

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164242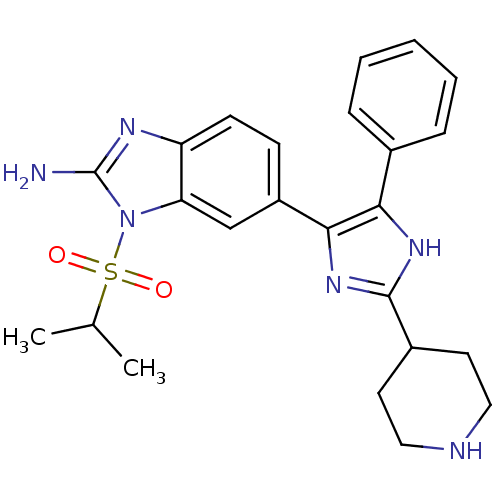 (6-(5-Phenyl-2-piperidin-4-yl-3H-imidazol-4-yl)-1-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164228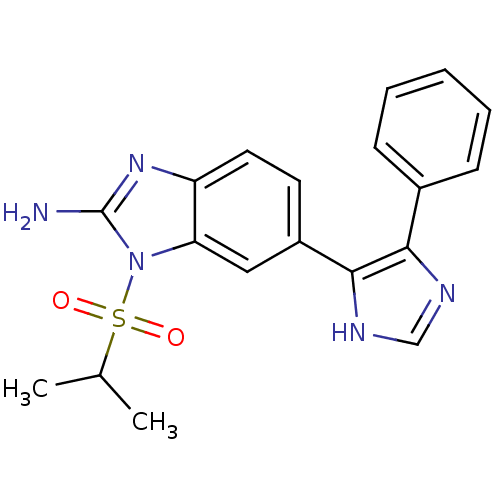 (1-(isopropylsulfonyl)-6-(4-phenyl-1H-imidazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164240 (6-[5-(4-Fluoro-phenyl)-3H-imidazol-4-yl]-1-(propan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164236 (6-[2-tert-Butyl-5-(2,4-difluoro-phenyl)-3H-imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164230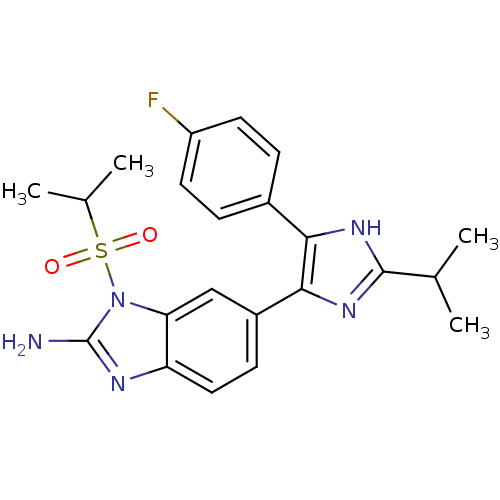 (6-[5-(4-Fluoro-phenyl)-2-isopropyl-3H-imidazol-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164239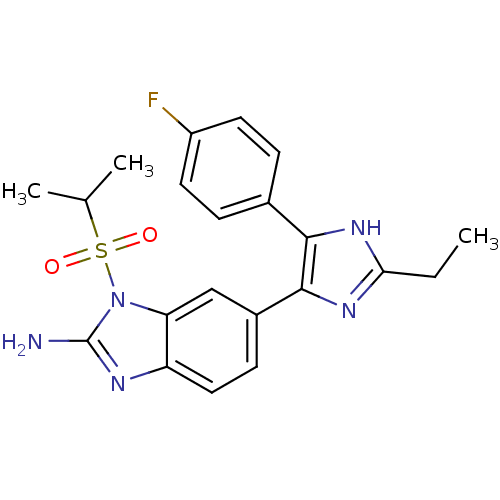 (6-[2-Ethyl-5-(4-fluoro-phenyl)-3H-imidazol-4-yl]-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164229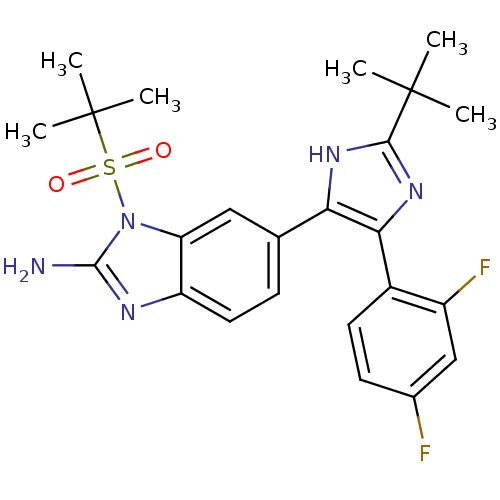 (6-[2-tert-Butyl-5-(2,4-difluoro-phenyl)-3H-imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164232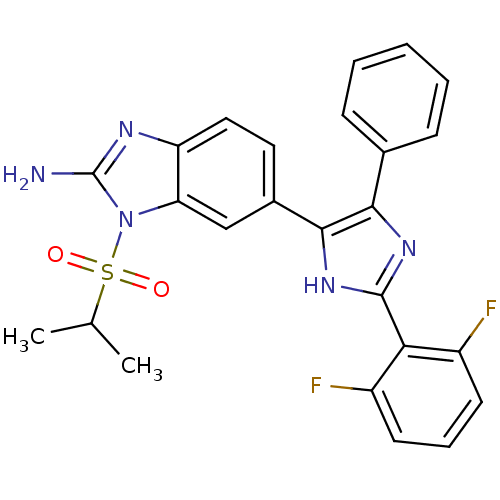 (6-(2-(2,6-difluorophenyl)-4-phenyl-1H-imidazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164231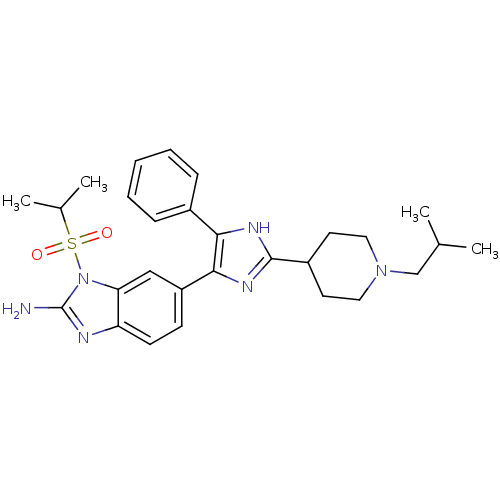 (6-[2-(1-Isobutyl-piperidin-4-yl)-5-phenyl-3H-imida...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164232 (6-(2-(2,6-difluorophenyl)-4-phenyl-1H-imidazol-5-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164233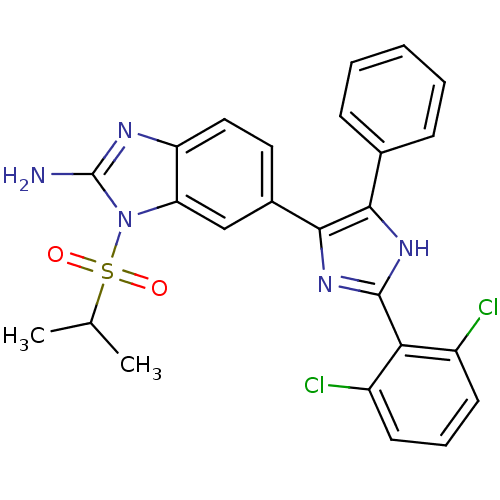 (6-(2-(2,6-dichlorophenyl)-4-phenyl-1H-imidazol-5-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164235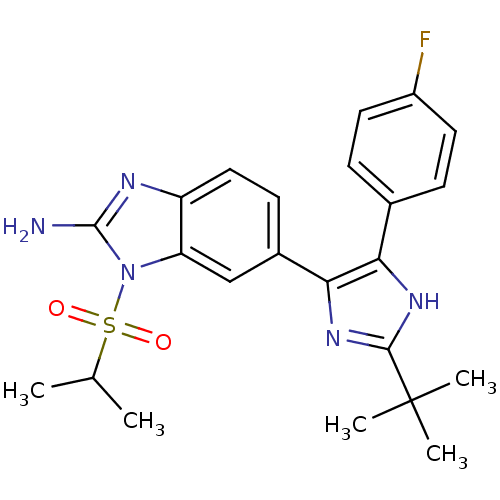 (6-[2-tert-Butyl-5-(4-fluoro-phenyl)-3H-imidazol-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164234 (1-Cyclopentanesulfonyl-6-[2-(2,6-difluoro-phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164238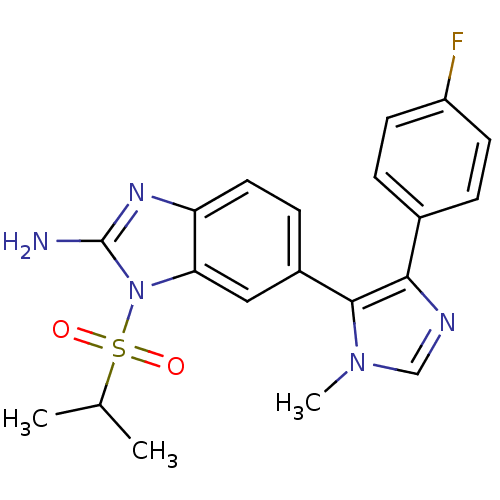 (6-[5-(4-Fluoro-phenyl)-3-methyl-3H-imidazol-4-yl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164237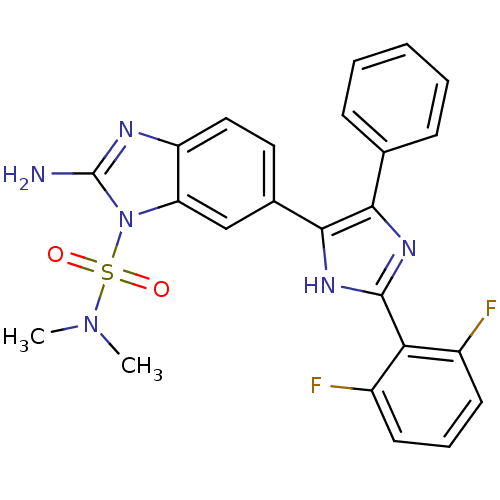 (2-Amino-6-[2-(2,6-difluoro-phenyl)-5-phenyl-3H-imi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50164241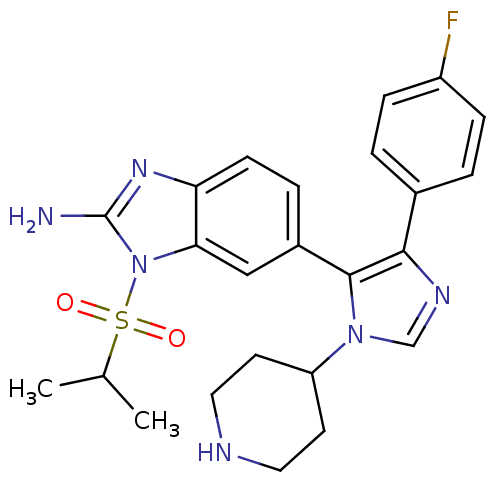 (6-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description Inhibition of human recombinant Mitogen activated protein kinase p38 alpha activity using ATP[gamma-33P] and EGFR 21mer-peptide | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164240 (6-[5-(4-Fluoro-phenyl)-3H-imidazol-4-yl]-1-(propan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164228 (1-(isopropylsulfonyl)-6-(4-phenyl-1H-imidazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164239 (6-[2-Ethyl-5-(4-fluoro-phenyl)-3H-imidazol-4-yl]-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164231 (6-[2-(1-Isobutyl-piperidin-4-yl)-5-phenyl-3H-imida...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164230 (6-[5-(4-Fluoro-phenyl)-2-isopropyl-3H-imidazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164234 (1-Cyclopentanesulfonyl-6-[2-(2,6-difluoro-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50164235 (6-[2-tert-Butyl-5-(4-fluoro-phenyl)-3H-imidazol-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Cytochrome P450 3A4 | J Med Chem 48: 2270-3 (2005) Article DOI: 10.1021/jm048978k BindingDB Entry DOI: 10.7270/Q2M90869 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||