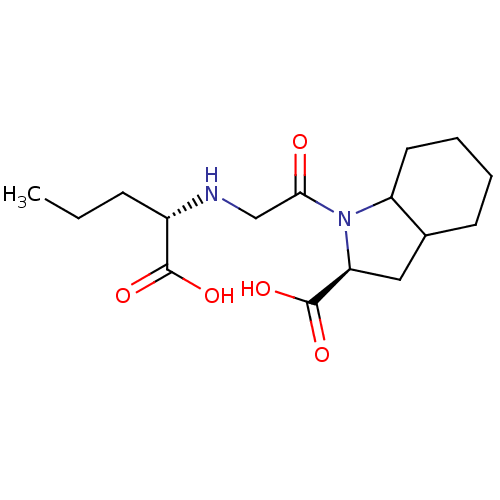 (1-[2-(1-Carboxy-butylamino)-acetyl]-octahydro-indo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description In vitro activity against angiotensin I converting enzyme especially against Hip-His-Leu residues | J Med Chem 34: 663-9 (1991) BindingDB Entry DOI: 10.7270/Q21V5FKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50017129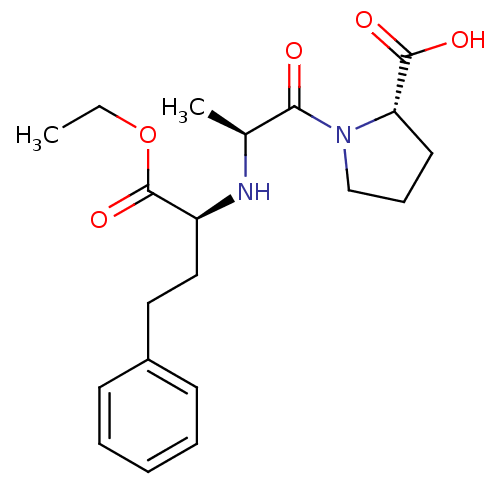 ((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021153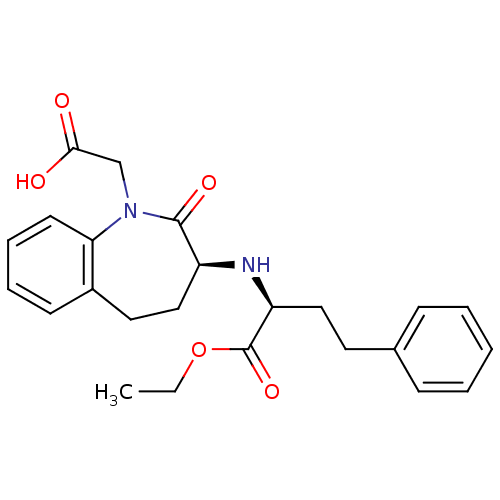 (1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021127 (2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested in vitro for the inhibition against neutral endopeptidase (NEP) from rat kidney | J Med Chem 36: 3829-33 (1994) BindingDB Entry DOI: 10.7270/Q2125RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered base. (reported from ref. 1b) | Bioorg Med Chem Lett 1: 581-584 (1991) Article DOI: 10.1016/S0960-894X(01)81155-0 BindingDB Entry DOI: 10.7270/Q2GF0TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021783 (1-(3-Mercapto-propionyl)-2,3-dihydro-1H-indole-2-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021790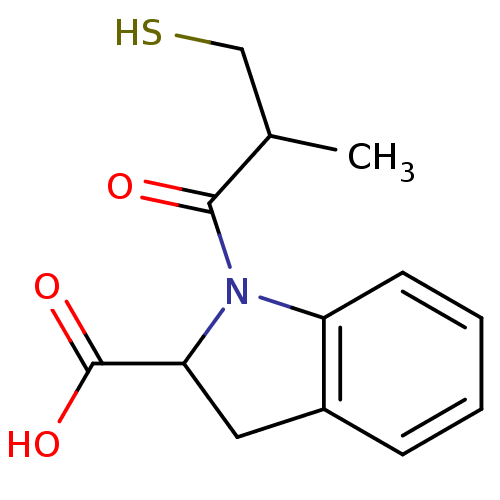 ((S,S)1-(3-Mercapto-2-methyl-propionyl)-2,3-dihydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of dihydrofolate reductase in Escherichia coli | J Med Chem 26: 1267-77 (1983) BindingDB Entry DOI: 10.7270/Q29024B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021790 ((S,S)1-(3-Mercapto-2-methyl-propionyl)-2,3-dihydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to inhibit Angiotensin I converting enzyme was determined | J Med Chem 27: 816-8 (1984) BindingDB Entry DOI: 10.7270/Q20K2956 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021184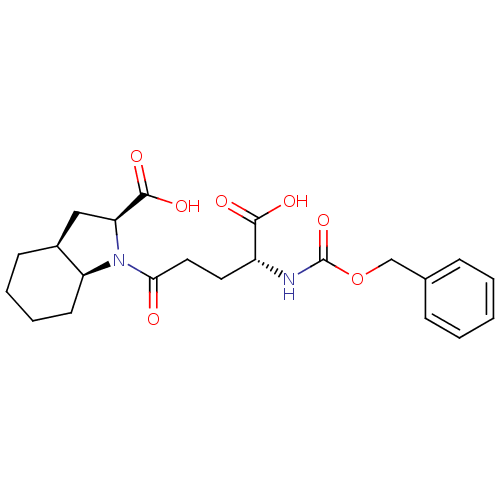 (1-(4-Benzyloxycarbonylamino-4-carboxy-butyryl)-oct...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of angiotensin I converting enzyme from rabbit lung | J Med Chem 28: 1606-11 (1985) BindingDB Entry DOI: 10.7270/Q2TT4RHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024714 (2-(4-Carboxymethyl-5-oxo-3-thiophen-2-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021127 (2-(1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydro-1H-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | J Med Chem 28: 1511-6 (1985) BindingDB Entry DOI: 10.7270/Q2BR8SRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023299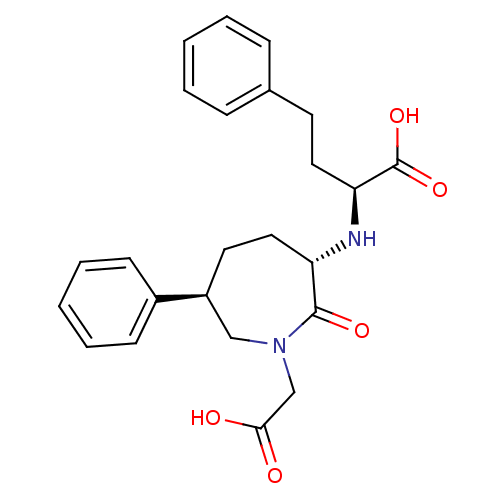 (2-(1-Carboxymethyl-2-oxo-6-phenyl-azepan-3-ylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023298 (2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023298 (2-(4-Carboxymethyl-5-oxo-3-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50085460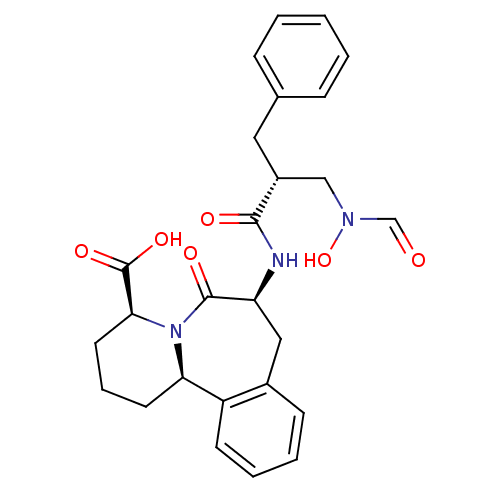 ((4S,7S,12bR)-7-[(R)-2-Benzyl-3-(formyl-hydroxy-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Angiotensin I converting enzyme | Bioorg Med Chem Lett 10: 257-60 (2000) BindingDB Entry DOI: 10.7270/Q2BK1BJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered base | Bioorg Med Chem Lett 1: 581-584 (1991) Article DOI: 10.1016/S0960-894X(01)81155-0 BindingDB Entry DOI: 10.7270/Q2GF0TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024710 (2-(4-Carboxymethyl-5-oxo-2-thiophen-2-yl-[1,4]thia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023296 (2-(1-Carboxymethyl-2-oxo-7-phenyl-azepan-3-ylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against angiotensin converting enzyme from rabbit in bovine buffered base | Bioorg Med Chem Lett 1: 581-584 (1991) Article DOI: 10.1016/S0960-894X(01)81155-0 BindingDB Entry DOI: 10.7270/Q2GF0TZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023295 (2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung Angiotensin I converting enzyme with 5 mM hippurylhistidylleucine as substrate | J Med Chem 30: 1984-91 (1987) BindingDB Entry DOI: 10.7270/Q2Q52NMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50023295 (2-(4-Carboxymethyl-5-oxo-2-phenyl-[1,4]thiazepan-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of rabbit lung Angiotensin I converting enzyme with 5 mM hippuryl-histidyl-leucine as substrate | J Med Chem 31: 422-8 (1988) BindingDB Entry DOI: 10.7270/Q2S75GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 594 total ) | Next | Last >> |