 Found 1059 hits of ic50 for UniProtKB: P20309
Found 1059 hits of ic50 for UniProtKB: P20309 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419522
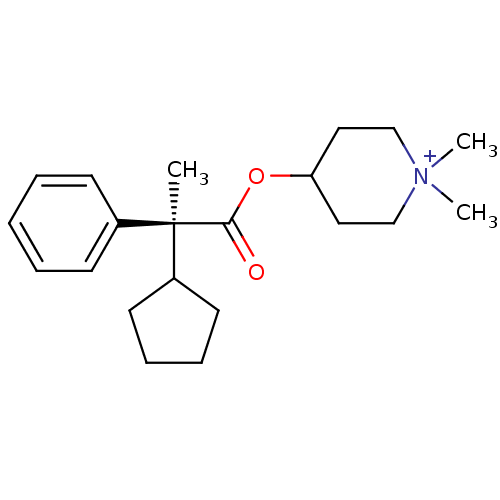
(CHEMBL1924027)Show SMILES C[C@@](C1CCCC1)(C(=O)OC1CC[N+](C)(C)CC1)c1ccccc1 |r| Show InChI InChI=1S/C21H32NO2/c1-21(18-11-7-8-12-18,17-9-5-4-6-10-17)20(23)24-19-13-15-22(2,3)16-14-19/h4-6,9-10,18-19H,7-8,11-16H2,1-3H3/q+1/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296345

((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...)Show SMILES C[N+]1(C)[C@@H]2C[C@H](C[C@@H]1[C@@H]1O[C@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 |TLB:11:5:1:8.10| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13-,16-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHO-K1 cells after 16 hrs by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7458-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.016
BindingDB Entry DOI: 10.7270/Q2GM88K4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419512
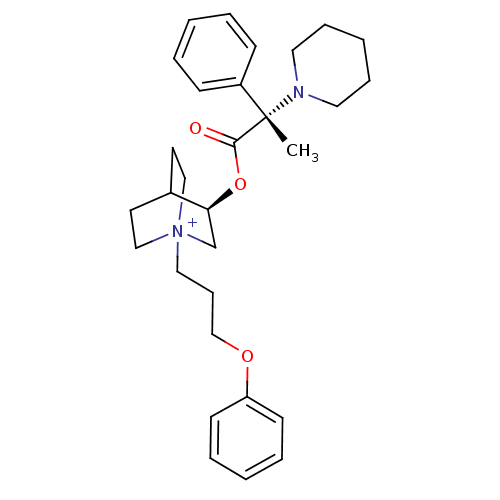
(CHEMBL1924040)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(6.96,-48.42,;6.97,-46.88,;6.97,-45.33,;8.31,-44.56,;8.31,-43.03,;6.97,-42.25,;5.63,-43.02,;5.63,-44.57,;8.31,-47.65,;8.32,-49.2,;9.65,-46.87,;10.99,-47.64,;10.99,-49.19,;12.33,-49.95,;12.37,-51.49,;11.07,-52.3,;11.11,-53.83,;9.8,-54.64,;8.45,-53.91,;8.41,-52.38,;7.06,-51.65,;5.75,-52.46,;5.8,-54,;7.15,-54.72,;13.67,-49.19,;13.67,-47.64,;12.33,-46.86,;11.55,-48.19,;13.03,-48.58,;5.63,-47.64,;4.31,-46.85,;2.97,-47.61,;2.96,-49.15,;4.29,-49.93,;5.62,-49.17,)| Show InChI InChI=1S/C30H41N2O3/c1-30(26-12-5-2-6-13-26,31-18-9-4-10-19-31)29(33)35-28-24-32(21-16-25(28)17-22-32)20-11-23-34-27-14-7-3-8-15-27/h2-3,5-8,12-15,25,28H,4,9-11,16-24H2,1H3/q+1/t25?,28-,30-,32?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419531
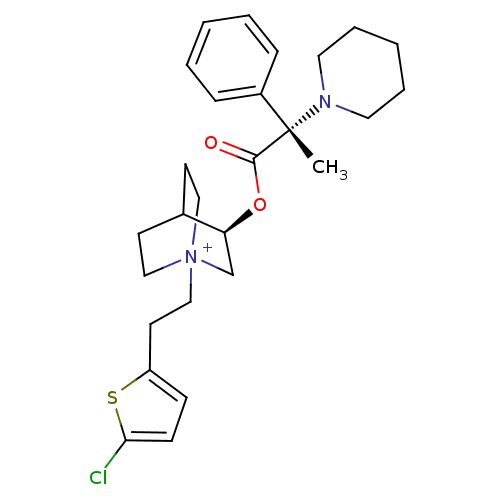
(CHEMBL1921908)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCc3ccc(Cl)s3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(43.85,-32.51,;43.86,-30.96,;43.86,-29.42,;45.2,-28.65,;45.19,-27.11,;43.86,-26.33,;42.52,-27.11,;42.51,-28.66,;45.2,-31.74,;45.21,-33.28,;46.54,-30.96,;47.88,-31.73,;47.88,-33.28,;49.22,-34.03,;49.26,-35.57,;50.62,-36.3,;51.89,-35.43,;51.93,-33.89,;53.4,-33.45,;54.27,-34.72,;55.81,-34.76,;53.34,-35.94,;50.56,-33.28,;50.56,-31.73,;49.22,-30.94,;48.44,-32.27,;49.92,-32.67,;42.52,-31.72,;41.2,-30.94,;39.86,-31.69,;39.85,-33.23,;41.18,-34.01,;42.52,-33.25,)| Show InChI InChI=1S/C27H36ClN2O2S/c1-27(22-8-4-2-5-9-22,29-15-6-3-7-16-29)26(31)32-24-20-30(17-12-21(24)13-18-30)19-14-23-10-11-25(28)33-23/h2,4-5,8-11,21,24H,3,6-7,12-20H2,1H3/q+1/t21?,24-,27-,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419493
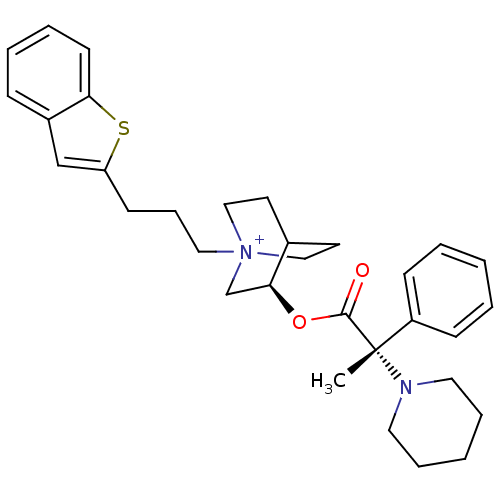
(CHEMBL1921925)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCCc3cc4ccccc4s3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(-8.09,-43.98,;-8.08,-42.44,;-8.08,-40.89,;-6.75,-40.12,;-6.75,-38.58,;-8.09,-37.8,;-9.43,-38.58,;-9.43,-40.13,;-6.74,-43.21,;-6.73,-44.76,;-5.4,-42.43,;-4.06,-43.2,;-4.06,-44.75,;-2.72,-45.51,;-2.68,-47.05,;-1.32,-47.78,;-.01,-46.97,;1.34,-47.7,;.87,-49.17,;2.13,-50.06,;2.3,-51.59,;3.71,-52.2,;4.95,-51.28,;4.77,-49.75,;3.36,-49.14,;2.87,-47.69,;-1.38,-44.75,;-1.38,-43.2,;-2.72,-42.42,;-3.5,-43.75,;-2.02,-44.14,;-9.41,-43.21,;-10.74,-42.45,;-12.07,-43.22,;-12.06,-44.76,;-10.71,-45.53,;-9.39,-44.75,)| Show InChI InChI=1S/C32H41N2O2S/c1-32(27-12-4-2-5-13-27,33-18-8-3-9-19-33)31(35)36-29-24-34(21-16-25(29)17-22-34)20-10-14-28-23-26-11-6-7-15-30(26)37-28/h2,4-7,11-13,15,23,25,29H,3,8-10,14,16-22,24H2,1H3/q+1/t25?,29-,32-,34?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419504
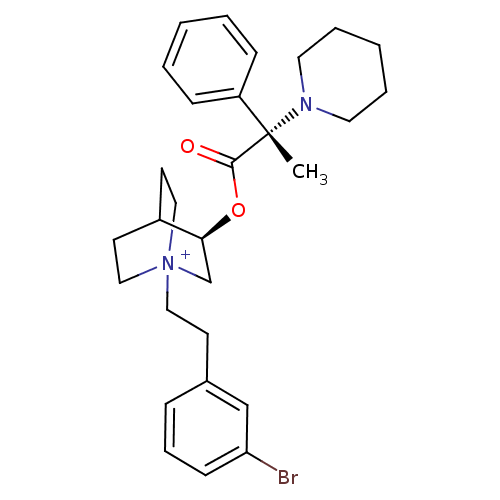
(CHEMBL1921907)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCc3cccc(Br)c3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(28.27,-31.58,;28.28,-30.04,;28.28,-28.49,;29.62,-27.73,;29.62,-26.19,;28.28,-25.41,;26.94,-26.19,;26.94,-27.74,;29.63,-30.81,;29.63,-32.36,;30.96,-30.03,;32.31,-30.81,;32.31,-32.35,;33.64,-33.11,;33.69,-34.65,;35.04,-35.38,;36.35,-34.57,;36.3,-33.04,;37.6,-32.23,;38.96,-32.96,;39.01,-34.5,;40.36,-35.23,;37.7,-35.31,;34.98,-32.35,;34.98,-30.81,;33.64,-30.02,;32.86,-31.35,;34.34,-31.75,;26.94,-30.8,;25.62,-30.01,;24.28,-30.77,;24.27,-32.31,;25.6,-33.09,;26.94,-32.33,)| Show InChI InChI=1S/C29H38BrN2O2/c1-29(25-10-4-2-5-11-25,31-16-6-3-7-17-31)28(33)34-27-22-32(19-14-24(27)15-20-32)18-13-23-9-8-12-26(30)21-23/h2,4-5,8-12,21,24,27H,3,6-7,13-20,22H2,1H3/q+1/t24?,27-,29-,32?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419538
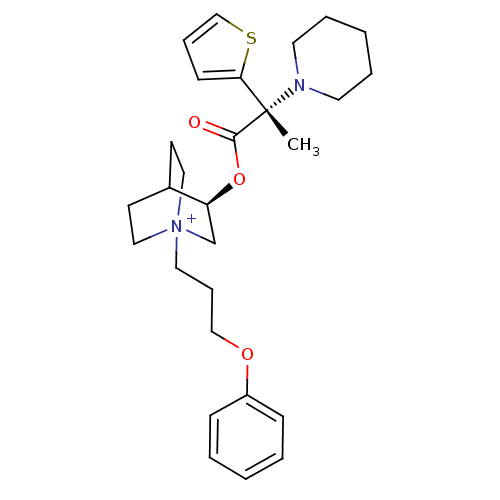
(CHEMBL1921935)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)c1cccs1 |r,wU:1.0,wD:1.8,11.11,(41.55,-11.62,;41.56,-10.08,;41.55,-8.53,;42.89,-7.77,;42.89,-6.23,;41.55,-5.45,;40.21,-6.23,;40.21,-7.78,;42.9,-10.85,;42.9,-12.4,;44.24,-10.07,;45.58,-10.85,;45.58,-12.39,;46.92,-13.15,;46.96,-14.69,;48.31,-15.42,;49.62,-14.61,;50.98,-15.34,;52.29,-14.53,;53.64,-15.27,;54.94,-14.46,;54.9,-12.92,;53.54,-12.19,;52.23,-13,;48.25,-12.39,;48.25,-10.85,;46.92,-10.06,;46.13,-11.39,;47.62,-11.79,;40.32,-10.99,;38.84,-10.53,;37.95,-11.79,;38.86,-13.02,;40.32,-12.54,)| Show InChI InChI=1S/C28H39N2O3S/c1-28(26-12-8-21-34-26,29-15-6-3-7-16-29)27(31)33-25-22-30(18-13-23(25)14-19-30)17-9-20-32-24-10-4-2-5-11-24/h2,4-5,8,10-12,21,23,25H,3,6-7,9,13-20,22H2,1H3/q+1/t23?,25-,28-,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419484
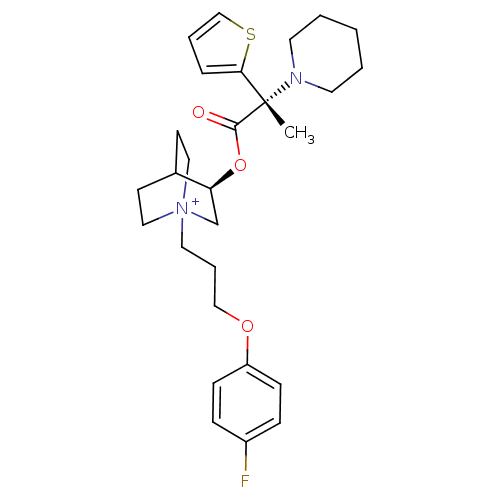
(CHEMBL1921937)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccc(F)cc3)CCC1CC2)c1cccs1 |r,wU:1.0,wD:1.8,11.11,(12.44,-21.32,;12.45,-19.78,;12.45,-18.23,;13.78,-17.46,;13.78,-15.93,;12.45,-15.15,;11.11,-15.92,;11.1,-17.48,;13.79,-20.55,;13.8,-22.1,;15.13,-19.77,;16.47,-20.54,;16.47,-22.09,;17.81,-22.85,;17.85,-24.39,;19.21,-25.12,;20.52,-24.31,;21.87,-25.04,;23.18,-24.23,;24.53,-24.97,;25.84,-24.16,;25.79,-22.62,;27.1,-21.81,;24.43,-21.89,;23.13,-22.7,;19.14,-22.09,;19.14,-20.54,;17.81,-19.76,;17.02,-21.09,;18.51,-21.48,;11.21,-20.69,;9.73,-20.23,;8.84,-21.48,;9.75,-22.72,;11.21,-22.23,)| Show InChI InChI=1S/C28H38FN2O3S/c1-28(26-7-5-20-35-26,30-14-3-2-4-15-30)27(32)34-25-21-31(17-12-22(25)13-18-31)16-6-19-33-24-10-8-23(29)9-11-24/h5,7-11,20,22,25H,2-4,6,12-19,21H2,1H3/q+1/t22?,25-,28-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419541
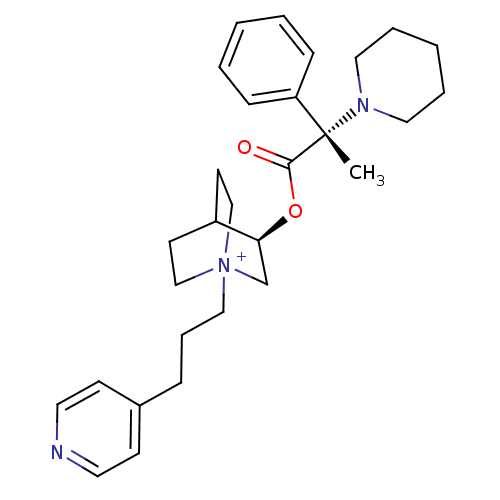
(CHEMBL1921917)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCCc3ccncc3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(9.05,-9.08,;9.06,-7.54,;9.05,-5.99,;10.39,-5.22,;10.39,-3.68,;9.05,-2.91,;7.71,-3.68,;7.71,-5.23,;10.4,-8.31,;10.41,-9.86,;11.74,-7.53,;13.08,-8.3,;13.08,-9.85,;14.42,-10.61,;14.46,-12.15,;15.82,-12.88,;17.13,-12.07,;18.48,-12.8,;18.52,-14.34,;19.88,-15.06,;21.19,-14.26,;21.14,-12.71,;19.78,-11.99,;15.75,-9.85,;15.75,-8.3,;14.42,-7.52,;13.63,-8.85,;15.12,-9.24,;7.73,-8.3,;6.39,-7.53,;5.06,-8.3,;5.06,-9.84,;6.4,-10.61,;7.73,-9.84,)| Show InChI InChI=1S/C29H40N3O2/c1-29(26-10-4-2-5-11-26,31-18-6-3-7-19-31)28(33)34-27-23-32(21-14-25(27)15-22-32)20-8-9-24-12-16-30-17-13-24/h2,4-5,10-13,16-17,25,27H,3,6-9,14-15,18-23H2,1H3/q+1/t25?,27-,29-,32?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419478

(CHEMBL1921945)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CC(=O)Nc3cccc(F)c3)CCC1CC2)c1cccs1 |r,wU:1.0,wD:1.8,11.11,(43.88,2.13,;43.89,3.67,;43.89,5.22,;45.23,5.98,;45.23,7.53,;43.89,8.31,;42.55,7.53,;42.55,5.97,;45.24,2.9,;45.24,1.35,;46.57,3.68,;47.92,2.91,;47.92,1.36,;49.25,.6,;49.26,-.94,;50.6,-1.7,;50.61,-3.24,;51.93,-.92,;53.26,-1.68,;53.27,-3.22,;54.6,-3.98,;55.93,-3.2,;55.92,-1.65,;57.24,-.87,;54.58,-.9,;50.59,1.36,;50.59,2.91,;49.25,3.69,;48.47,2.36,;49.95,1.97,;42.65,2.76,;41.2,3.23,;40.3,1.99,;41.21,.75,;42.67,1.23,)| Show InChI InChI=1S/C27H34FN3O3S/c1-27(24-9-6-16-35-24,30-12-3-2-4-13-30)26(33)34-23-18-31(14-10-20(23)11-15-31)19-25(32)29-22-8-5-7-21(28)17-22/h5-9,16-17,20,23H,2-4,10-15,18-19H2,1H3/p+1/t20?,23-,27-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419533
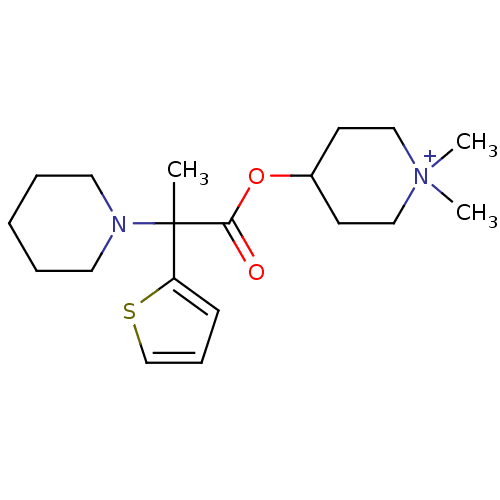
(CHEMBL1924036)Show SMILES CC(N1CCCCC1)(C(=O)OC1CC[N+](C)(C)CC1)c1cccs1 Show InChI InChI=1S/C19H31N2O2S/c1-19(17-8-7-15-24-17,20-11-5-4-6-12-20)18(22)23-16-9-13-21(2,3)14-10-16/h7-8,15-16H,4-6,9-14H2,1-3H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419535
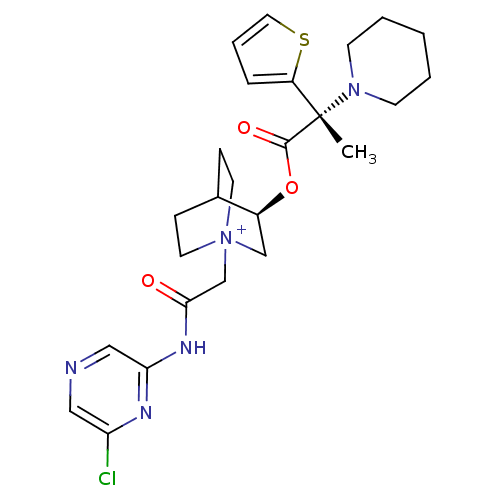
(CHEMBL1922053)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CC(=O)Nc3cncc(Cl)n3)CCC1CC2)c1cccs1 |r,wU:1.0,wD:1.8,11.11,(-7.42,-34.35,;-7.41,-32.8,;-7.41,-31.26,;-6.07,-30.49,;-6.07,-28.95,;-7.41,-28.18,;-8.75,-28.95,;-8.75,-30.5,;-6.06,-33.58,;-6.06,-35.12,;-4.73,-32.8,;-3.39,-33.57,;-3.39,-35.11,;-2.05,-35.87,;-2.04,-37.41,;-.7,-38.17,;-.69,-39.71,;.62,-37.39,;1.96,-38.15,;3.27,-37.37,;4.61,-38.12,;4.63,-39.67,;3.3,-40.45,;3.31,-41.99,;1.96,-39.69,;-.72,-35.11,;-.72,-33.57,;-2.05,-32.78,;-2.83,-34.11,;-1.35,-34.51,;-8.74,-33.57,;-10.19,-33.09,;-11.11,-34.32,;-10.22,-35.57,;-8.75,-35.12,)| Show InChI InChI=1S/C25H32ClN5O3S/c1-25(20-6-5-13-35-20,30-9-3-2-4-10-30)24(33)34-19-16-31(11-7-18(19)8-12-31)17-23(32)29-22-15-27-14-21(26)28-22/h5-6,13-15,18-19H,2-4,7-12,16-17H2,1H3/p+1/t18?,19-,25-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419506
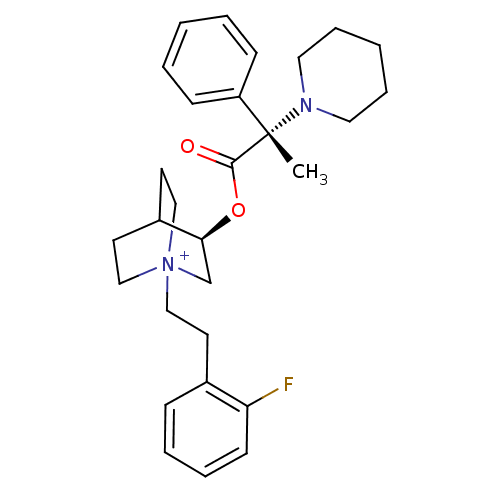
(CHEMBL1924049)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCc3ccccc3F)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(38.09,-9.95,;38.1,-8.41,;38.1,-6.86,;39.44,-6.1,;39.43,-4.56,;38.1,-3.78,;36.76,-4.56,;36.75,-6.11,;39.44,-9.18,;39.45,-10.73,;40.78,-8.4,;42.12,-9.18,;42.12,-10.72,;43.46,-11.48,;43.5,-13.02,;44.86,-13.75,;46.17,-12.94,;46.11,-11.41,;47.41,-10.6,;48.78,-11.33,;48.82,-12.87,;47.51,-13.68,;47.55,-15.21,;44.79,-10.72,;44.79,-9.18,;43.46,-8.39,;42.67,-9.72,;44.16,-10.12,;36.76,-9.17,;35.44,-8.38,;34.1,-9.14,;34.09,-10.68,;35.42,-11.46,;36.75,-10.7,)| Show InChI InChI=1S/C29H38FN2O2/c1-29(25-11-4-2-5-12-25,31-17-8-3-9-18-31)28(33)34-27-22-32(20-15-24(27)16-21-32)19-14-23-10-6-7-13-26(23)30/h2,4-7,10-13,24,27H,3,8-9,14-22H2,1H3/q+1/t24?,27-,29-,32?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296331
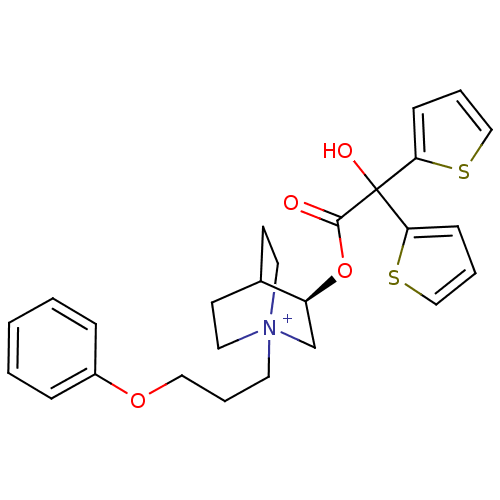
((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(-4.87,-33.87,;-5.96,-34.96,;-4.62,-35.72,;-4.61,-37.26,;-3.29,-34.95,;-1.95,-35.72,;-1.95,-37.26,;-.62,-38.02,;-.64,-39.56,;.69,-40.34,;2.03,-39.58,;3.36,-40.37,;4.7,-39.61,;6.02,-40.39,;7.36,-39.64,;7.37,-38.1,;6.04,-37.31,;4.7,-38.07,;.71,-37.26,;.71,-35.72,;-.62,-34.94,;-.2,-36.18,;-1.25,-36.53,;-5.96,-33.42,;-7.21,-32.51,;-6.74,-31.04,;-5.2,-31.04,;-4.72,-32.5,;-7.29,-35.73,;-8.7,-35.11,;-9.73,-36.25,;-8.96,-37.59,;-7.45,-37.26,)| Show InChI InChI=1S/C26H30NO4S2/c28-25(26(29,23-9-4-17-32-23)24-10-5-18-33-24)31-22-19-27(14-11-20(22)12-15-27)13-6-16-30-21-7-2-1-3-8-21/h1-5,7-10,17-18,20,22,29H,6,11-16,19H2/q+1/t20?,22-,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHO-K1 cells after 16 hrs by scintillation proximity assay |
Bioorg Med Chem Lett 20: 7458-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.016
BindingDB Entry DOI: 10.7270/Q2GM88K4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419527
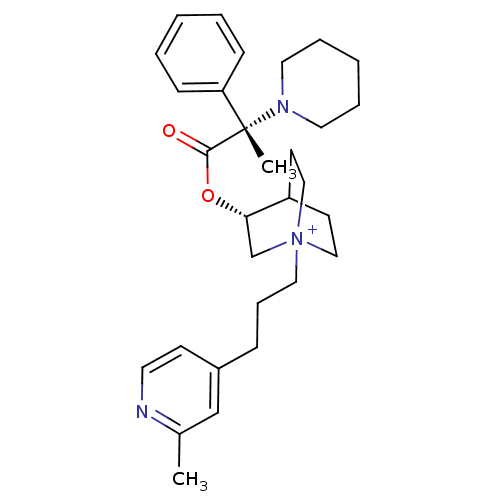
(CHEMBL1921919)Show SMILES Cc1cc(CCC[N+]23CCC(CC2)[C@H](C3)OC(=O)[C@@](C)(N2CCCCC2)c2ccccc2)ccn1 |r,wU:18.20,wD:18.19,13.16,(4.91,-28.76,;4.86,-27.22,;3.51,-26.49,;3.47,-24.96,;2.11,-24.23,;.8,-25.03,;-.55,-24.3,;-.6,-22.76,;.74,-22.01,;.74,-20.46,;-.6,-19.67,;-1.38,-21,;.1,-21.4,;-1.94,-20.46,;-1.94,-22.01,;-3.28,-19.68,;-4.62,-20.47,;-4.61,-22.01,;-5.96,-19.69,;-5.97,-21.24,;-5.97,-18.14,;-4.63,-17.37,;-4.63,-15.83,;-5.97,-15.05,;-7.31,-15.83,;-7.31,-17.38,;-7.29,-20.47,;-8.62,-19.7,;-9.95,-20.48,;-9.94,-22.02,;-8.6,-22.78,;-7.27,-22,;4.77,-24.14,;6.12,-24.87,;6.17,-26.41,)| Show InChI InChI=1S/C30H42N3O2/c1-24-22-25(13-16-31-24)10-9-19-33-20-14-26(15-21-33)28(23-33)35-29(34)30(2,27-11-5-3-6-12-27)32-17-7-4-8-18-32/h3,5-6,11-13,16,22,26,28H,4,7-10,14-15,17-21,23H2,1-2H3/q+1/t26?,28-,30-,33?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419481
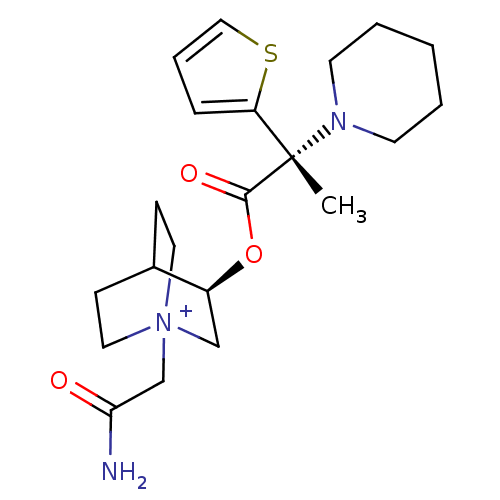
(CHEMBL1921941)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CC(N)=O)CCC1CC2)c1cccs1 |r,wU:1.0,wD:11.11,1.8,(27.7,-33.54,;27.71,-32,;27.7,-30.46,;29.04,-29.69,;29.04,-28.15,;27.7,-27.38,;26.37,-28.15,;26.36,-29.7,;29.05,-32.77,;29.05,-34.31,;30.38,-31.99,;31.72,-32.76,;31.72,-34.31,;33.06,-35.06,;33.07,-36.6,;34.4,-37.36,;35.73,-36.58,;34.41,-38.9,;34.39,-34.31,;34.39,-32.76,;33.06,-31.98,;32.27,-33.31,;33.76,-33.7,;26.48,-32.92,;25.02,-32.47,;24.13,-33.73,;25.05,-34.96,;26.51,-34.46,)| Show InChI InChI=1S/C21H31N3O3S/c1-21(18-6-5-13-28-18,23-9-3-2-4-10-23)20(26)27-17-14-24(15-19(22)25)11-7-16(17)8-12-24/h5-6,13,16-17H,2-4,7-12,14-15H2,1H3,(H-,22,25)/p+1/t16?,17-,21-,24?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419477
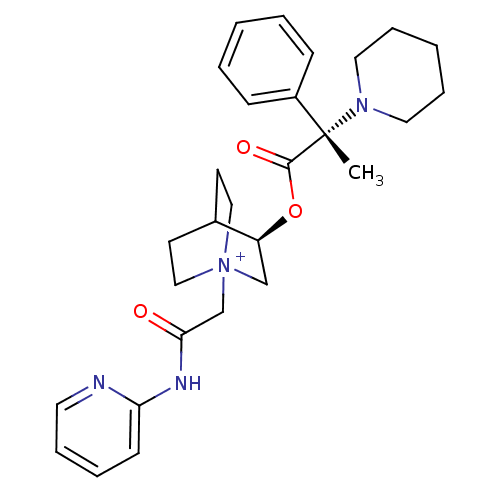
(CHEMBL1921946)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CC(=O)Nc3ccccn3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(-5.78,-11.14,;-5.77,-9.6,;-5.77,-8.05,;-4.43,-7.28,;-4.44,-5.75,;-5.77,-4.97,;-7.11,-5.74,;-7.12,-7.29,;-4.43,-10.37,;-4.42,-11.92,;-3.09,-9.59,;-1.75,-10.36,;-1.75,-11.91,;-.41,-12.67,;-.4,-14.21,;.93,-14.97,;.94,-16.5,;2.26,-14.19,;3.6,-14.95,;3.6,-16.49,;4.94,-17.25,;6.27,-16.47,;6.25,-14.92,;4.91,-14.17,;.92,-11.91,;.92,-10.36,;-.41,-9.58,;-1.2,-10.91,;.29,-11.3,;-7.1,-10.37,;-8.43,-9.6,;-9.76,-10.36,;-9.76,-11.9,;-8.42,-12.67,;-7.09,-11.9,)| Show InChI InChI=1S/C28H36N4O3/c1-28(23-10-4-2-5-11-23,31-16-8-3-9-17-31)27(34)35-24-20-32(18-13-22(24)14-19-32)21-26(33)30-25-12-6-7-15-29-25/h2,4-7,10-12,15,22,24H,3,8-9,13-14,16-21H2,1H3/p+1/t22?,24-,28-,32?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM624956
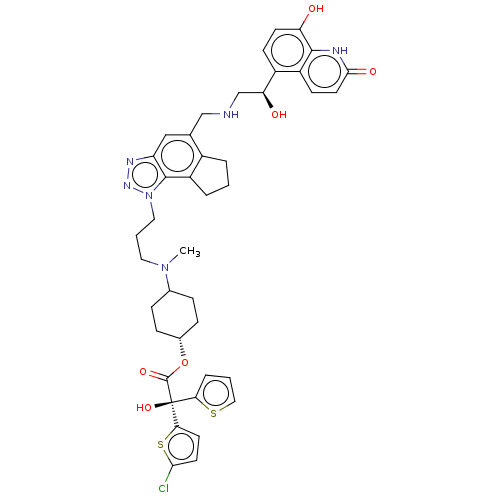
(US20230322745, Compound 28)Show SMILES CN(CCCn1nnc2cc(CNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)c3CCCc3c12)C1CC[C@@H](CC1)OC(=O)[C@](O)(c1cccs1)c1ccc(Cl)s1 |r,wU:37.45,43.49,wD:14.14,43.50,(3.77,3.25,;4.17,4.74,;3.08,5.83,;1.59,5.43,;.5,6.52,;-.98,6.12,;-1.61,4.72,;-3.14,4.88,;-3.46,6.38,;-4.8,7.15,;-4.8,8.69,;-6.13,9.46,;-7.46,8.69,;-8.8,9.46,;-10.13,8.69,;-10.13,7.15,;-11.46,9.46,;-12.8,8.69,;-14.13,9.46,;-14.13,11,;-15.46,11.77,;-12.8,11.77,;-12.8,13.31,;-11.46,14.08,;-11.46,15.62,;-10.13,13.31,;-10.13,11.77,;-11.46,11,;-3.46,9.46,;-3.14,10.97,;-1.61,11.13,;-.98,9.72,;-2.13,8.69,;-2.13,7.15,;5.66,5.14,;6.75,4.05,;8.23,4.45,;8.63,5.94,;7.54,7.03,;6.06,6.63,;10.12,6.34,;10.52,7.82,;9.43,8.91,;12.01,8.22,;13.49,8.62,;12.4,9.71,;11.44,10.91,;12.27,12.2,;13.76,11.8,;13.84,10.26,;13.09,7.13,;14.56,7.61,;15.46,6.36,;14.56,5.12,;15.04,3.65,;13.09,5.59,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.136 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419501
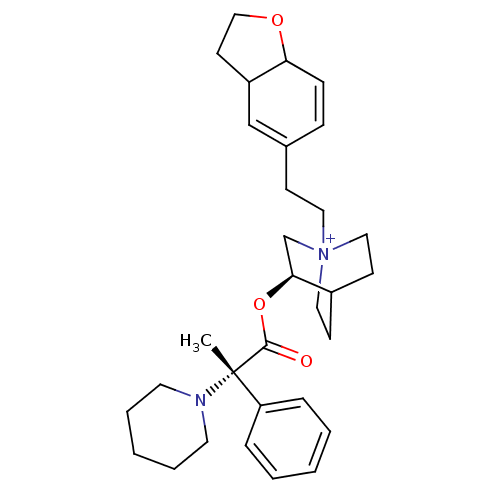
(CHEMBL1921911)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCC3=CC4CCOC4C=C3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,c:25,t:17,(45.85,-43.25,;45.86,-41.71,;45.85,-40.16,;47.19,-39.39,;47.19,-37.85,;45.85,-37.08,;44.51,-37.85,;44.51,-39.4,;47.2,-42.48,;47.2,-44.03,;48.54,-41.7,;49.88,-42.47,;49.88,-44.02,;51.22,-44.78,;51.26,-46.32,;52.62,-47.05,;53.95,-46.28,;55.28,-47.05,;56.61,-46.28,;58.07,-46.75,;58.98,-45.51,;58.07,-44.27,;56.61,-44.74,;55.28,-43.97,;53.95,-44.74,;52.55,-44.02,;52.55,-42.47,;51.22,-41.69,;50.43,-43.02,;51.92,-43.41,;44.52,-42.46,;43.19,-41.68,;41.85,-42.44,;41.84,-43.98,;43.18,-44.76,;44.51,-44,)| Show InChI InChI=1S/C31H43N2O3/c1-31(27-8-4-2-5-9-27,32-16-6-3-7-17-32)30(34)36-29-23-33(19-13-25(29)14-20-33)18-12-24-10-11-28-26(22-24)15-21-35-28/h2,4-5,8-11,22,25-26,28-29H,3,6-7,12-21,23H2,1H3/q+1/t25?,26?,28?,29-,31-,33?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419528

(CHEMBL1924025)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCc3ccccc3F)CCC1CC2)c1cccs1 |r,wU:1.0,wD:1.8,11.11,(-7.52,-20.69,;-7.51,-19.15,;-7.51,-17.6,;-6.18,-16.83,;-6.18,-15.29,;-7.52,-14.52,;-8.85,-15.29,;-8.85,-16.84,;-6.17,-19.92,;-6.16,-21.46,;-4.83,-19.14,;-3.49,-19.91,;-3.49,-21.46,;-2.16,-22.21,;-2.11,-23.75,;-.76,-24.48,;.55,-23.67,;.49,-22.15,;1.79,-21.34,;3.15,-22.06,;3.2,-23.6,;1.89,-24.41,;1.82,-25.94,;-.82,-21.46,;-.82,-19.91,;-2.16,-19.13,;-2.94,-20.45,;-1.46,-20.85,;-8.76,-20.04,;-10.22,-19.55,;-11.14,-20.79,;-10.25,-22.04,;-8.78,-21.58,)| Show InChI InChI=1S/C27H36FN2O2S/c1-27(25-10-7-19-33-25,29-14-5-2-6-15-29)26(31)32-24-20-30(17-12-22(24)13-18-30)16-11-21-8-3-4-9-23(21)28/h3-4,7-10,19,22,24H,2,5-6,11-18,20H2,1H3/q+1/t22?,24-,27-,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419474
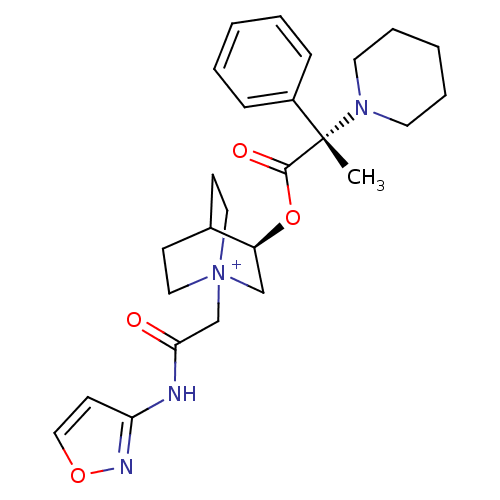
(CHEMBL1922051)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CC(=O)Nc3ccon3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:11.11,1.31,(9,-23.29,;9.01,-21.75,;9,-20.2,;10.34,-19.43,;10.34,-17.89,;9,-17.12,;7.66,-17.89,;7.66,-19.44,;10.35,-22.52,;10.36,-24.07,;11.69,-21.74,;13.03,-22.51,;13.03,-24.06,;14.37,-24.82,;14.38,-26.36,;15.72,-27.12,;15.73,-28.66,;17.04,-26.34,;18.38,-27.1,;19.84,-26.62,;20.75,-27.86,;19.85,-29.11,;18.39,-28.64,;15.7,-24.06,;15.7,-22.51,;14.37,-21.73,;13.58,-23.06,;15.07,-23.45,;7.68,-22.52,;6.35,-21.75,;5.02,-22.51,;5.02,-24.05,;6.36,-24.82,;7.69,-24.05,)| Show InChI InChI=1S/C26H34N4O4/c1-26(21-8-4-2-5-9-21,29-13-6-3-7-14-29)25(32)34-22-18-30(15-10-20(22)11-16-30)19-24(31)27-23-12-17-33-28-23/h2,4-5,8-9,12,17,20,22H,3,6-7,10-11,13-16,18-19H2,1H3/p+1/t20?,22-,26-,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419505
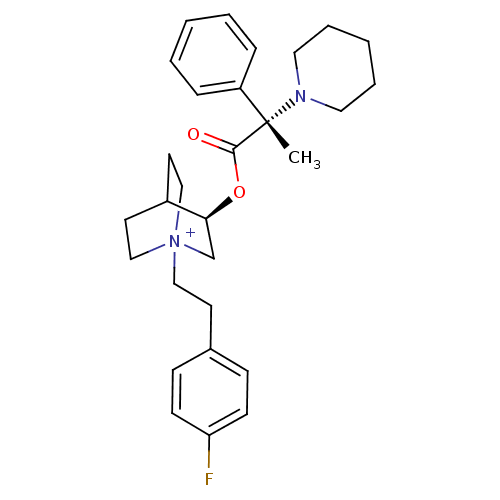
(CHEMBL1921904)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCc3ccc(F)cc3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(42.03,-22.57,;42.04,-21.03,;42.04,-19.49,;43.38,-18.72,;43.38,-17.18,;42.04,-16.4,;40.7,-17.18,;40.7,-18.73,;43.39,-21.81,;43.39,-23.35,;44.72,-21.02,;46.07,-21.8,;46.07,-23.34,;47.4,-24.1,;47.45,-25.64,;48.8,-26.37,;50.11,-25.56,;51.46,-26.3,;52.77,-25.49,;52.72,-23.95,;54.03,-23.14,;51.36,-23.22,;50.06,-24.03,;48.74,-23.34,;48.74,-21.8,;47.4,-21.01,;46.62,-22.34,;48.1,-22.74,;40.7,-21.79,;39.38,-21,;38.04,-21.76,;38.03,-23.3,;39.36,-24.08,;40.69,-23.32,)| Show InChI InChI=1S/C29H38FN2O2/c1-29(25-8-4-2-5-9-25,31-17-6-3-7-18-31)28(33)34-27-22-32(20-15-24(27)16-21-32)19-14-23-10-12-26(30)13-11-23/h2,4-5,8-13,24,27H,3,6-7,14-22H2,1H3/q+1/t24?,27-,29-,32?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419498
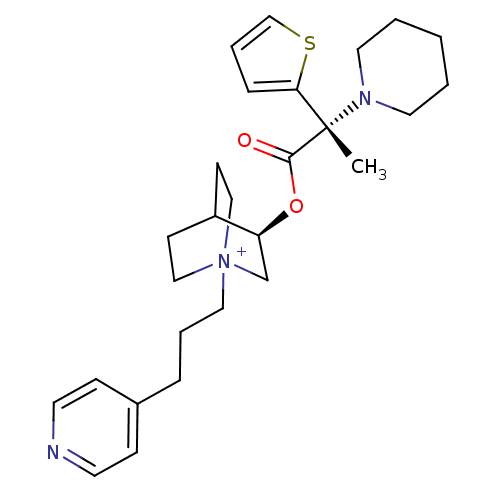
(CHEMBL1921918)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCCc3ccncc3)CCC1CC2)c1cccs1 |r,wU:1.0,wD:1.8,11.11,(24.92,-9.35,;24.93,-7.81,;24.92,-6.26,;26.26,-5.49,;26.26,-3.95,;24.93,-3.18,;23.58,-3.95,;23.58,-5.51,;26.27,-8.58,;26.28,-10.13,;27.61,-7.8,;28.95,-8.58,;28.95,-10.12,;30.29,-10.88,;30.34,-12.42,;31.69,-13.15,;33,-12.34,;34.36,-13.07,;34.4,-14.61,;35.75,-15.34,;37.06,-14.53,;37.01,-12.99,;35.66,-12.26,;31.63,-10.12,;31.63,-8.58,;30.29,-7.79,;29.51,-9.12,;30.99,-9.52,;23.67,-8.69,;22.22,-8.19,;21.29,-9.42,;22.17,-10.68,;23.64,-10.24,)| Show InChI InChI=1S/C27H38N3O2S/c1-27(25-8-6-20-33-25,29-15-3-2-4-16-29)26(31)32-24-21-30(18-11-23(24)12-19-30)17-5-7-22-9-13-28-14-10-22/h6,8-10,13-14,20,23-24H,2-5,7,11-12,15-19,21H2,1H3/q+1/t23?,24-,27-,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419472
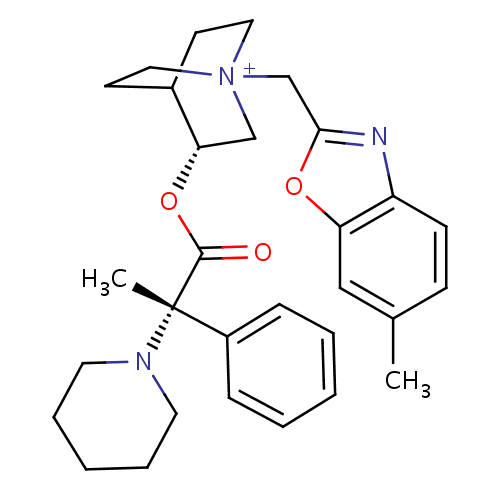
(CHEMBL1922054)Show SMILES Cc1ccc2nc(C[N+]34CCC(CC3)[C@H](C4)OC(=O)[C@@](C)(N3CCCCC3)c3ccccc3)oc2c1 |r| Show InChI InChI=1S/C30H38N3O3/c1-22-11-12-25-26(19-22)35-28(31-25)21-33-17-13-23(14-18-33)27(20-33)36-29(34)30(2,24-9-5-3-6-10-24)32-15-7-4-8-16-32/h3,5-6,9-12,19,23,27H,4,7-8,13-18,20-21H2,1-2H3/q+1/t23?,27-,30-,33?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296331

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(-4.87,-33.87,;-5.96,-34.96,;-4.62,-35.72,;-4.61,-37.26,;-3.29,-34.95,;-1.95,-35.72,;-1.95,-37.26,;-.62,-38.02,;-.64,-39.56,;.69,-40.34,;2.03,-39.58,;3.36,-40.37,;4.7,-39.61,;6.02,-40.39,;7.36,-39.64,;7.37,-38.1,;6.04,-37.31,;4.7,-38.07,;.71,-37.26,;.71,-35.72,;-.62,-34.94,;-.2,-36.18,;-1.25,-36.53,;-5.96,-33.42,;-7.21,-32.51,;-6.74,-31.04,;-5.2,-31.04,;-4.72,-32.5,;-7.29,-35.73,;-8.7,-35.11,;-9.73,-36.25,;-8.96,-37.59,;-7.45,-37.26,)| Show InChI InChI=1S/C26H30NO4S2/c28-25(26(29,23-9-4-17-32-23)24-10-5-18-33-24)31-22-19-27(14-11-20(22)12-15-27)13-6-16-30-21-7-2-1-3-8-21/h1-5,7-10,17-18,20,22,29H,6,11-16,19H2/q+1/t20?,22-,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHOK1 cells by microplate scintillation counting |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM624955

(US20230322745, Compound 25)Show SMILES O[C@@H](CNCc1cc2nnn(CCCN3CCC4(C[C@@H](CO4)OC(=O)C(O)(c4cccs4)c4cccs4)CC3)c2c2CCCc12)c1ccc(O)c2[nH]c(=O)ccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.179 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296336
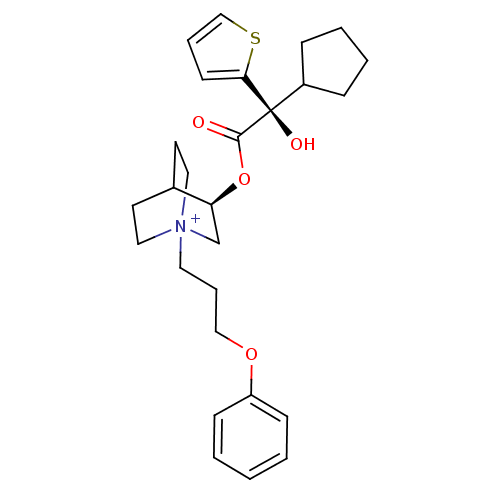
((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...)Show SMILES O[C@@](C1CCCC1)(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)c1cccs1 |r,wU:1.31,wD:10.10,1.0,(16.18,-14.66,;15.09,-15.75,;13.76,-16.53,;12.35,-15.91,;11.32,-17.05,;12.1,-18.39,;13.6,-18.06,;16.43,-16.52,;16.43,-18.06,;17.76,-15.75,;19.09,-16.51,;19.09,-18.05,;20.42,-18.81,;20.41,-20.35,;21.74,-21.14,;23.08,-20.38,;24.4,-21.16,;25.74,-20.41,;27.07,-21.19,;28.41,-20.43,;28.42,-18.89,;27.09,-18.11,;25.75,-18.87,;21.75,-18.05,;21.75,-16.51,;20.42,-15.73,;20.84,-16.98,;19.79,-17.33,;15.09,-14.21,;13.83,-13.31,;14.3,-11.85,;15.84,-11.84,;16.32,-13.31,)| Show InChI InChI=1S/C27H36NO4S/c29-26(27(30,22-8-4-5-9-22)25-12-6-19-33-25)32-24-20-28(16-13-21(24)14-17-28)15-7-18-31-23-10-2-1-3-11-23/h1-3,6,10-12,19,21-22,24,30H,4-5,7-9,13-18,20H2/q+1/t21?,24-,27+,28?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHOK1 cells by microplate scintillation counting |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM624948

(US20230322745, Compound 15 | US20230322745, Compou...)Show SMILES CN(CCCn1nnc2cc(CNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)c3CCCc3c12)[C@H]1CC[C@@H](CC1)OC(=O)[C@](O)(c1cccs1)c1ccc(Br)s1 |r,wU:34.38,43.49,wD:14.14,37.45,43.56,(4.5,4.18,;4.5,5.72,;3.16,6.49,;1.83,5.72,;.5,6.49,;-.84,5.72,;-1.16,4.21,;-2.69,4.05,;-3.32,5.46,;-4.78,5.93,;-5.1,7.44,;-6.59,7.84,;-7.68,6.75,;-9.16,7.15,;-10.25,6.06,;-9.85,4.57,;-11.74,6.46,;-12.83,5.37,;-14.32,5.77,;-14.72,7.26,;-16.2,7.65,;-13.63,8.34,;-14.03,9.83,;-12.94,10.92,;-13.33,12.41,;-11.45,10.52,;-11.05,9.03,;-12.14,7.95,;-3.96,8.47,;-3.96,10.01,;-2.49,10.49,;-1.59,9.24,;-2.49,7.99,;-2.17,6.49,;5.83,6.49,;5.83,8.03,;7.16,8.8,;8.5,8.03,;8.5,6.49,;7.16,5.72,;9.83,8.8,;11.17,8.03,;11.17,6.49,;12.5,8.8,;13.83,9.57,;12.5,10.34,;11.25,11.24,;11.73,12.71,;13.27,12.71,;13.75,11.24,;13.83,8.03,;15.3,8.5,;16.2,7.26,;15.3,6.01,;15.7,4.53,;13.83,6.49,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.198 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM200747
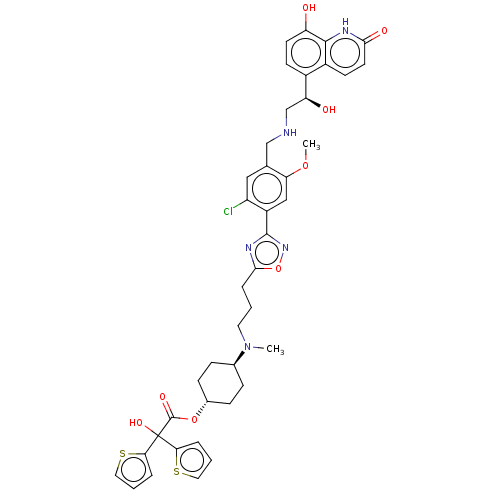
(US9233108, 9 | US9757383, Example 9)Show SMILES COc1cc(-c2noc(CCCN(C)[C@H]3CC[C@@H](CC3)OC(=O)C(O)(c3cccs3)c3cccs3)n2)c(Cl)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |wU:17.20,wD:43.48,14.13,(3.51,-4.33,;4.85,-5.1,;6.18,-4.33,;6.18,-2.79,;7.51,-2.02,;7.51,-.48,;8.76,.43,;8.28,1.89,;6.74,1.89,;5.84,3.14,;6.46,4.54,;5.56,5.79,;6.18,7.2,;7.72,7.36,;5.28,8.44,;5.91,9.85,;5,11.09,;3.47,10.93,;2.84,9.53,;3.75,8.28,;2.56,12.18,;1.03,12.02,;.41,10.61,;.13,13.26,;1.37,14.17,;-.78,14.51,;-2.32,14.51,;-2.79,15.98,;-1.55,16.88,;-.3,15.98,;-1.12,12.36,;-1.12,10.82,;-2.58,10.34,;-3.49,11.59,;-2.58,12.84,;6.27,.43,;8.85,-2.79,;10.18,-2.02,;8.85,-4.33,;7.51,-5.1,;7.51,-6.64,;8.85,-7.41,;8.85,-8.95,;10.18,-9.72,;11.51,-8.95,;10.18,-11.26,;8.85,-12.03,;8.85,-13.57,;10.18,-14.34,;10.18,-15.88,;11.51,-13.57,;12.85,-14.34,;14.18,-13.57,;15.51,-14.34,;14.18,-12.03,;12.85,-11.26,;11.51,-12.03,)| Show InChI InChI=1S/C41H44ClN5O8S2/c1-47(25-9-11-26(12-10-25)54-40(51)41(52,34-6-4-18-56-34)35-7-5-19-57-35)17-3-8-37-45-39(46-55-37)29-21-33(53-2)24(20-30(29)42)22-43-23-32(49)27-13-15-31(48)38-28(27)14-16-36(50)44-38/h4-7,13-16,18-21,25-26,32,43,48-49,52H,3,8-12,17,22-23H2,1-2H3,(H,44,50)/t25-,26-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A.
US Patent
| Assay Description
The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... |
US Patent US9757383 (2017)
BindingDB Entry DOI: 10.7270/Q2HX1FSN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419482

(CHEMBL1921940)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CC(N)=O)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:11.11,1.8,(12.77,-33.6,;12.78,-32.06,;12.77,-30.52,;14.11,-29.75,;14.11,-28.22,;12.77,-27.44,;11.44,-28.22,;11.43,-29.76,;14.12,-32.84,;14.12,-34.38,;15.45,-32.06,;16.79,-32.83,;16.79,-34.37,;18.13,-35.13,;18.14,-36.67,;19.47,-37.42,;20.8,-36.65,;19.48,-38.96,;19.46,-34.37,;19.46,-32.83,;18.13,-32.04,;17.34,-33.37,;18.83,-33.77,;11.45,-32.83,;10.13,-32.06,;8.8,-32.83,;8.8,-34.37,;10.14,-35.13,;11.46,-34.36,)| Show InChI InChI=1S/C23H33N3O3/c1-23(19-8-4-2-5-9-19,25-12-6-3-7-13-25)22(28)29-20-16-26(17-21(24)27)14-10-18(20)11-15-26/h2,4-5,8-9,18,20H,3,6-7,10-17H2,1H3,(H-,24,27)/p+1/t18?,20-,23-,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296329
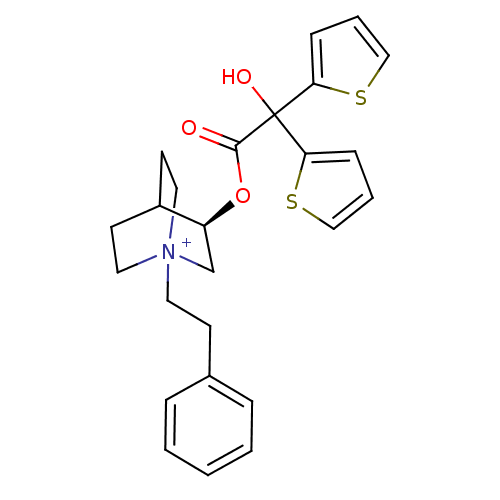
((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(12.26,-17.08,;11.17,-18.17,;12.51,-18.94,;12.51,-20.48,;13.84,-18.17,;15.17,-18.93,;15.17,-20.47,;16.5,-21.23,;16.49,-22.77,;17.82,-23.56,;19.16,-22.8,;20.48,-23.59,;21.82,-22.83,;21.84,-21.29,;20.5,-20.51,;19.17,-21.27,;17.83,-20.47,;17.83,-18.93,;16.5,-18.15,;16.92,-19.4,;15.87,-19.75,;11.17,-16.63,;9.92,-15.73,;10.39,-14.26,;11.93,-14.26,;12.41,-15.72,;9.84,-18.95,;8.42,-18.32,;7.4,-19.47,;8.17,-20.8,;9.68,-20.48,)| Show InChI InChI=1S/C25H28NO3S2/c27-24(25(28,22-8-4-16-30-22)23-9-5-17-31-23)29-21-18-26(14-11-20(21)12-15-26)13-10-19-6-2-1-3-7-19/h1-9,16-17,20-21,28H,10-15,18H2/q+1/t20?,21-,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHOK1 cells by microplate scintillation counting |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419491

(CHEMBL1921927)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCCCc3ccccc3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(27.45,-44.75,;27.46,-43.2,;27.46,-41.66,;28.8,-40.89,;28.79,-39.35,;27.46,-38.57,;26.12,-39.35,;26.11,-40.9,;28.8,-43.98,;28.81,-45.52,;30.14,-43.2,;31.48,-43.97,;31.48,-45.52,;32.82,-46.28,;32.87,-47.82,;34.22,-48.55,;35.53,-47.74,;36.89,-48.46,;38.19,-47.64,;39.55,-48.37,;40.85,-47.56,;40.8,-46.02,;39.43,-45.29,;38.13,-46.11,;34.16,-45.52,;34.16,-43.97,;32.82,-43.18,;32.04,-44.51,;33.52,-44.91,;26.13,-43.98,;24.8,-43.22,;23.47,-43.99,;23.48,-45.53,;24.82,-46.29,;26.15,-45.51,)| Show InChI InChI=1S/C31H43N2O2/c1-31(28-16-7-3-8-17-28,32-20-10-4-11-21-32)30(34)35-29-25-33(23-18-27(29)19-24-33)22-12-9-15-26-13-5-2-6-14-26/h2-3,5-8,13-14,16-17,27,29H,4,9-12,15,18-25H2,1H3/q+1/t27?,29-,31-,33?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419537
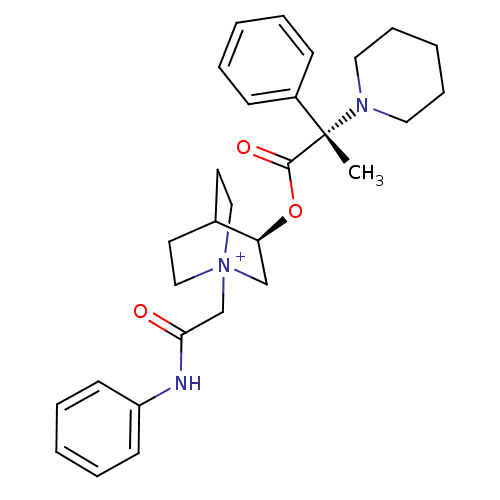
(CHEMBL1921942)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CC(=O)Nc3ccccc3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(-5.89,.59,;-5.88,2.13,;-5.89,3.68,;-4.55,4.45,;-4.55,5.99,;-5.88,6.76,;-7.22,5.99,;-7.23,4.44,;-4.54,1.36,;-4.53,-.19,;-3.2,2.14,;-1.86,1.37,;-1.86,-.18,;-.52,-.94,;-.51,-2.48,;.83,-3.24,;.84,-4.78,;2.15,-2.46,;3.49,-3.22,;3.49,-4.76,;4.83,-5.52,;6.16,-4.74,;6.14,-3.19,;4.81,-2.44,;.81,-.18,;.81,1.37,;-.52,2.15,;-1.31,.82,;.18,.43,;-7.21,1.36,;-8.54,2.13,;-9.87,1.37,;-9.87,-.17,;-8.53,-.94,;-7.2,-.17,)| Show InChI InChI=1S/C29H37N3O3/c1-29(24-11-5-2-6-12-24,31-17-9-4-10-18-31)28(34)35-26-21-32(19-15-23(26)16-20-32)22-27(33)30-25-13-7-3-8-14-25/h2-3,5-8,11-14,23,26H,4,9-10,15-22H2,1H3/p+1/t23?,26-,29-,32?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419499
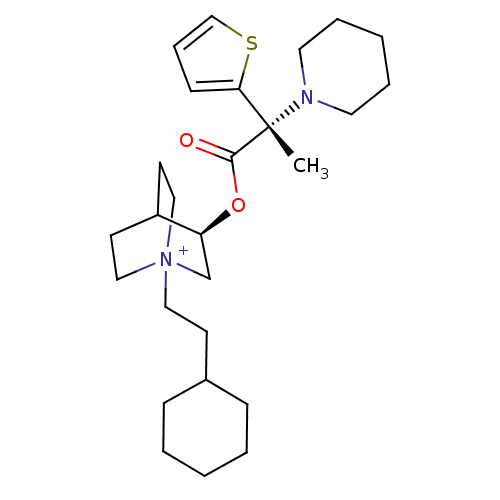
(CHEMBL1921914)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCC3CCCCC3)CCC1CC2)c1cccs1 |r,wU:1.0,wD:1.8,11.11,(27.17,1.58,;27.18,3.12,;27.18,4.67,;28.52,5.44,;28.52,6.98,;27.18,7.76,;25.84,6.98,;25.84,5.43,;28.52,2.35,;28.53,.8,;29.86,3.13,;31.2,2.36,;31.2,.81,;32.54,.05,;32.59,-1.49,;33.94,-2.22,;35.25,-1.41,;36.6,-2.15,;37.91,-1.35,;37.87,.19,;36.51,.93,;35.2,.12,;33.88,.81,;33.88,2.36,;32.54,3.14,;31.76,1.81,;33.24,1.41,;25.93,2.23,;24.47,2.72,;23.55,1.49,;24.44,.23,;25.91,.69,)| Show InChI InChI=1S/C27H43N2O2S/c1-27(25-11-8-20-32-25,28-15-6-3-7-16-28)26(30)31-24-21-29(18-13-23(24)14-19-29)17-12-22-9-4-2-5-10-22/h8,11,20,22-24H,2-7,9-10,12-19,21H2,1H3/q+1/t23?,24-,27-,29?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419475

(CHEMBL1922050)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CC(=O)Nc3cnccn3)CCC1CC2)c1ccccc1 |r| Show InChI InChI=1S/C27H35N5O3/c1-27(22-8-4-2-5-9-22,31-14-6-3-7-15-31)26(34)35-23-19-32(16-10-21(23)11-17-32)20-25(33)30-24-18-28-12-13-29-24/h2,4-5,8-9,12-13,18,21,23H,3,6-7,10-11,14-17,19-20H2,1H3/p+1/t21?,23-,27-,32?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419503
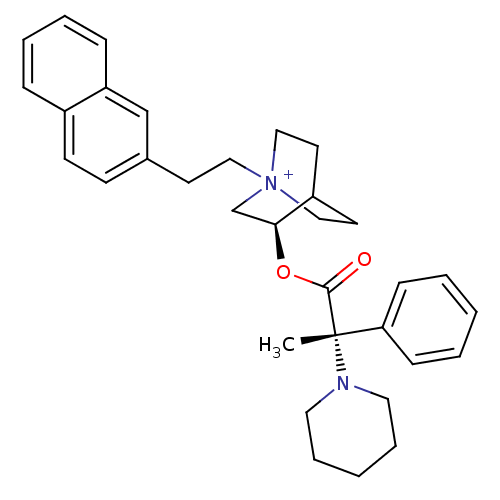
(CHEMBL1921910)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CCc3ccc4ccccc4c3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(10.47,-40.88,;10.48,-39.34,;10.47,-37.79,;11.81,-37.02,;11.81,-35.48,;10.47,-34.7,;9.13,-35.48,;9.13,-37.03,;11.82,-40.11,;11.83,-41.66,;13.16,-39.33,;14.51,-40.1,;14.51,-41.65,;15.84,-42.41,;15.89,-43.95,;17.24,-44.68,;18.58,-43.91,;18.57,-42.38,;19.89,-41.6,;21.24,-42.37,;22.56,-41.6,;23.89,-42.37,;23.9,-43.91,;22.56,-44.68,;21.24,-43.91,;19.91,-44.68,;17.18,-41.65,;17.18,-40.1,;15.84,-39.32,;15.06,-40.65,;16.54,-41.04,;9.14,-40.09,;7.81,-39.31,;6.48,-40.07,;6.46,-41.61,;7.8,-42.39,;9.13,-41.63,)| Show InChI InChI=1S/C33H41N2O2/c1-33(30-12-4-2-5-13-30,34-19-8-3-9-20-34)32(36)37-31-25-35(22-17-28(31)18-23-35)21-16-26-14-15-27-10-6-7-11-29(27)24-26/h2,4-7,10-15,24,28,31H,3,8-9,16-23,25H2,1H3/q+1/t28?,31-,33-,35?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419473
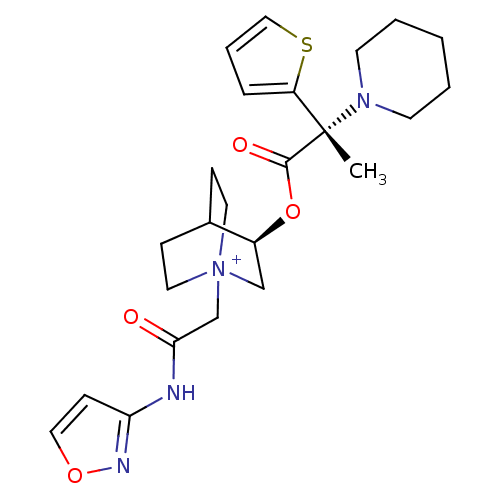
(CHEMBL1922052)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CC(=O)Nc3ccon3)CCC1CC2)c1cccs1 |r,wU:1.0,wD:11.11,1.31,(23.99,-24.2,;24,-22.66,;24,-21.12,;25.34,-20.35,;25.33,-18.81,;24,-18.04,;22.66,-18.81,;22.66,-20.36,;25.34,-23.43,;25.35,-24.98,;26.68,-22.65,;28.02,-23.43,;28.02,-24.97,;29.36,-25.73,;29.37,-27.27,;30.7,-28.03,;30.71,-29.56,;32.03,-27.25,;33.37,-28.01,;34.82,-27.53,;35.73,-28.77,;34.83,-30.01,;33.37,-29.55,;30.69,-24.97,;30.69,-23.43,;29.36,-22.64,;28.57,-23.97,;30.06,-24.37,;22.67,-23.43,;21.22,-22.94,;20.3,-24.18,;21.19,-25.43,;22.66,-24.97,)| Show InChI InChI=1S/C24H32N4O4S/c1-24(20-6-5-15-33-20,27-10-3-2-4-11-27)23(30)32-19-16-28(12-7-18(19)8-13-28)17-22(29)25-21-9-14-31-26-21/h5-6,9,14-15,18-19H,2-4,7-8,10-13,16-17H2,1H3/p+1/t18?,19-,24-,28?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419471
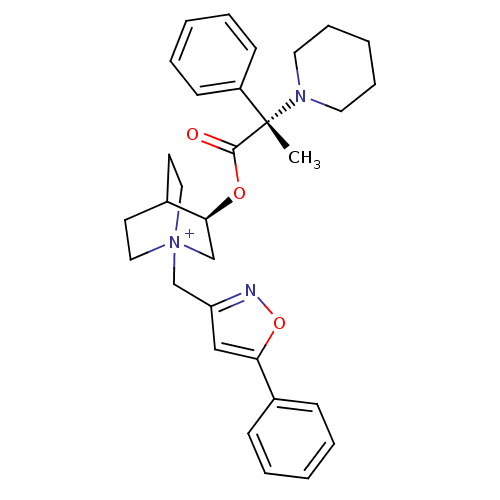
(CHEMBL1922055)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(Cc3cc(on3)-c3ccccc3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(23.03,-34.84,;23.05,-33.3,;23.04,-31.76,;24.38,-30.99,;24.38,-29.45,;23.04,-28.67,;21.7,-29.45,;21.7,-31,;24.39,-34.07,;24.39,-35.62,;25.72,-33.29,;27.07,-34.07,;27.07,-35.61,;28.4,-36.37,;28.41,-37.91,;29.75,-38.67,;31.22,-38.19,;32.12,-39.43,;31.22,-40.67,;29.76,-40.21,;33.66,-39.43,;34.43,-40.76,;35.97,-40.76,;36.73,-39.43,;35.95,-38.09,;34.42,-38.1,;29.74,-35.61,;29.74,-34.07,;28.4,-33.28,;27.62,-34.61,;29.1,-35.01,;21.71,-34.07,;20.38,-33.3,;19.05,-34.07,;19.05,-35.6,;20.39,-36.37,;21.72,-35.6,)| Show InChI InChI=1S/C31H38N3O3/c1-31(26-13-7-3-8-14-26,33-17-9-4-10-18-33)30(35)36-29-23-34(19-15-25(29)16-20-34)22-27-21-28(37-32-27)24-11-5-2-6-12-24/h2-3,5-8,11-14,21,25,29H,4,9-10,15-20,22-23H2,1H3/q+1/t25?,29-,31-,34?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50344294
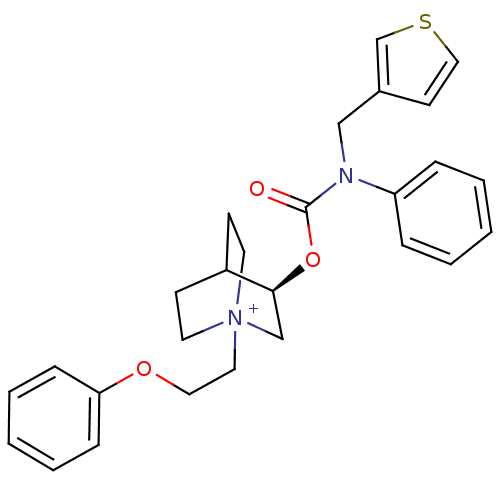
((R)-1-(2-phenoxyethyl)-3-(phenyl(thiophen-3-ylmeth...)Show SMILES O=C(O[C@H]1C[N+]2(CCOc3ccccc3)CCC1CC2)N(Cc1ccsc1)c1ccccc1 |r,wD:3.2,(.53,-12.66,;.53,-14.2,;1.87,-14.97,;3.2,-14.2,;4.53,-14.98,;5.86,-14.22,;7.19,-14.98,;8.52,-14.21,;9.85,-14.98,;11.19,-14.21,;12.51,-14.98,;13.85,-14.21,;13.85,-12.67,;12.5,-11.9,;11.18,-12.68,;5.87,-12.68,;4.54,-11.9,;3.19,-12.67,;3.95,-13.99,;5.08,-12.87,;-.8,-14.97,;-.8,-16.51,;-2.13,-17.28,;-3.54,-16.66,;-4.57,-17.8,;-3.8,-19.14,;-2.29,-18.82,;-2.13,-14.2,;-2.13,-12.66,;-3.46,-11.89,;-4.79,-12.66,;-4.79,-14.21,;-3.46,-14.97,)| Show InChI InChI=1S/C27H31N2O3S/c30-27(28(19-22-13-18-33-21-22)24-7-3-1-4-8-24)32-26-20-29(14-11-23(26)12-15-29)16-17-31-25-9-5-2-6-10-25/h1-10,13,18,21,23,26H,11-12,14-17,19-20H2/q+1/t23?,26-,29?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHO-K1 cells after 1 hr by scintillation counting |
Bioorg Med Chem Lett 21: 3457-61 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.096
BindingDB Entry DOI: 10.7270/Q25H7GMV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM221898
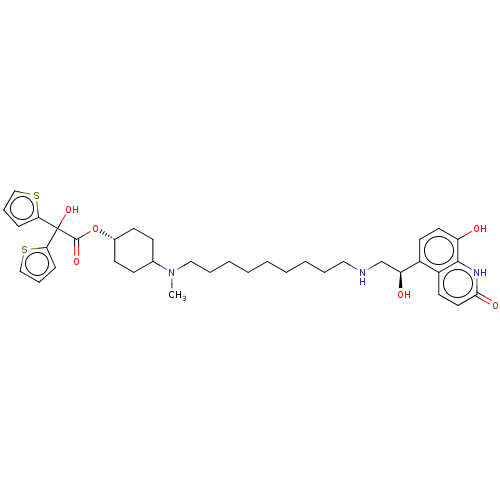
(US9315463, 1)Show SMILES CN(CCCCCCCCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12)C1CC[C@@H](CC1)OC(=O)C(O)(c1cccs1)c1cccs1 |r,wU:13.13,30.35,(5.28,4.23,;5.28,2.69,;3.94,1.93,;2.61,2.69,;1.27,1.93,;-.06,2.69,;-1.39,1.93,;-2.73,2.69,;-4.06,1.93,;-5.39,2.69,;-6.73,1.93,;-8.06,2.69,;-9.39,1.93,;-10.73,2.69,;-10.73,4.23,;-12.06,1.93,;-13.4,2.69,;-14.73,1.93,;-14.73,.38,;-16.06,-.38,;-13.4,-.38,;-13.4,-1.93,;-12.06,-2.69,;-12.06,-4.23,;-10.73,-1.93,;-10.73,-.38,;-12.06,.38,;6.61,1.93,;6.61,.38,;7.94,-.38,;9.28,.38,;9.28,1.93,;7.94,2.69,;10.61,-.38,;11.94,.38,;11.94,1.93,;13.28,-.38,;14.61,-1.15,;12.51,-1.72,;10.97,-1.72,;10.49,-3.18,;11.74,-4.09,;12.98,-3.18,;14.05,.95,;15.59,.95,;16.06,2.41,;14.82,3.32,;13.57,2.41,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Almirall, S.A.
US Patent
| Assay Description
The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... |
US Patent US9315463 (2016)
BindingDB Entry DOI: 10.7270/Q24X56MM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419480

(CHEMBL1921943)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CC(=O)Nc3ccccc3)CCC1CC2)c1cccs1 |r,wU:1.0,wD:1.8,11.11,(10.86,1.34,;10.87,2.89,;10.87,4.43,;12.2,5.2,;12.2,6.74,;10.87,7.53,;9.53,6.74,;9.52,5.19,;12.21,2.11,;12.22,.57,;13.55,2.89,;14.89,2.12,;14.89,.57,;16.23,-.18,;16.24,-1.72,;17.57,-2.48,;17.58,-4.02,;18.9,-1.71,;20.24,-2.47,;20.24,-4,;21.58,-4.76,;22.9,-3.98,;22.89,-2.44,;21.55,-1.69,;17.56,.57,;17.56,2.12,;16.23,2.91,;15.44,1.58,;16.93,1.18,;9.63,1.98,;8.17,2.45,;7.27,1.2,;8.18,-.04,;9.64,.44,)| Show InChI InChI=1S/C27H35N3O3S/c1-27(24-11-8-18-34-24,29-14-6-3-7-15-29)26(32)33-23-19-30(16-12-21(23)13-17-30)20-25(31)28-22-9-4-2-5-10-22/h2,4-5,8-11,18,21,23H,3,6-7,12-17,19-20H2,1H3/p+1/t21?,23-,27-,30?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50419479
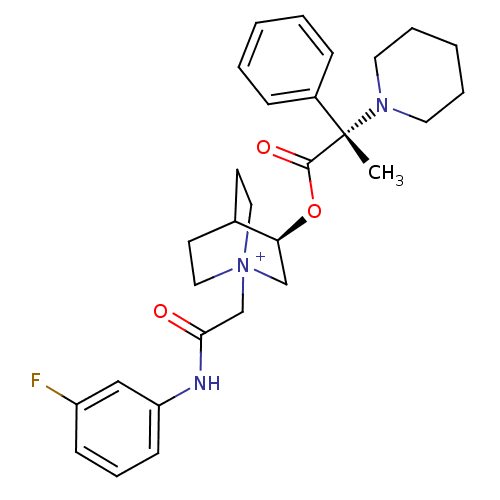
(CHEMBL1921944)Show SMILES C[C@@](N1CCCCC1)(C(=O)O[C@H]1C[N+]2(CC(=O)Nc3cccc(F)c3)CCC1CC2)c1ccccc1 |r,wU:1.0,wD:1.8,11.11,(26.17,1.93,;26.18,3.47,;26.18,5.02,;27.52,5.78,;27.52,7.32,;26.18,8.11,;24.84,7.32,;24.84,5.77,;27.53,2.7,;27.53,1.15,;28.86,3.48,;30.21,2.7,;30.21,1.16,;31.54,.4,;31.55,-1.14,;32.89,-1.9,;32.9,-3.44,;34.22,-1.12,;35.55,-1.88,;35.56,-3.42,;36.89,-4.18,;38.22,-3.4,;38.21,-1.86,;39.53,-1.07,;36.87,-1.1,;32.88,1.16,;32.88,2.7,;31.54,3.49,;30.76,2.16,;32.24,1.76,;24.85,2.7,;23.53,3.47,;22.2,2.7,;22.19,1.16,;23.54,.39,;24.86,1.17,)| Show InChI InChI=1S/C29H36FN3O3/c1-29(23-9-4-2-5-10-23,32-15-6-3-7-16-32)28(35)36-26-20-33(17-13-22(26)14-18-33)21-27(34)31-25-12-8-11-24(30)19-25/h2,4-5,8-12,19,22,26H,3,6-7,13-18,20-21H2,1H3/p+1/t22?,26-,29-,33?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from recombinant human M3 receptor expressed in CHO-K1 cells after 16 hrs |
Bioorg Med Chem Lett 21: 7440-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.002
BindingDB Entry DOI: 10.7270/Q2H41SPH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM200747

(US9233108, 9 | US9757383, Example 9)Show SMILES COc1cc(-c2noc(CCCN(C)[C@H]3CC[C@@H](CC3)OC(=O)C(O)(c3cccs3)c3cccs3)n2)c(Cl)cc1CNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |wU:17.20,wD:43.48,14.13,(3.51,-4.33,;4.85,-5.1,;6.18,-4.33,;6.18,-2.79,;7.51,-2.02,;7.51,-.48,;8.76,.43,;8.28,1.89,;6.74,1.89,;5.84,3.14,;6.46,4.54,;5.56,5.79,;6.18,7.2,;7.72,7.36,;5.28,8.44,;5.91,9.85,;5,11.09,;3.47,10.93,;2.84,9.53,;3.75,8.28,;2.56,12.18,;1.03,12.02,;.41,10.61,;.13,13.26,;1.37,14.17,;-.78,14.51,;-2.32,14.51,;-2.79,15.98,;-1.55,16.88,;-.3,15.98,;-1.12,12.36,;-1.12,10.82,;-2.58,10.34,;-3.49,11.59,;-2.58,12.84,;6.27,.43,;8.85,-2.79,;10.18,-2.02,;8.85,-4.33,;7.51,-5.1,;7.51,-6.64,;8.85,-7.41,;8.85,-8.95,;10.18,-9.72,;11.51,-8.95,;10.18,-11.26,;8.85,-12.03,;8.85,-13.57,;10.18,-14.34,;10.18,-15.88,;11.51,-13.57,;12.85,-14.34,;14.18,-13.57,;15.51,-14.34,;14.18,-12.03,;12.85,-11.26,;11.51,-12.03,)| Show InChI InChI=1S/C41H44ClN5O8S2/c1-47(25-9-11-26(12-10-25)54-40(51)41(52,34-6-4-18-56-34)35-7-5-19-57-35)17-3-8-37-45-39(46-55-37)29-21-33(53-2)24(20-30(29)42)22-43-23-32(49)27-13-15-31(48)38-28(27)14-16-36(50)44-38/h4-7,13-16,18-21,25-26,32,43,48-49,52H,3,8-12,17,22-23H2,1-2H3,(H,44,50)/t25-,26-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMIRALL, S.A.
US Patent
| Assay Description
The study of binding to human muscarinic M1, M2, M3, M4 and M5 receptors was performed using commercial membranes (Perkin Elmer) prepared from CHO-K1... |
US Patent US9233108 (2016)
BindingDB Entry DOI: 10.7270/Q23J3BSN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM624969

(US20230322745, Compound 41)Show SMILES CN(CCCn1cnc2cc(CNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)c3CCCc3c12)C1CC[C@@H](CC1)OC(=O)C(O)(c1cccs1)c1cccs1 |r,wD:14.14,37.45,(3.77,-6.18,;4.17,-4.7,;3.08,-3.61,;1.59,-4.01,;.5,-2.92,;-.98,-3.32,;-1.61,-4.72,;-3.14,-4.56,;-3.46,-3.06,;-4.8,-2.29,;-4.8,-.75,;-6.13,.02,;-7.46,-.75,;-8.8,.02,;-10.13,-.75,;-10.13,-2.29,;-11.46,.02,;-12.8,-.75,;-14.13,.02,;-14.13,1.56,;-15.46,2.33,;-12.8,2.33,;-12.8,3.87,;-11.46,4.64,;-11.46,6.18,;-10.13,3.87,;-10.13,2.33,;-11.46,1.56,;-3.46,.02,;-3.14,1.53,;-1.61,1.69,;-.98,.28,;-2.13,-.75,;-2.13,-2.29,;5.66,-4.3,;6.06,-2.81,;7.54,-2.41,;8.63,-3.5,;8.23,-4.99,;6.75,-5.39,;10.12,-3.1,;10.52,-1.62,;9.43,-.53,;12.01,-1.22,;13.49,-.82,;12.4,.27,;11.44,1.47,;12.27,2.76,;13.76,2.36,;13.84,.82,;13.09,-2.31,;14.56,-1.83,;15.46,-3.08,;14.56,-4.32,;13.09,-3.85,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.201 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM624962

(US20230322745, Compound 35)Show SMILES CN(CCCn1nnc2cc(CNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)c3OCOc3c12)C1CC[C@@H](CC1)OC(=O)[C@@](O)(c1cccs1)c1ccc(C)s1 |r,wU:43.50,wD:14.14,37.45,43.49,(3.77,1.47,;4.17,2.96,;3.08,4.04,;1.59,3.65,;.5,4.73,;-.98,4.34,;-1.61,2.93,;-3.14,3.09,;-3.46,4.6,;-4.8,5.37,;-4.8,6.91,;-6.13,7.68,;-7.46,6.91,;-8.8,7.68,;-10.13,6.91,;-10.13,5.37,;-11.46,7.68,;-12.8,6.91,;-14.13,7.68,;-14.13,9.22,;-15.46,9.99,;-12.8,9.99,;-12.8,11.53,;-11.46,12.3,;-11.46,13.84,;-10.13,11.53,;-10.13,9.99,;-11.46,9.22,;-3.46,7.68,;-3.14,9.18,;-1.61,9.34,;-.98,7.94,;-2.13,6.91,;-2.13,5.37,;5.66,3.35,;6.06,4.84,;7.54,5.24,;8.63,4.15,;8.23,2.66,;6.75,2.26,;10.12,4.55,;10.52,6.04,;9.43,7.13,;12.01,6.44,;13.49,6.83,;12.4,7.92,;11.44,9.12,;12.27,10.41,;13.76,10.01,;13.84,8.47,;13.09,5.35,;14.56,5.82,;15.46,4.58,;14.56,3.33,;15.04,1.87,;13.09,3.81,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.213 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM624953
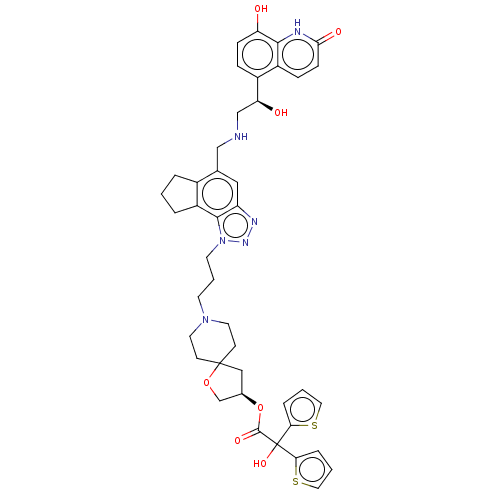
(US20230322745, Compound 24)Show SMILES O[C@@H](CNCc1cc2nnn(CCCN3CCC4(C[C@H](CO4)OC(=O)C(O)(c4cccs4)c4cccs4)CC3)c2c2CCCc12)c1ccc(O)c2[nH]c(=O)ccc12 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM123054

(US8742134, (1))Show SMILES C[C@@](C1CCCC1)(C(=O)O[C@@H]1CC2CCC1[N+]2(C)C)c1ccccc1 |r| Show InChI InChI=1S/C22H32NO2/c1-22(17-11-7-8-12-17,16-9-5-4-6-10-16)21(24)25-20-15-18-13-14-19(20)23(18,2)3/h4-6,9-10,17-20H,7-8,11-15H2,1-3H3/q+1/t18?,19?,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.160 | -13.2 | 0.230 | n/a | n/a | n/a | n/a | n/a | 22 |
Theron Pharmaceuticals, Inc.
US Patent
| Assay Description
Radioligand binding studies were carried out with M3 receptor cell homogenates as described (Peralta et al., The EMBO Journal 6, 3923-3929, (1987)). ... |
US Patent US8742134 (2014)
BindingDB Entry DOI: 10.7270/Q20000SX |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296317
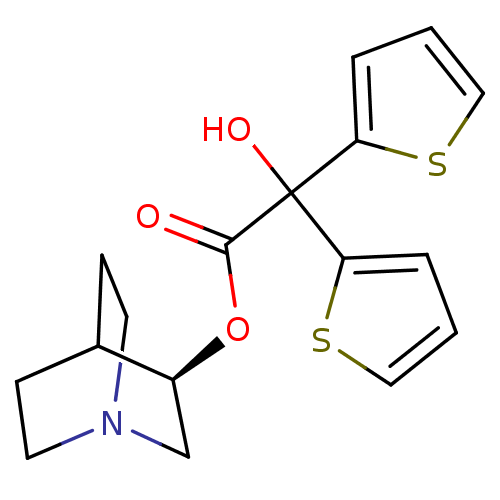
((3R)-1-Azabicyclo[2.2.2]oct-3-ylhydroxy(di-2-thien...)Show SMILES OC(C(=O)O[C@H]1CN2CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(-5.41,-13.09,;-6.49,-14.18,;-5.16,-14.95,;-5.15,-16.49,;-3.82,-14.18,;-2.49,-14.94,;-2.49,-16.48,;-1.16,-17.24,;.17,-16.48,;.17,-14.94,;-1.16,-14.16,;-.74,-15.41,;-1.79,-15.76,;-6.5,-12.64,;-7.75,-11.74,;-7.27,-10.27,;-5.73,-10.27,;-5.25,-11.73,;-7.82,-14.96,;-9.24,-14.33,;-10.27,-15.48,;-9.49,-16.81,;-7.99,-16.49,)| Show InChI InChI=1S/C17H19NO3S2/c19-16(21-13-11-18-7-5-12(13)6-8-18)17(20,14-3-1-9-22-14)15-4-2-10-23-15/h1-4,9-10,12-13,20H,5-8,11H2/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHOK1 cells by microplate scintillation counting |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296341
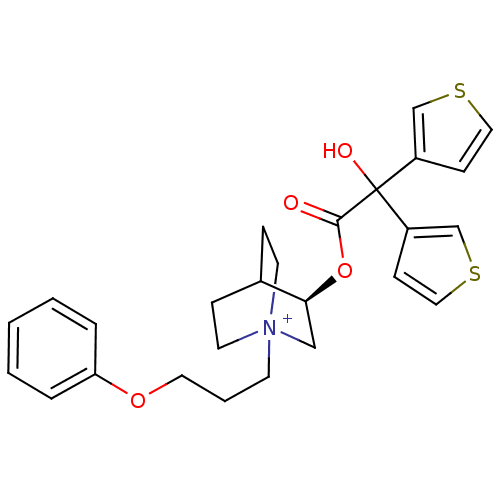
((3R)-3-{[Hydroxy(di-3-thienyl)acetyl]oxy}-1-(3-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCOc3ccccc3)CCC1CC2)(c1ccsc1)c1ccsc1 |r,wD:5.4,(-3.84,-.23,;-5.17,-1.01,;-3.83,-1.77,;-3.83,-3.31,;-2.5,-1,;-1.17,-1.77,;-1.17,-3.31,;.16,-4.07,;.15,-5.61,;1.47,-6.39,;2.82,-5.63,;4.14,-6.42,;5.48,-5.66,;6.81,-6.44,;8.15,-5.69,;8.16,-4.15,;6.82,-3.36,;5.49,-4.12,;1.49,-3.31,;1.49,-1.77,;.16,-.99,;.58,-2.23,;-.47,-2.58,;-6.5,-1.78,;-7.91,-1.16,;-8.94,-2.31,;-8.16,-3.64,;-6.66,-3.31,;-5.17,.53,;-6.43,1.43,;-5.96,2.9,;-4.42,2.9,;-3.94,1.44,)| Show InChI InChI=1S/C26H30NO4S2/c28-25(26(29,21-9-15-32-18-21)22-10-16-33-19-22)31-24-17-27(12-7-20(24)8-13-27)11-4-14-30-23-5-2-1-3-6-23/h1-3,5-6,9-10,15-16,18-20,24,29H,4,7-8,11-14,17H2/q+1/t20?,24-,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHOK1 cells by microplate scintillation counting |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296338
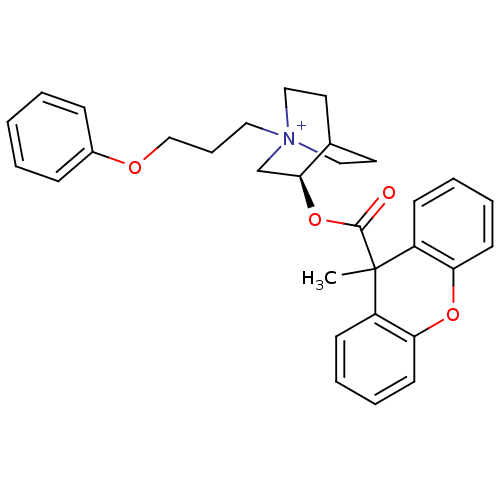
((3R)-3-{[(9-Methyl-9H-xanthen-9-yl)carbonyl]oxy}-1...)Show SMILES CC1(C(=O)O[C@H]2C[N+]3(CCCOc4ccccc4)CCC2CC3)c2ccccc2Oc2ccccc12 |r,wD:5.4,(16.43,-28.66,;15.1,-29.44,;16.44,-30.21,;16.44,-31.75,;17.77,-29.43,;19.11,-30.2,;19.11,-31.74,;20.44,-32.5,;20.43,-34.05,;21.75,-34.83,;23.1,-34.07,;24.42,-34.85,;25.77,-34.1,;27.09,-34.88,;28.43,-34.13,;28.45,-32.58,;27.11,-31.8,;25.77,-32.56,;21.77,-31.74,;21.77,-30.2,;20.44,-29.42,;20.86,-30.67,;19.81,-31.02,;15.1,-27.9,;16.42,-27.13,;16.42,-25.6,;15.09,-24.84,;13.77,-25.61,;13.77,-27.13,;12.44,-27.9,;12.44,-29.44,;11.11,-30.21,;11.1,-31.75,;12.44,-32.52,;13.77,-31.75,;13.78,-30.22,)| Show InChI InChI=1S/C31H34NO4/c1-31(25-12-5-7-14-27(25)35-28-15-8-6-13-26(28)31)30(33)36-29-22-32(19-16-23(29)17-20-32)18-9-21-34-24-10-3-2-4-11-24/h2-8,10-15,23,29H,9,16-22H2,1H3/q+1/t23?,29-,32?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHOK1 cells by microplate scintillation counting |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data