 Found 406 hits of ic50 data for polymerid = 5039,7622,50007716,50007728
Found 406 hits of ic50 data for polymerid = 5039,7622,50007716,50007728 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosinase
(Homo sapiens (Human)) | BDBM50031467

(5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...)Show InChI InChI=1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 0.613 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50241367
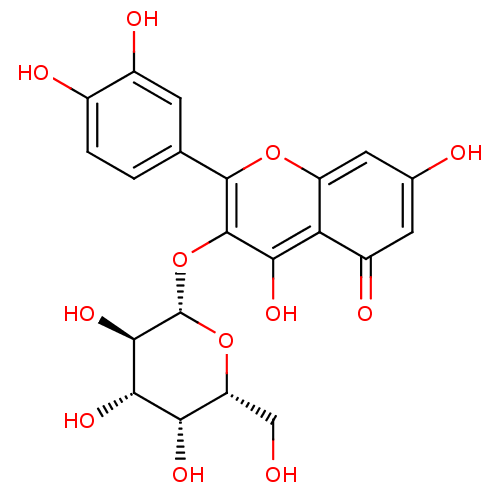
(2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-3-((2S,4R,5...)Show SMILES OC[C@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-25,27-30H,6H2/t13-,15+,17+,18-,21+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.762 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50217942
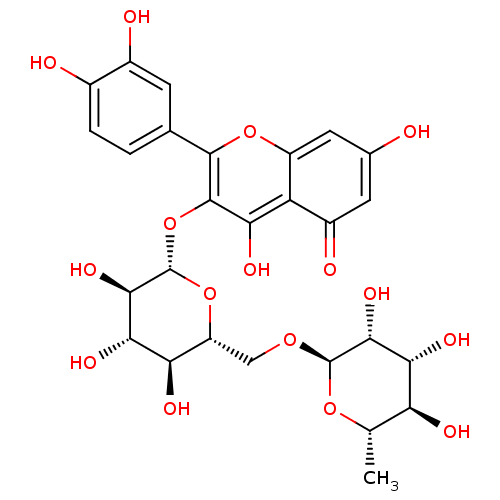
(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 0.856 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50378581

(NICOTIFLOROSIDE)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)cc3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O15/c1-9-17(31)20(34)22(36)26(39-9)38-8-15-18(32)21(35)23(37)27(41-15)42-25-19(33)16-13(30)6-12(29)7-14(16)40-24(25)10-2-4-11(28)5-3-10/h2-7,9,15,17-18,20-23,26-29,31-37H,8H2,1H3/t9-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.908 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50559076
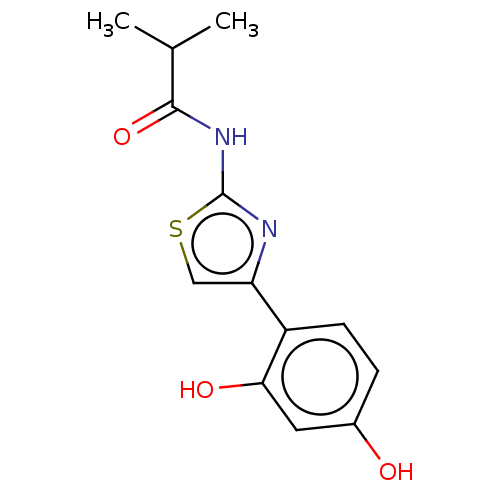
(CHEMBL4743159) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50269559
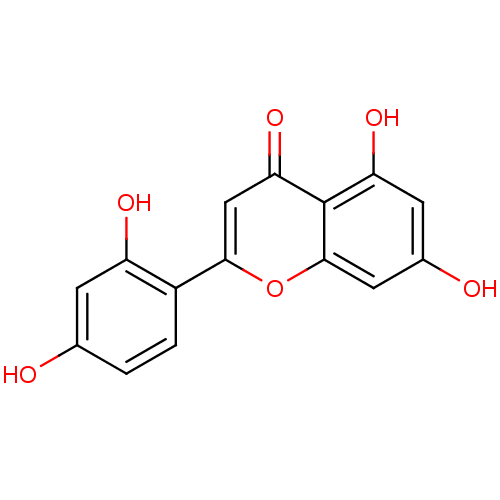
(2-(2,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-6,16-19H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima University
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) using L-dihydroxyphenylalanine as substrate preincubated for 30 mins followed by substrate addition measure... |
J Nat Prod 80: 3172-3178 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00453
BindingDB Entry DOI: 10.7270/Q2VD71Z4 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50044784
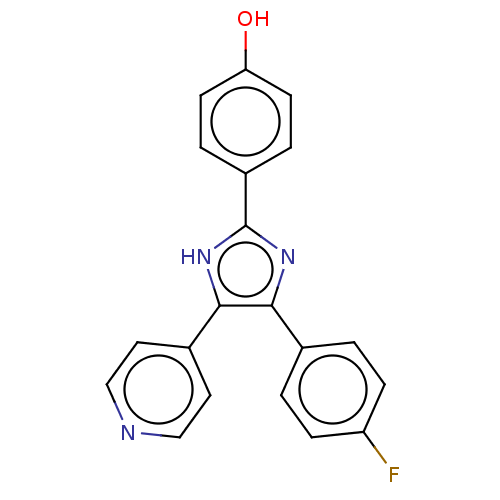
(CHEBI:79090 | SB-202190 | US10865384, Compound SB2...)Show SMILES Oc1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C20H14FN3O/c21-16-5-1-13(2-6-16)18-19(14-9-11-22-12-10-14)24-20(23-18)15-3-7-17(25)8-4-15/h1-12,25H,(H,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50044784

(CHEBI:79090 | SB-202190 | US10865384, Compound SB2...)Show SMILES Oc1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C20H14FN3O/c21-16-5-1-13(2-6-16)18-19(14-9-11-22-12-10-14)24-20(23-18)15-3-7-17(25)8-4-15/h1-12,25H,(H,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50045333
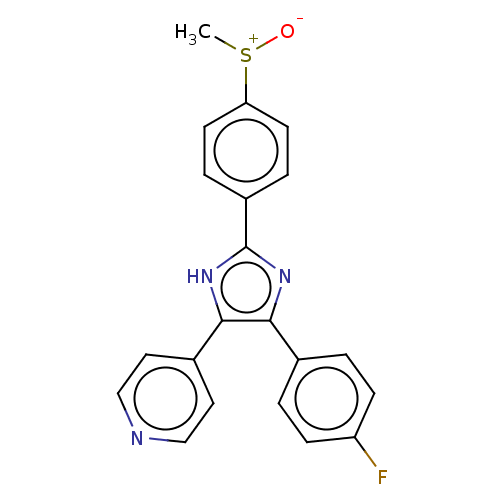
(CHEBI:90705 | SB-203580)Show SMILES C[S+]([O-])c1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50045333

(CHEBI:90705 | SB-203580)Show SMILES C[S+]([O-])c1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50287127
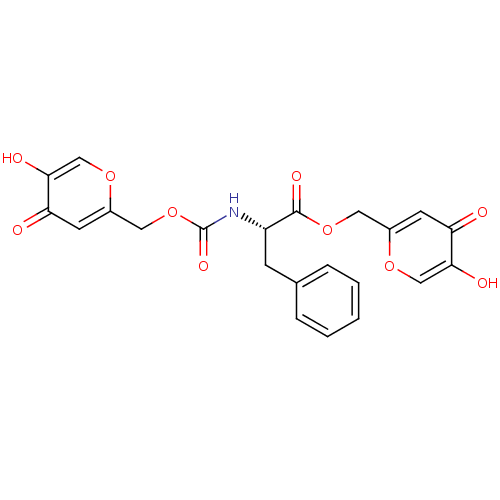
((S)-2-(5-Hydroxy-4-oxo-4H-pyran-2-ylmethoxycarbony...)Show SMILES Oc1coc(COC(=O)N[C@@H](Cc2ccccc2)C(=O)OCc2cc(=O)c(O)co2)cc1=O Show InChI InChI=1S/C22H19NO10/c24-17-7-14(30-11-19(17)26)9-32-21(28)16(6-13-4-2-1-3-5-13)23-22(29)33-10-15-8-18(25)20(27)12-31-15/h1-5,7-8,11-12,16,26-27H,6,9-10H2,(H,23,29)/t16-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of tyrosinase enzyme. |
Bioorg Med Chem Lett 6: 1303-1308 (1996)
Article DOI: 10.1016/0960-894X(96)00221-1
BindingDB Entry DOI: 10.7270/Q2V1259K |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50203985
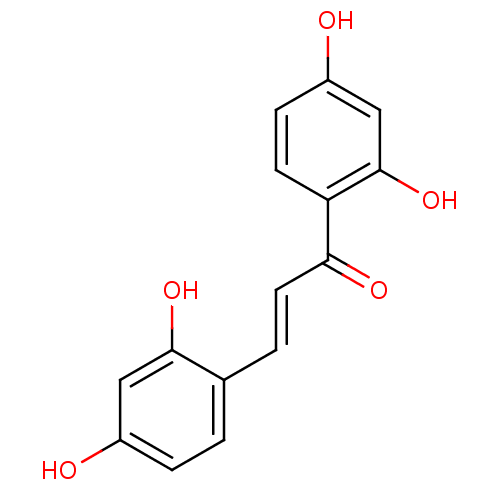
((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...)Show InChI InChI=1S/C15H12O5/c16-10-3-1-9(14(19)7-10)2-6-13(18)12-5-4-11(17)8-15(12)20/h1-8,16-17,19-20H/b6-2+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50203985

((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...)Show InChI InChI=1S/C15H12O5/c16-10-3-1-9(14(19)7-10)2-6-13(18)12-5-4-11(17)8-15(12)20/h1-8,16-17,19-20H/b6-2+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50031467

(5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...)Show InChI InChI=1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50251013
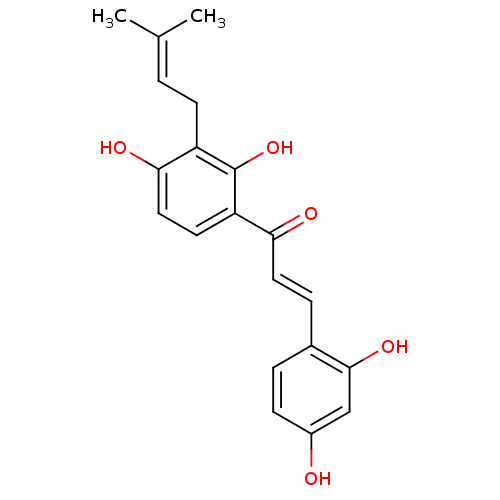
(2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8])cc2-[#8])c1-[#8] Show InChI InChI=1S/C20H20O5/c1-12(2)3-7-15-18(23)10-8-16(20(15)25)17(22)9-5-13-4-6-14(21)11-19(13)24/h3-6,8-11,21,23-25H,7H2,1-2H3/b9-5+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50251013

(2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8])cc2-[#8])c1-[#8] Show InChI InChI=1S/C20H20O5/c1-12(2)3-7-15-18(23)10-8-16(20(15)25)17(22)9-5-13-4-6-14(21)11-19(13)24/h3-6,8-11,21,23-25H,7H2,1-2H3/b9-5+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50230001

(4-(4-(4-fluorophenyl)-2-(4-nitrophenyl)-1H-imidazo...)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C20H13FN4O2/c21-16-5-1-13(2-6-16)18-19(14-9-11-22-12-10-14)24-20(23-18)15-3-7-17(8-4-15)25(26)27/h1-12H,(H,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50230001

(4-(4-(4-fluorophenyl)-2-(4-nitrophenyl)-1H-imidazo...)Show SMILES [O-][N+](=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C20H13FN4O2/c21-16-5-1-13(2-6-16)18-19(14-9-11-22-12-10-14)24-20(23-18)15-3-7-17(8-4-15)25(26)27/h1-12H,(H,23,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50441626
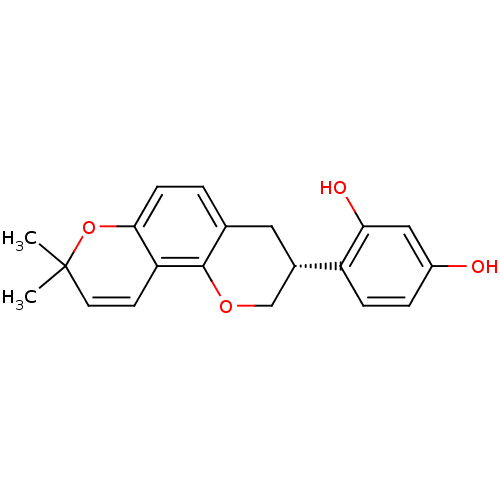
(GLABRIDIN | US9783551, Compound 1)Show SMILES CC1(C)Oc2ccc3C[C@@H](COc3c2C=C1)c1ccc(O)cc1O |r,c:16| Show InChI InChI=1S/C20H20O4/c1-20(2)8-7-16-18(24-20)6-3-12-9-13(11-23-19(12)16)15-5-4-14(21)10-17(15)22/h3-8,10,13,21-22H,9,11H2,1-2H3/t13-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) using L-tyrosine as substrate preincubated for 5 mins followed by substrate addition |
J Nat Prod 80: 334-346 (2017)
Article DOI: 10.1021/acs.jnatprod.6b00783
BindingDB Entry DOI: 10.7270/Q2XD146V |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50108046
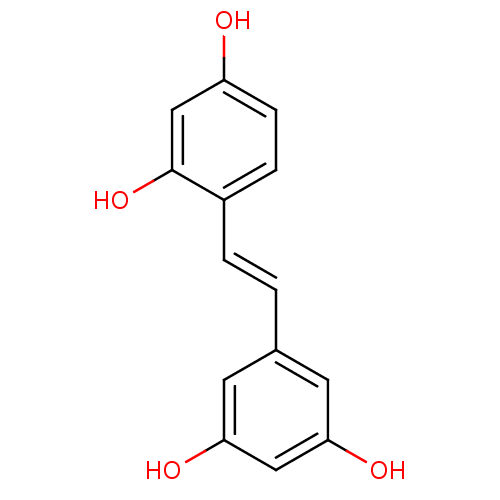
((oxyresveratrol)4-[(E)-2-(3,5-dihydroxyphenyl)viny...)Show InChI InChI=1S/C14H12O4/c15-11-4-3-10(14(18)8-11)2-1-9-5-12(16)7-13(17)6-9/h1-8,15-18H/b2-1+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human tyrosinase expressed in HEK293 cells using L-tyrosine and DOPA as substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00994
BindingDB Entry DOI: 10.7270/Q2PV6Q29 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50172095
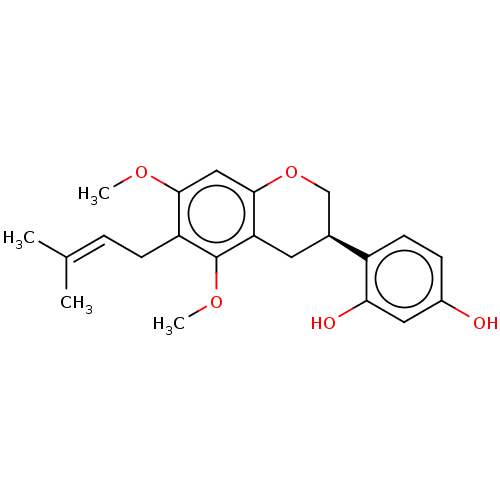
(CHEBI:69084 | Glyasperin D)Show SMILES [#6]-[#8]-c1cc2-[#8]-[#6]-[#6@H](-[#6]-c2c(-[#8]-[#6])c1-[#6]\[#6]=[#6](\[#6])-[#6])-c1ccc(-[#8])cc1-[#8] Show InChI InChI=1S/C22H26O5/c1-13(2)5-7-17-20(25-3)11-21-18(22(17)26-4)9-14(12-27-21)16-8-6-15(23)10-19(16)24/h5-6,8,10-11,14,23-24H,7,9,12H2,1-4H3/t14-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) using L-tyrosine as substrate assessed as oxidation of L-tyrosine after 15 mins |
J Nat Prod 79: 281-92 (2016)
Article DOI: 10.1021/acs.jnatprod.5b00877
BindingDB Entry DOI: 10.7270/Q20V8FPM |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50529589
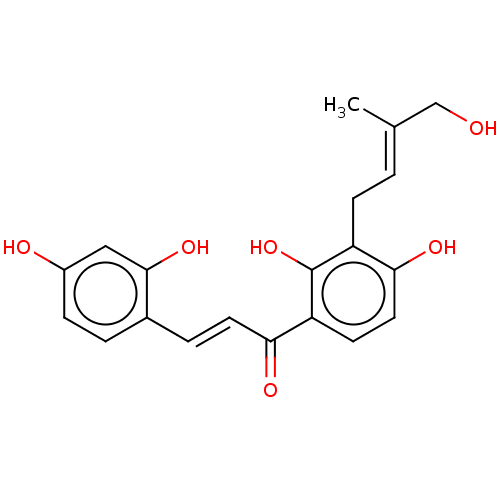
(CHEMBL4239110)Show SMILES C\C(CO)=C/Cc1c(O)ccc(C(=O)\C=C\c2ccc(O)cc2O)c1O Show InChI InChI=1S/C20H20O6/c1-12(11-21)2-6-15-18(24)9-7-16(20(15)26)17(23)8-4-13-3-5-14(22)10-19(13)25/h2-5,7-10,21-22,24-26H,6,11H2,1H3/b8-4+,12-2+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50529589

(CHEMBL4239110)Show SMILES C\C(CO)=C/Cc1c(O)ccc(C(=O)\C=C\c2ccc(O)cc2O)c1O Show InChI InChI=1S/C20H20O6/c1-12(11-21)2-6-15-18(24)9-7-16(20(15)26)17(23)8-4-13-3-5-14(22)10-19(13)25/h2-5,7-10,21-22,24-26H,6,11H2,1H3/b8-4+,12-2+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50172096
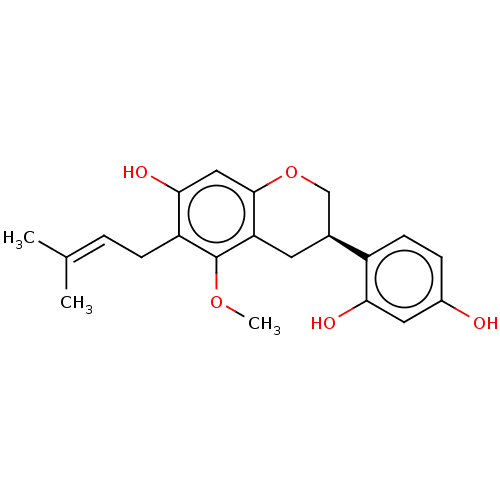
(CHEBI:69089 | Glyasperin C)Show SMILES [#6]-[#8]-c1c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])cc2-[#8]-[#6]-[#6@H](-[#6]-c12)-c1ccc(-[#8])cc1-[#8] |r| Show InChI InChI=1S/C21H24O5/c1-12(2)4-6-16-19(24)10-20-17(21(16)25-3)8-13(11-26-20)15-7-5-14(22)9-18(15)23/h4-5,7,9-10,13,22-24H,6,8,11H2,1-3H3/t13-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) using L-tyrosine as substrate assessed as oxidation of L-tyrosine after 15 mins |
J Nat Prod 79: 281-92 (2016)
Article DOI: 10.1021/acs.jnatprod.5b00877
BindingDB Entry DOI: 10.7270/Q20V8FPM |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50146416
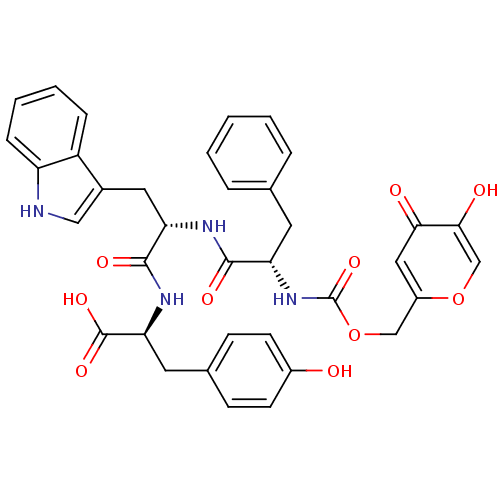
((S)-2-[(S)-2-[(S)-2-(5-Hydroxy-4-oxo-4H-pyran-2-yl...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1cc(=O)c(O)co1 Show InChI InChI=1S/C36H34N4O10/c41-24-12-10-22(11-13-24)15-30(35(46)47)39-34(45)29(16-23-18-37-27-9-5-4-8-26(23)27)38-33(44)28(14-21-6-2-1-3-7-21)40-36(48)50-19-25-17-31(42)32(43)20-49-25/h1-13,17-18,20,28-30,37,41,43H,14-16,19H2,(H,38,44)(H,39,45)(H,40,48)(H,46,47)/t28-,29-,30-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against mushroom tyrosinase |
Bioorg Med Chem Lett 14: 2843-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.046
BindingDB Entry DOI: 10.7270/Q2N015ZH |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50193667
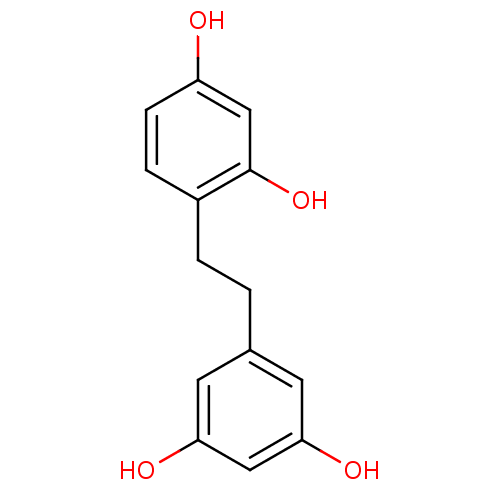
(2,4,3',5'-tetrahydroxybibenzyl | CHEMBL221291)Show InChI InChI=1S/C14H14O4/c15-11-4-3-10(14(18)8-11)2-1-9-5-12(16)7-13(17)6-9/h3-8,15-18H,1-2H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16 cells |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50193667

(2,4,3',5'-tetrahydroxybibenzyl | CHEMBL221291)Show InChI InChI=1S/C14H14O4/c15-11-4-3-10(14(18)8-11)2-1-9-5-12(16)7-13(17)6-9/h3-8,15-18H,1-2H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16 cells |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM85807

(Beta-PEA | CAS_60-12-8 | NSC_6054)Show InChI InChI=1S/C8H10O/c9-7-6-8-4-2-1-3-5-8/h1-5,9H,6-7H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| | n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50146418
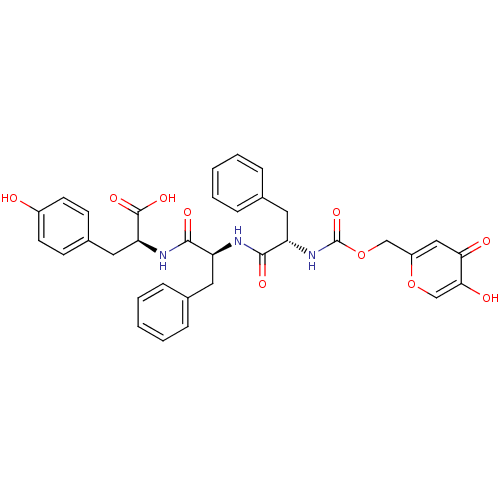
((S)-2-{(S)-2-[(S)-2-(5-Hydroxy-4-oxo-4H-pyran-2-yl...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1cc(=O)c(O)co1 Show InChI InChI=1S/C34H33N3O10/c38-24-13-11-23(12-14-24)17-28(33(43)44)36-31(41)26(15-21-7-3-1-4-8-21)35-32(42)27(16-22-9-5-2-6-10-22)37-34(45)47-19-25-18-29(39)30(40)20-46-25/h1-14,18,20,26-28,38,40H,15-17,19H2,(H,35,42)(H,36,41)(H,37,45)(H,43,44)/t26-,27-,28-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against mushroom tyrosinase |
Bioorg Med Chem Lett 14: 2843-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.046
BindingDB Entry DOI: 10.7270/Q2N015ZH |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50263336
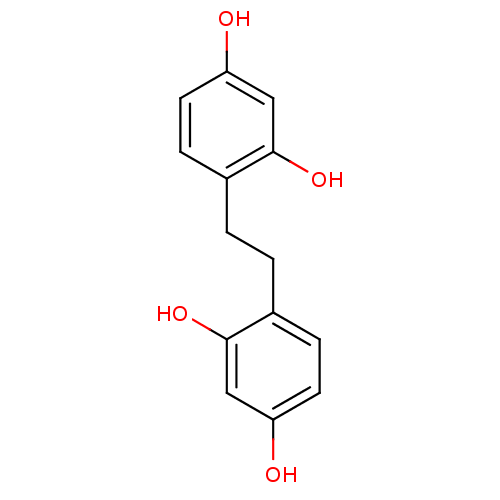
(4,4'-(ethane-1,2-diyl)dibenzene-1,3-diol | CHEMBL4...)Show InChI InChI=1S/C14H14O4/c15-11-5-3-9(13(17)7-11)1-2-10-4-6-12(16)8-14(10)18/h3-8,15-18H,1-2H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University
Curated by ChEMBL
| Assay Description
Inhibition of Tyrosinase |
Eur J Med Chem 46: 1374-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.065
BindingDB Entry DOI: 10.7270/Q20V8D41 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50146415
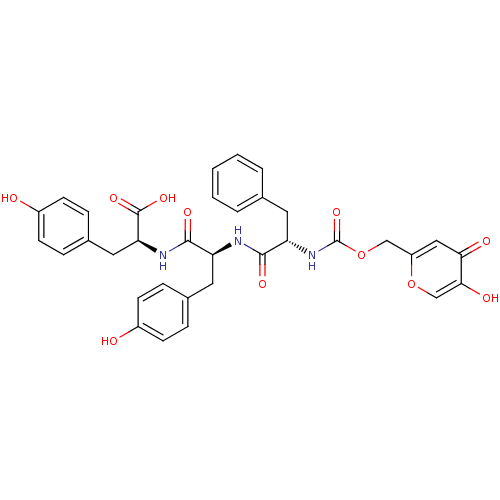
((S)-2-[(S)-2-[(S)-2-(5-Hydroxy-4-oxo-4H-pyran-2-yl...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1cc(=O)c(O)co1 Show InChI InChI=1S/C34H33N3O11/c38-23-10-6-21(7-11-23)15-26(31(42)36-28(33(44)45)16-22-8-12-24(39)13-9-22)35-32(43)27(14-20-4-2-1-3-5-20)37-34(46)48-18-25-17-29(40)30(41)19-47-25/h1-13,17,19,26-28,38-39,41H,14-16,18H2,(H,35,43)(H,36,42)(H,37,46)(H,44,45)/t26-,27-,28-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against mushroom tyrosinase |
Bioorg Med Chem Lett 14: 2843-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.046
BindingDB Entry DOI: 10.7270/Q2N015ZH |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50264086
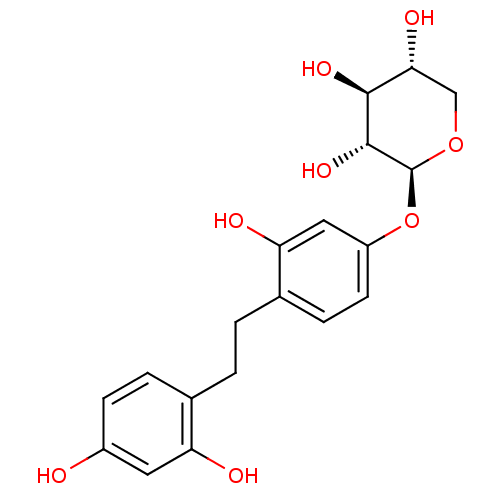
((2S,3R,4S,5R)-2-(4-(2,4-dihydroxyphenethyl)-3-hydr...)Show SMILES O[C@@H]1CO[C@@H](Oc2ccc(CCc3ccc(O)cc3O)c(O)c2)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H22O8/c20-12-5-3-10(14(21)7-12)1-2-11-4-6-13(8-15(11)22)27-19-18(25)17(24)16(23)9-26-19/h3-8,16-25H,1-2,9H2/t16-,17+,18-,19+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University
Curated by ChEMBL
| Assay Description
Inhibition of Tyrosinase |
Eur J Med Chem 46: 1374-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.065
BindingDB Entry DOI: 10.7270/Q20V8D41 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50292636
(4-hexyl resorcinol | ACRISORCIN | CHEMBL443605)Show InChI InChI=1S/C12H18O2/c1-2-3-4-5-6-10-7-8-11(13)9-12(10)14/h7-9,13-14H,2-6H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Agriculture and Technology
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) using L-DOPA as substrate assessed as formation of dopachrome by spectrophotometric analysis |
Bioorg Med Chem 23: 6650-8 (2015)
Article DOI: 10.1016/j.bmc.2015.09.014
BindingDB Entry DOI: 10.7270/Q2DR2X9W |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50608337

((+)-Anymol | CHEMBL477832)Show SMILES [H][C@@]1([#6]-[#6]-[#6](-[#6])=[#6]-[#6]1)[C@]([#6])([#8])[#6]-[#6]\[#6]=[#6](\[#6])-[#6] |r,c:5| | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 635 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50340788
(4-(beta-D-Cellobiopyranosyl)-2,2',4'-trihydroxybib...)Show SMILES OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](Oc3ccc(CCc4ccc(O)cc4O)c(O)c3)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C26H34O14/c27-9-17-19(32)20(33)22(35)26(38-17)40-24-18(10-28)39-25(23(36)21(24)34)37-14-6-4-12(16(31)8-14)2-1-11-3-5-13(29)7-15(11)30/h3-8,17-36H,1-2,9-10H2/t17-,18-,19-,20+,21-,22-,23-,24-,25-,26+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University
Curated by ChEMBL
| Assay Description
Inhibition of Tyrosinase |
Eur J Med Chem 46: 1374-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.065
BindingDB Entry DOI: 10.7270/Q20V8D41 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50366430
(CHEMBL1794031)Show SMILES CC[C@H](C)[C@H](NC(=O)OCc1cc(=O)c(O)co1)C(=O)OCc1cc(=O)c(O)co1 Show InChI InChI=1S/C19H21NO10/c1-3-10(2)17(18(25)29-6-11-4-13(21)15(23)8-27-11)20-19(26)30-7-12-5-14(22)16(24)9-28-12/h4-5,8-10,17,23-24H,3,6-7H2,1-2H3,(H,20,26)/t10-,17-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of tyrosinase enzyme. |
Bioorg Med Chem Lett 6: 1303-1308 (1996)
Article DOI: 10.1016/0960-894X(96)00221-1
BindingDB Entry DOI: 10.7270/Q2V1259K |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50264087
((2S,2'S,3R,3'R,4S,4'S,5R,5'R)-2,2'-(4-(2,4-dihydro...)Show SMILES O[C@@H]1CO[C@@H](Oc2ccc(CCc3ccc(O)cc3O)c(O[C@@H]3OC[C@@H](O)[C@H](O)[C@H]3O)c2)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C24H30O12/c25-13-5-3-11(15(26)7-13)1-2-12-4-6-14(35-23-21(31)19(29)16(27)9-33-23)8-18(12)36-24-22(32)20(30)17(28)10-34-24/h3-8,16-17,19-32H,1-2,9-10H2/t16-,17-,19+,20+,21-,22-,23+,24+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University
Curated by ChEMBL
| Assay Description
Inhibition of Tyrosinase |
Eur J Med Chem 46: 1374-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.065
BindingDB Entry DOI: 10.7270/Q20V8D41 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50237231
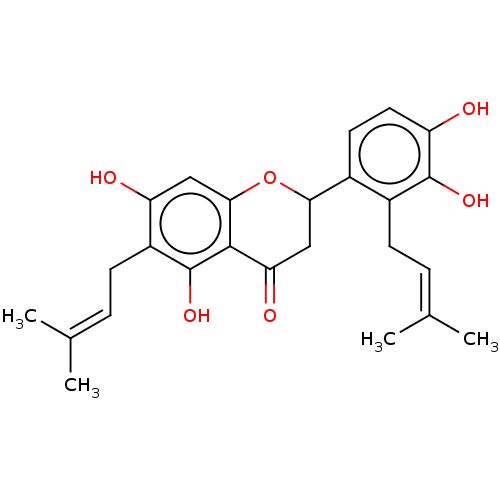
(CHEMBL4063130)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc2-[#8]-[#6](-[#6]-[#6](=O)-c2c1-[#8])-c1ccc(-[#8])c(-[#8])c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C25H28O6/c1-13(2)5-7-16-15(9-10-18(26)24(16)29)21-12-20(28)23-22(31-21)11-19(27)17(25(23)30)8-6-14(3)4/h5-6,9-11,21,26-27,29-30H,7-8,12H2,1-4H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de C£rdoba
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16 cells assessed as reduction in melanin production measured after 24 hrs |
Bioorg Med Chem Lett 27: 1789-1794 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.058
BindingDB Entry DOI: 10.7270/Q2MW2KDB |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50340787
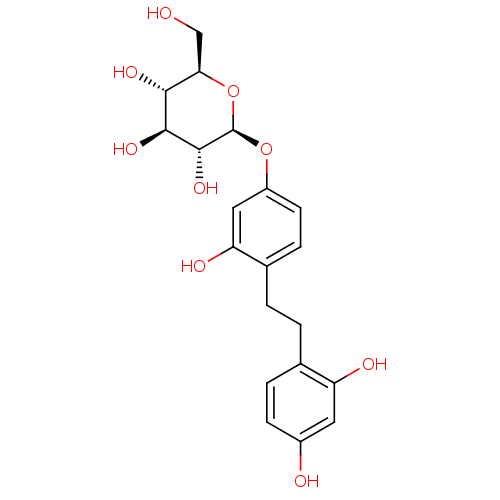
(4-(beta-D-Glucopyranosyl)-2,2',4'-trihydroxybibenz...)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(CCc3ccc(O)cc3O)c(O)c2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C20H24O9/c21-9-16-17(25)18(26)19(27)20(29-16)28-13-6-4-11(15(24)8-13)2-1-10-3-5-12(22)7-14(10)23/h3-8,16-27H,1-2,9H2/t16-,17-,18+,19-,20-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University
Curated by ChEMBL
| Assay Description
Inhibition of Tyrosinase |
Eur J Med Chem 46: 1374-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.065
BindingDB Entry DOI: 10.7270/Q20V8D41 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50146414
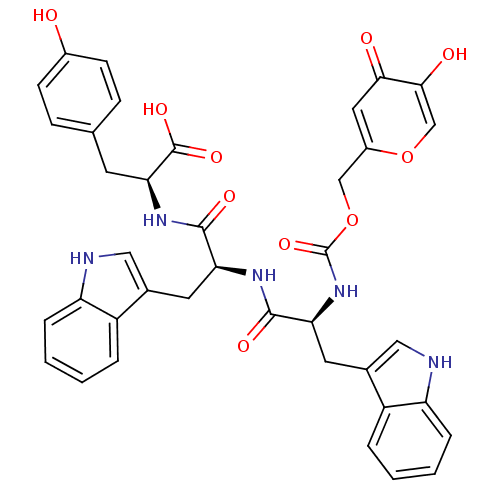
((2S)-2-[(2S)-2-[(2S)-2-({[(5-hydroxy-4-oxo-4H-pyra...)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1cc(=O)c(O)co1 Show InChI InChI=1S/C38H35N5O10/c44-24-11-9-21(10-12-24)13-32(37(49)50)42-35(47)30(14-22-17-39-28-7-3-1-5-26(22)28)41-36(48)31(15-23-18-40-29-8-4-2-6-27(23)29)43-38(51)53-19-25-16-33(45)34(46)20-52-25/h1-12,16-18,20,30-32,39-40,44,46H,13-15,19H2,(H,41,48)(H,42,47)(H,43,51)(H,49,50)/t30-,31-,32-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibitory activity against mushroom tyrosinase |
Bioorg Med Chem Lett 14: 2843-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.046
BindingDB Entry DOI: 10.7270/Q2N015ZH |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50529600
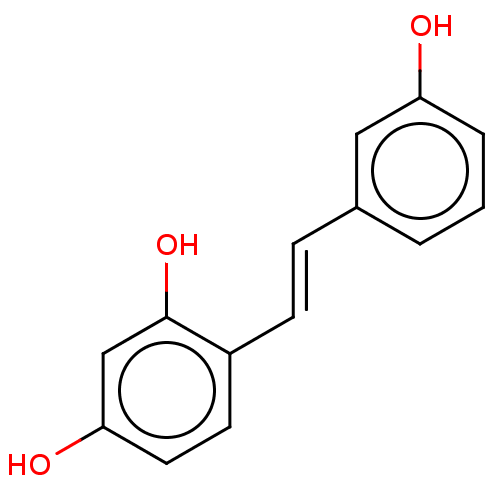
(CHEMBL4445400)Show InChI InChI=1S/C14H12O3/c15-12-3-1-2-10(8-12)4-5-11-6-7-13(16)9-14(11)17/h1-9,15-17H/b5-4+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16 cells |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50529600

(CHEMBL4445400)Show InChI InChI=1S/C14H12O3/c15-12-3-1-2-10(8-12)4-5-11-6-7-13(16)9-14(11)17/h1-9,15-17H/b5-4+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16 cells |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50340789
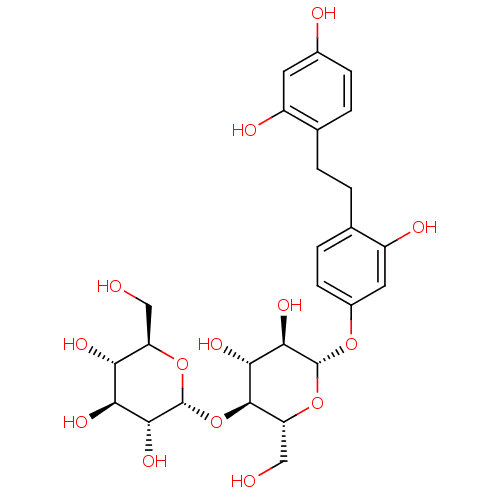
(2,2',4'-Trihydroxy-4-(beta-D-maltopyranosyl)bibenz...)Show SMILES OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](Oc3ccc(CCc4ccc(O)cc4O)c(O)c3)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C26H34O14/c27-9-17-19(32)20(33)22(35)26(38-17)40-24-18(10-28)39-25(23(36)21(24)34)37-14-6-4-12(16(31)8-14)2-1-11-3-5-13(29)7-15(11)30/h3-8,17-36H,1-2,9-10H2/t17-,18-,19-,20+,21-,22-,23-,24-,25-,26-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Utsunomiya University
Curated by ChEMBL
| Assay Description
Inhibition of Tyrosinase |
Eur J Med Chem 46: 1374-81 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.065
BindingDB Entry DOI: 10.7270/Q20V8D41 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50251005
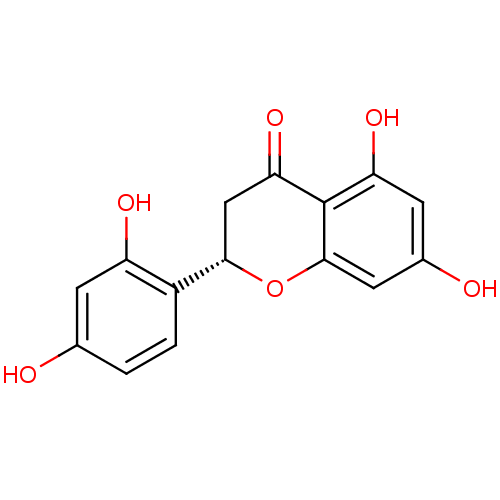
((2S)-5,7,2',4'-tetrahydroxyflavanone | (S)-2-(2,4-...)Show SMILES Oc1ccc([C@@H]2CC(=O)c3c(O)cc(O)cc3O2)c(O)c1 |r| Show InChI InChI=1S/C15H12O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-5,13,16-19H,6H2/t13-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50251005

((2S)-5,7,2',4'-tetrahydroxyflavanone | (S)-2-(2,4-...)Show SMILES Oc1ccc([C@@H]2CC(=O)c3c(O)cc(O)cc3O2)c(O)c1 |r| Show InChI InChI=1S/C15H12O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-5,13,16-19H,6H2/t13-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) |
J Med Chem 61: 7395-7418 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00967
BindingDB Entry DOI: 10.7270/Q2CN77C2 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50559078
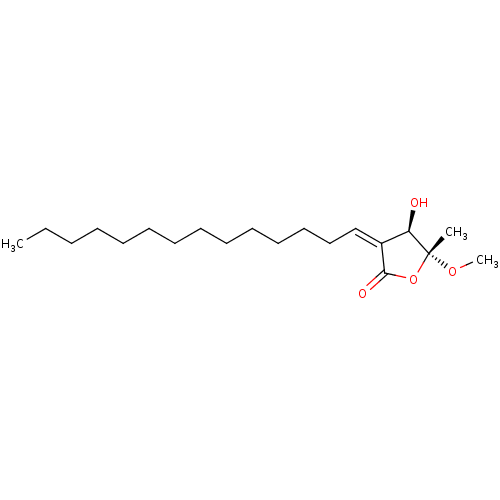
(SUBAMOLIDE A)Show SMILES CCCCCCCCCCCCC\C=C1\[C@@H](O)[C@](C)(OC)OC1=O |r| | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of tyrosinase in human neonatal foreskin epidermal melanocyte cells using L-tyrosine and L-DOPA as substrate preincubated for 2 days follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00994
BindingDB Entry DOI: 10.7270/Q2PV6Q29 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50559077
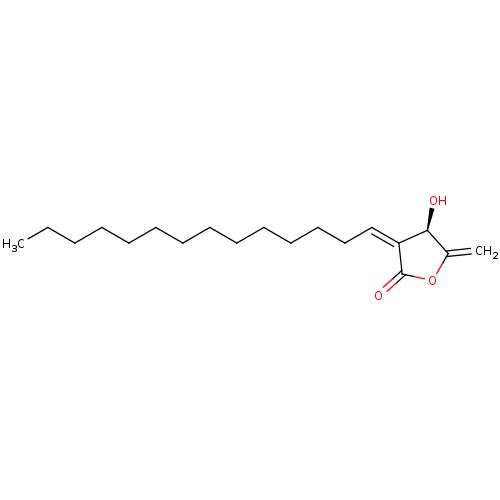
(CHEMBL4473314) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of tyrosinase in human neonatal foreskin epidermal melanocyte cells using L-tyrosine and L-DOPA as substrate preincubated for 2 days follo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00994
BindingDB Entry DOI: 10.7270/Q2PV6Q29 |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Mus musculus (Mouse)) | BDBM50237232
(CHEMBL516671)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(-[#8])c2-[#6](=O)-[#6]-[#6](-[#8]-c12)-c1cc(c(-[#8])cc1-[#8])C([#6])([#6])[#6]=[#6] Show InChI InChI=1S/C25H28O6/c1-6-25(4,5)16-9-15(18(27)10-19(16)28)22-12-21(30)23-20(29)11-17(26)14(24(23)31-22)8-7-13(2)3/h6-7,9-11,22,26-29H,1,8,12H2,2-5H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Nacional de C£rdoba
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase in mouse B16 cells assessed as reduction in melanin production measured after 24 hrs |
Bioorg Med Chem Lett 27: 1789-1794 (2017)
Article DOI: 10.1016/j.bmcl.2017.02.058
BindingDB Entry DOI: 10.7270/Q2MW2KDB |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM7462

(3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...)Show InChI InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) using L-tyrosine as substrate assessed as oxidation of L-tyrosine after 15 mins |
J Nat Prod 79: 281-92 (2016)
Article DOI: 10.1021/acs.jnatprod.5b00877
BindingDB Entry DOI: 10.7270/Q20V8FPM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data