

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203626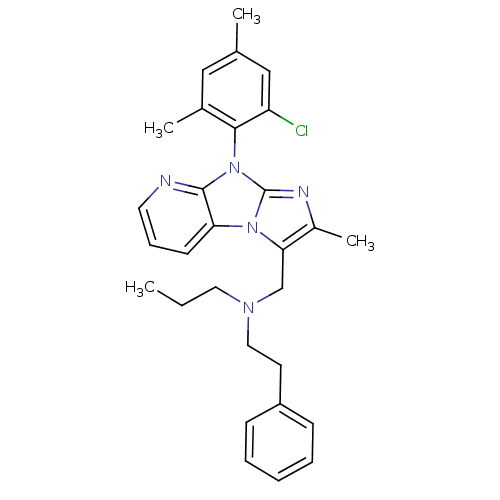 (CHEMBL247557 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203611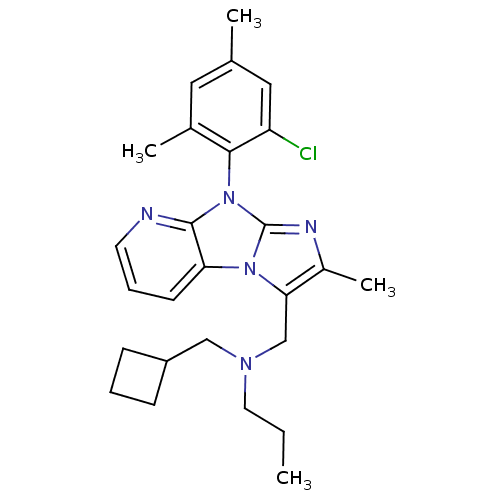 (CHEMBL393044 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203587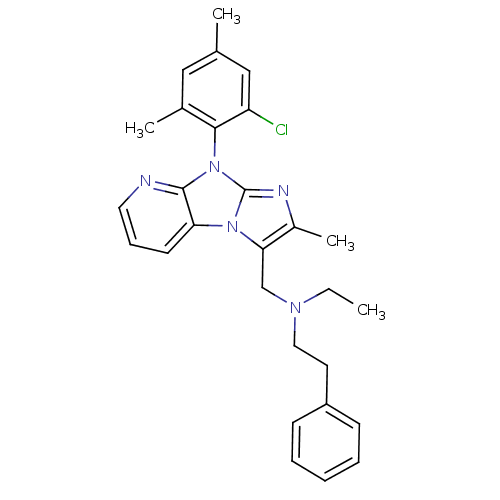 (CHEMBL392655 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203625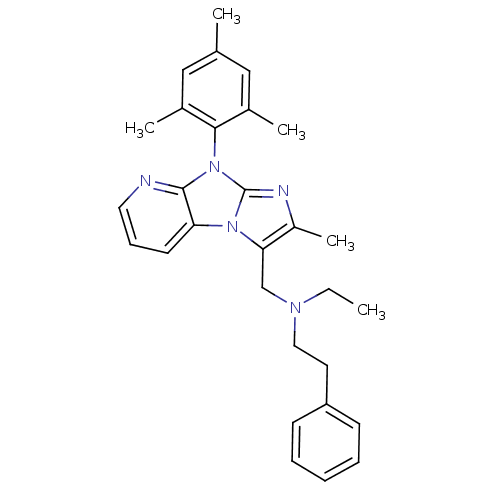 (CHEMBL246501 | ethyl-[2-methyl-8-(2,4,6-trimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203598 (CHEMBL397389 | cyclobutylmethyl-[2-methyl-8-(2,4,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203621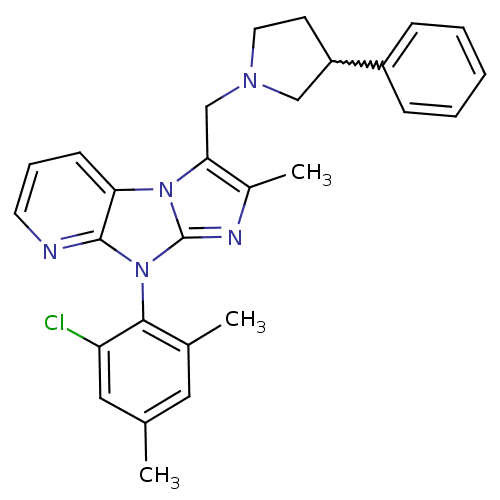 (8-(2-chloro-4,6-dimethyl-phenyl)-2-methyl-3-(3-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203590 (CHEMBL395929 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203609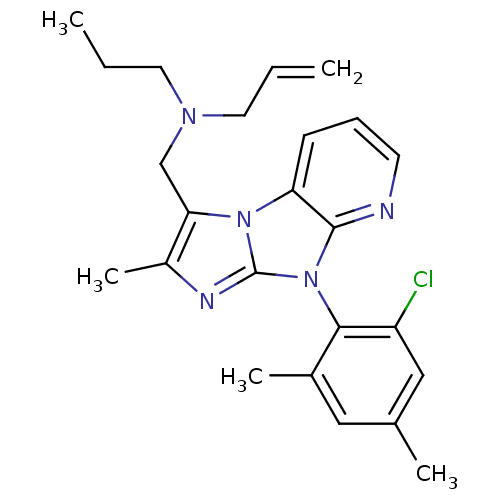 (CHEMBL247151 | allyl-[8-(2-chloro-4,6-dimethyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203589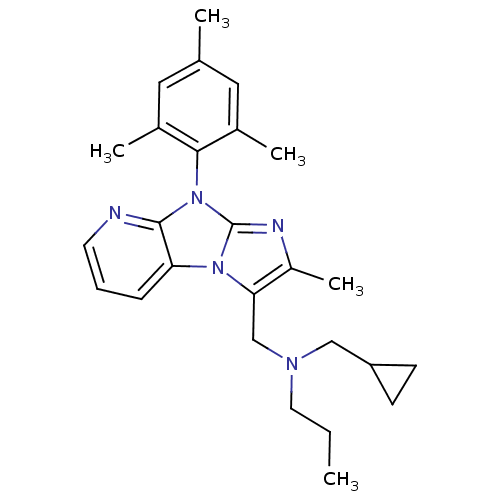 (CHEMBL391141 | cyclopropylmethyl-[2-methyl-8-(2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203624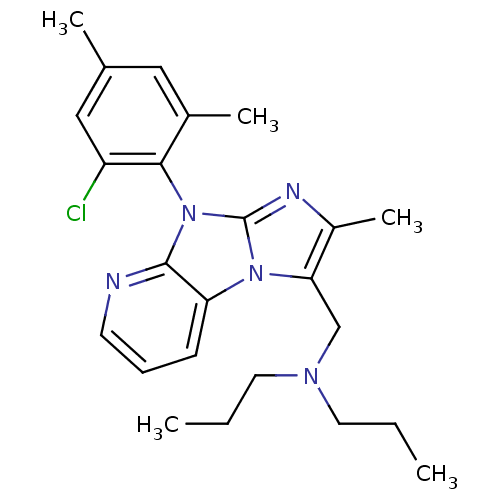 (CHEMBL393250 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203610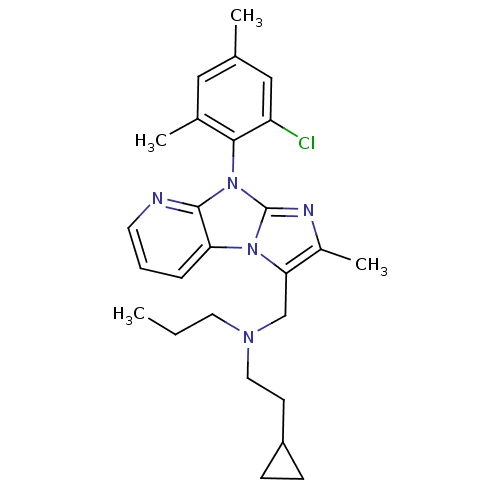 (CHEMBL247359 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203608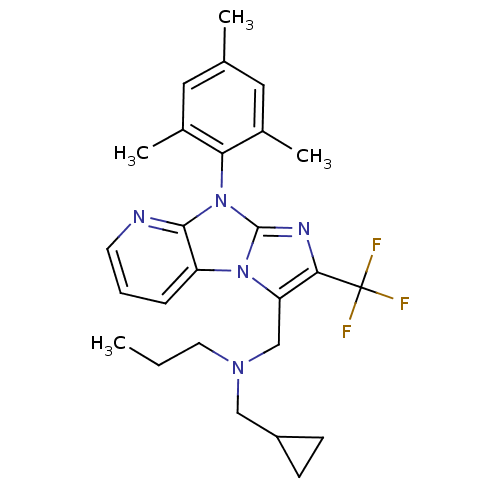 (CHEMBL245671 | cyclopropylmethyl-propyl-[2-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203617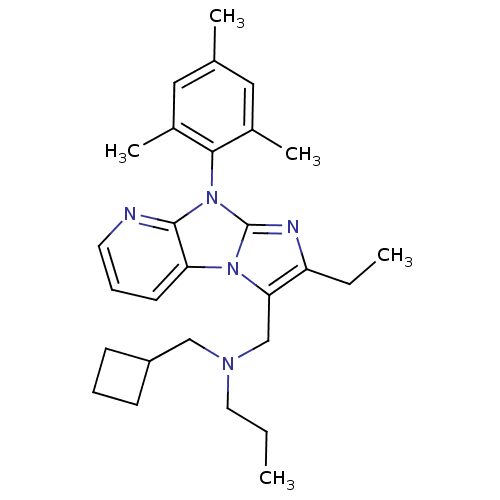 (CHEMBL395833 | cyclobutylmethyl-[2-ethyl-8-(2,4,6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203597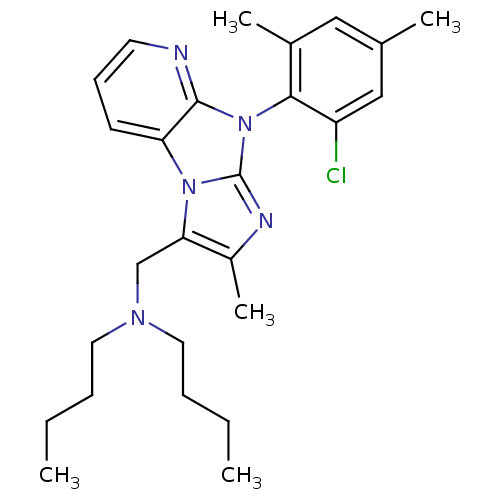 (CHEMBL556060 | dibutyl-[8-(2-chloro-4,6-dimethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203599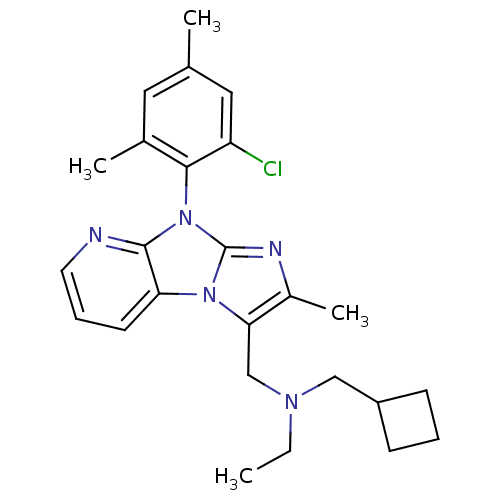 (CHEMBL247558 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203594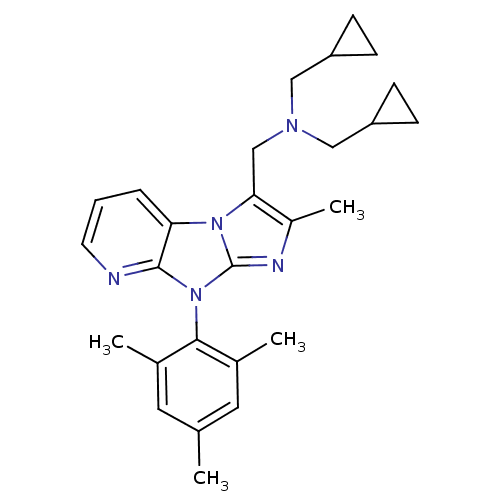 (CHEMBL391169 | bis-cyclopropylmethyl-[2-methyl-8-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203619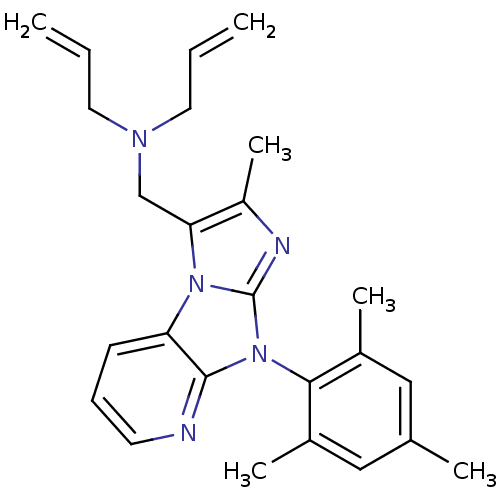 (CHEMBL430391 | diallyl-[2-methyl-8-(2,4,6-trimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203602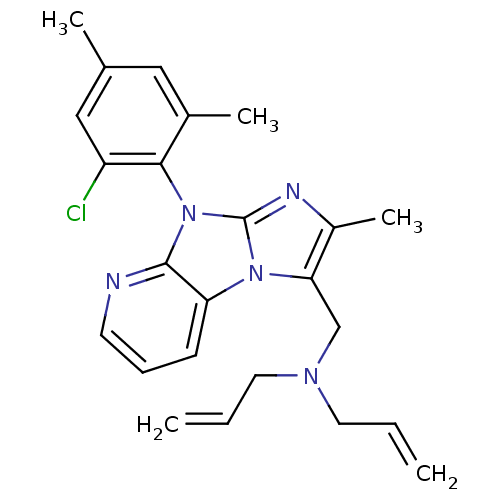 (CHEMBL247358 | diallyl-[8-(2-chloro-4,6-dimethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203600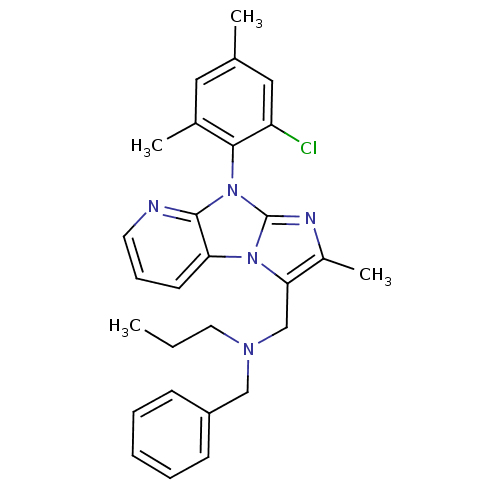 (CHEMBL246944 | benzyl-[8-(2-chloro-4,6-dimethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203588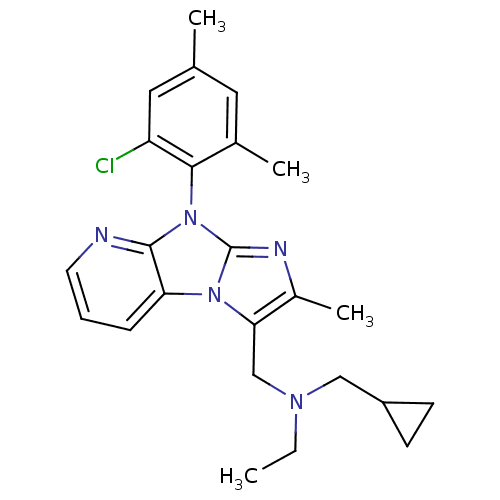 (CHEMBL392654 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203606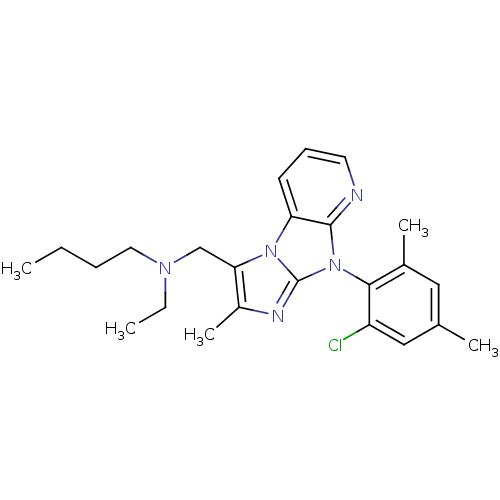 (CHEMBL247755 | butyl-[8-(2-chloro-4,6-dimethyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203604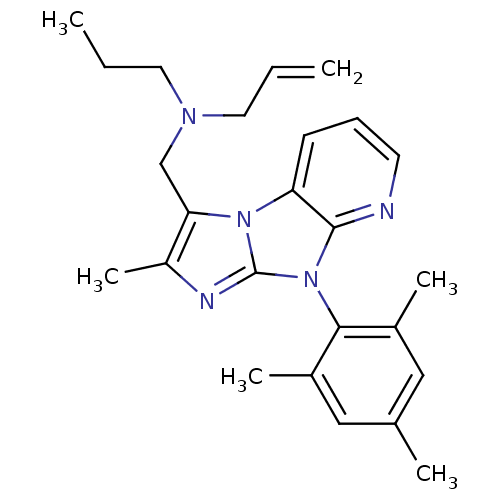 (CHEMBL247152 | allyl-[2-methyl-8-(2,4,6-trimethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203612 ((2-cyclopropyl-ethyl)-[2-methyl-8-(2,4,6-trimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203615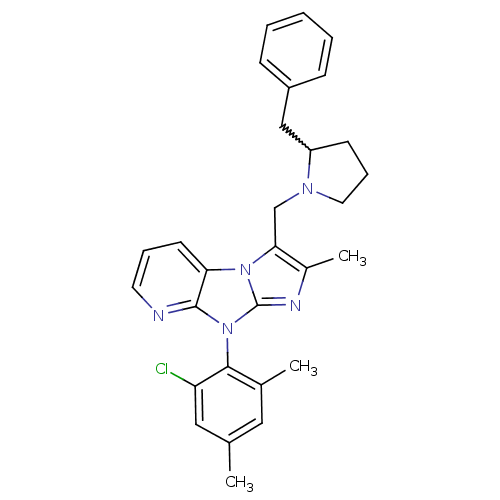 (3-(2-benzyl-pyrrolidin-1-ylmethyl)-8-(2-chloro-4,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203614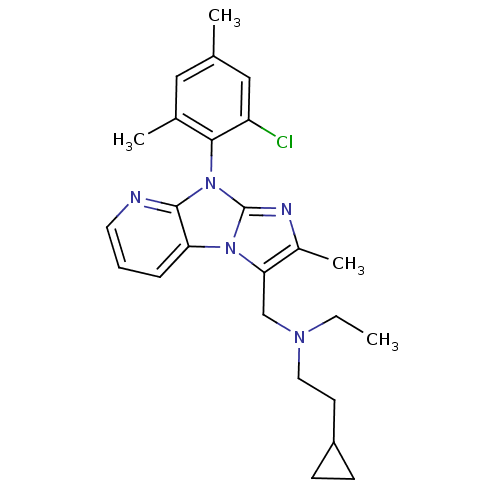 (CHEMBL247753 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203605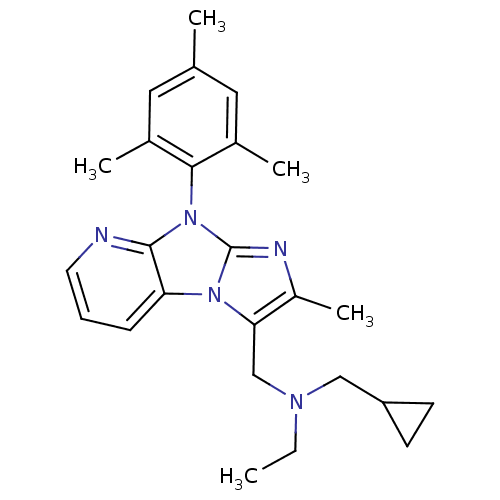 (CHEMBL428048 | cyclopropylmethyl-ethyl-[2-methyl-8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203596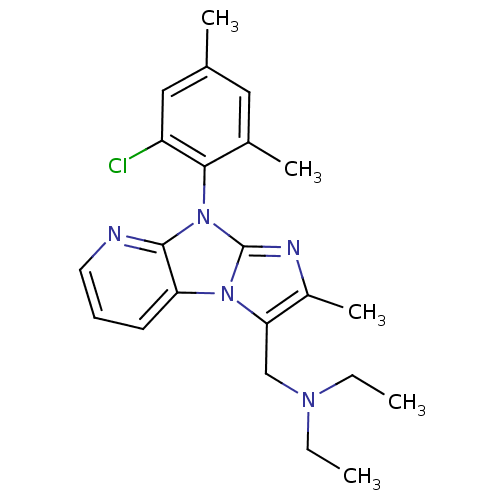 (CHEMBL247957 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203613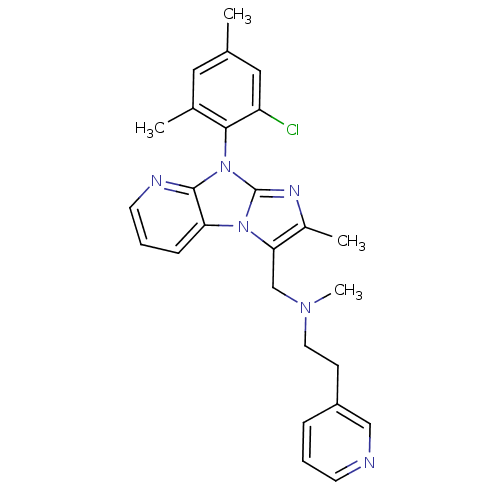 (CHEMBL245297 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203622 (8-(2-chloro-4,6-dimethyl-phenyl)-2-methyl-3-(2-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203616 (8-(2-chloro-4,6-dimethyl-phenyl)-2-methyl-3-(3-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203592 (3-(3-benzyl-pyrrolidin-1-ylmethyl)-8-(2-chloro-4,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203627 (CHEMBL392044 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203607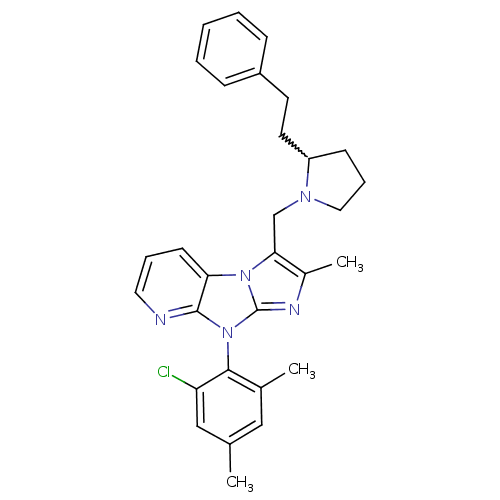 (8-(2-chloro-4,6-dimethyl-phenyl)-2-methyl-3-(2-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203620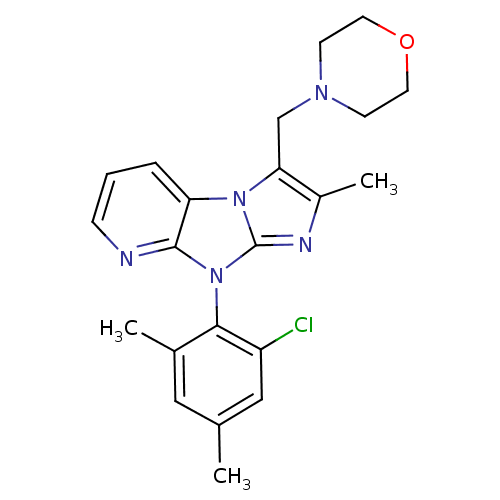 (8-(2-chloro-4,6-dimethyl-phenyl)-2-methyl-3-morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203593 (CHEMBL248367 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203591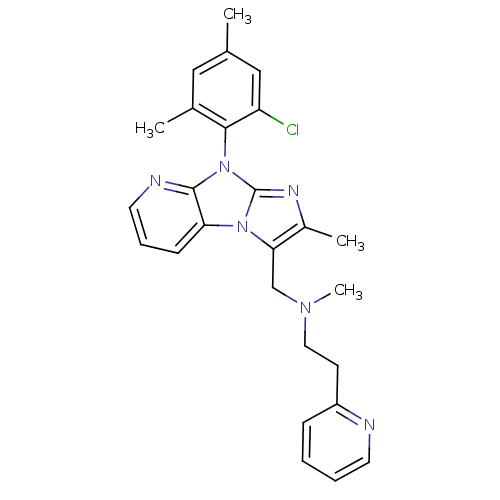 (CHEMBL396821 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203623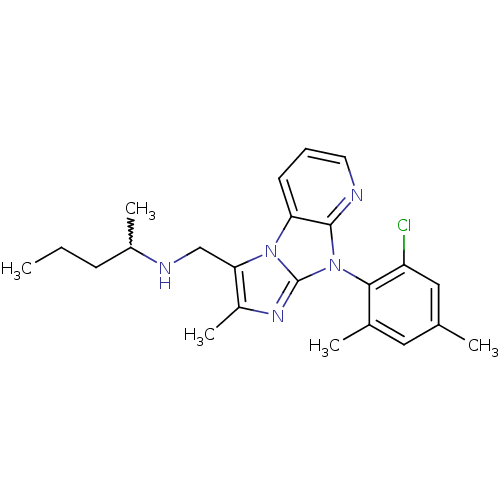 (CHEMBL396822 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203603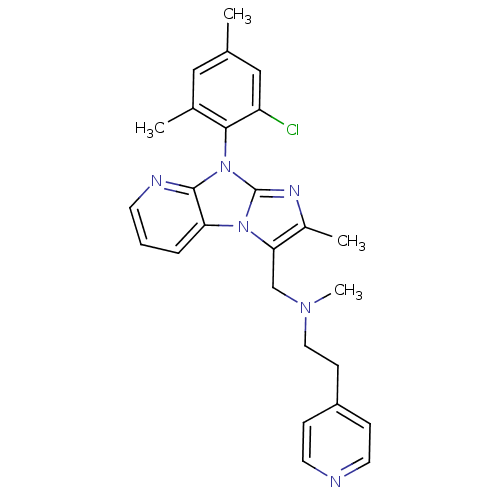 (CHEMBL428044 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203601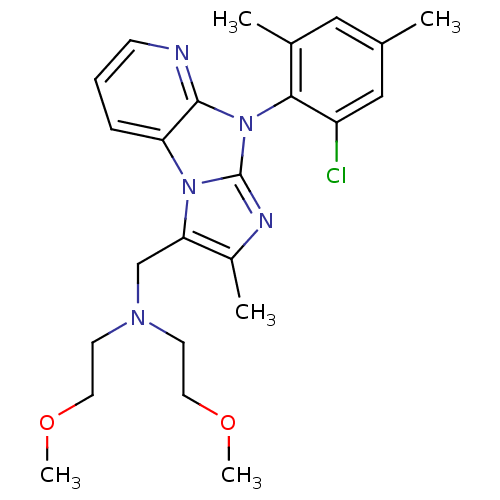 (CHEMBL245298 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203595 (CHEMBL245506 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50203618 (CHEMBL248368 | [8-(2-chloro-4,6-dimethyl-phenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from CRF1 receptor expressed in IMR32 cells assessed as inhibition of CRF stimulated cAMP production | Bioorg Med Chem Lett 17: 2026-30 (2007) Article DOI: 10.1016/j.bmcl.2007.01.008 BindingDB Entry DOI: 10.7270/Q21R6Q5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||