

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262329 (2-(2-chloro-3-fluorophenyl)-5-(4-(6-methoxypyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262271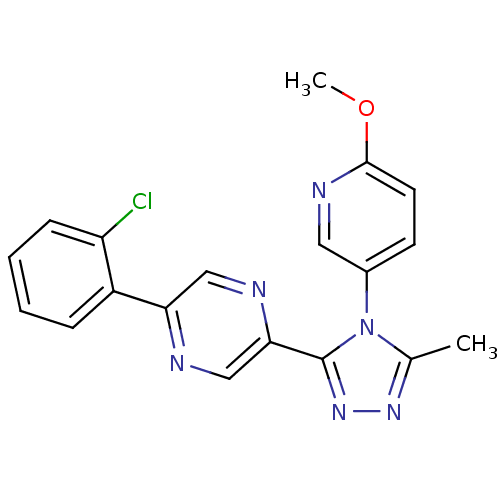 (2-(2-chlorophenyl)-5-(4-(6-methoxypyridin-3-yl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262151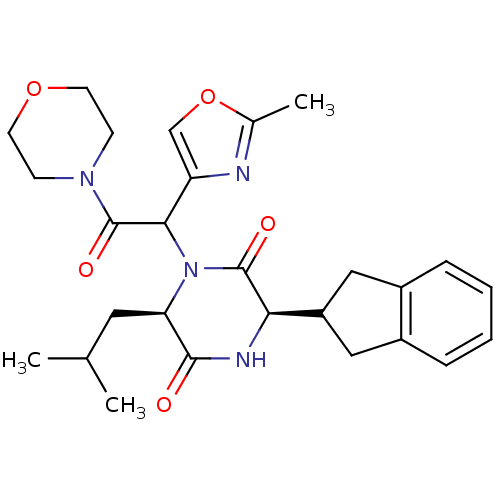 ((3R,6R)-3-(2,3-dihydro-1H-inden-2-yl)-6-isobutyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262209 (2-(2,3-dimethylphenyl)-5-(5-(methoxymethyl)-4-(6-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262270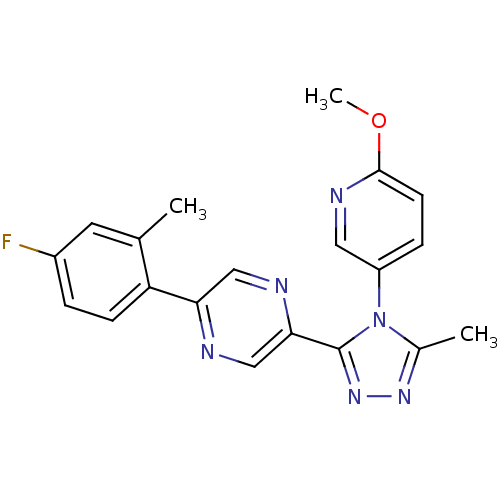 (2-(4-fluoro-2-methylphenyl)-5-(4-(6-methoxypyridin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50326719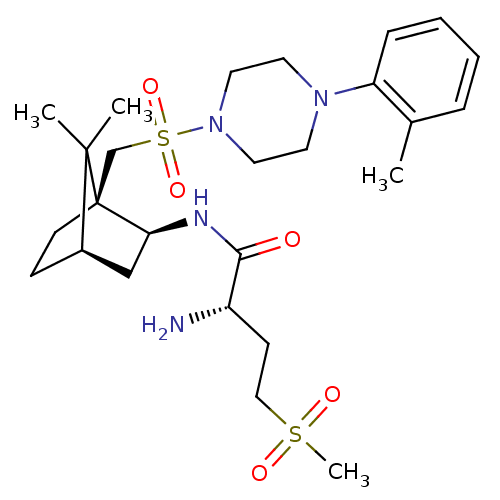 ((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262327 (4-(5-(5-(methoxymethyl)-4-(6-methoxypyridin-3-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262207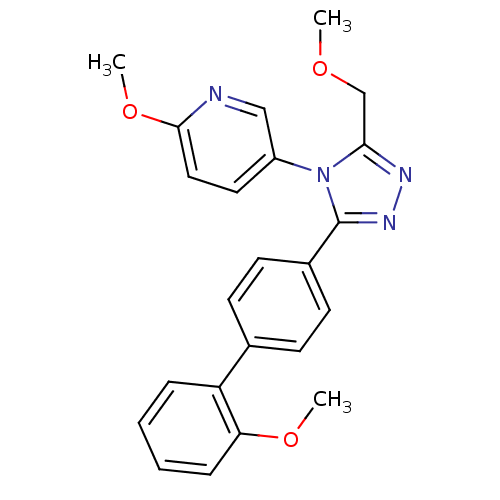 (2-Methoxy-5-[3-(2'-methoxy-biphenyl-4-yl)-5-methox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262210 (2-(4-fluoro-3-methylphenyl)-5-(5-(methoxymethyl)-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50029649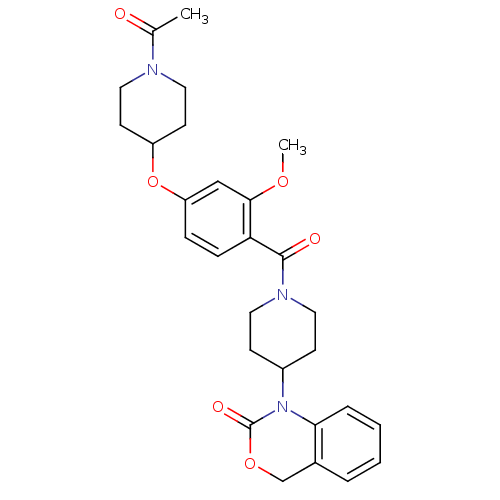 (1-(1-(4-(1-acetylpiperidin-4-yloxy)-2-methoxybenzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262153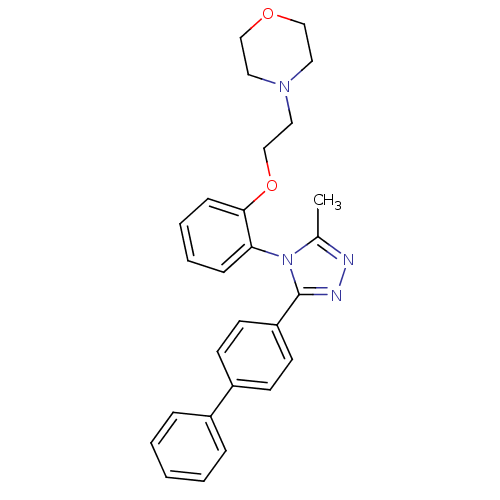 (4-{2-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262208 (2-(5-(methoxymethyl)-4-(6-methoxypyridin-3-yl)-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262208 (2-(5-(methoxymethyl)-4-(6-methoxypyridin-3-yl)-4H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262152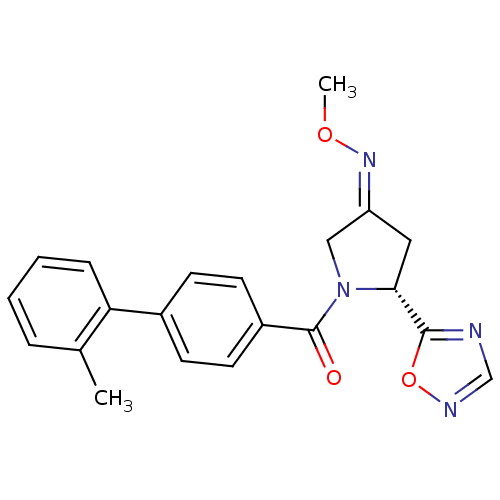 ((R)-1-(2'-Methyl-biphenyl-4-carbonyl)-5-[1,2,4]oxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262206 (3-(2'-Methoxy-biphenyl-4-yl)-4-(4-methoxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262269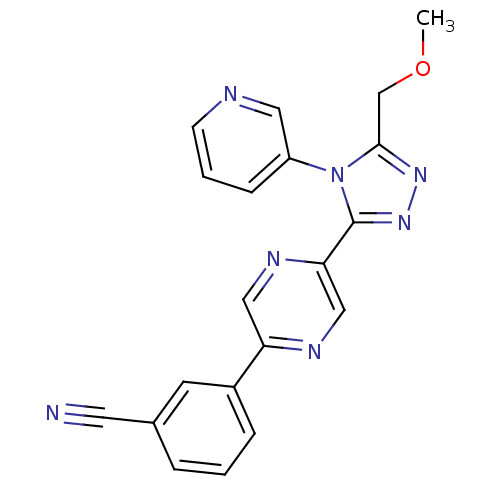 (3-(5-(5-(methoxymethyl)-4-(pyridin-3-yl)-4H-1,2,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50262209 (2-(2,3-dimethylphenyl)-5-(5-(methoxymethyl)-4-(6-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262209 (2-(2,3-dimethylphenyl)-5-(5-(methoxymethyl)-4-(6-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262154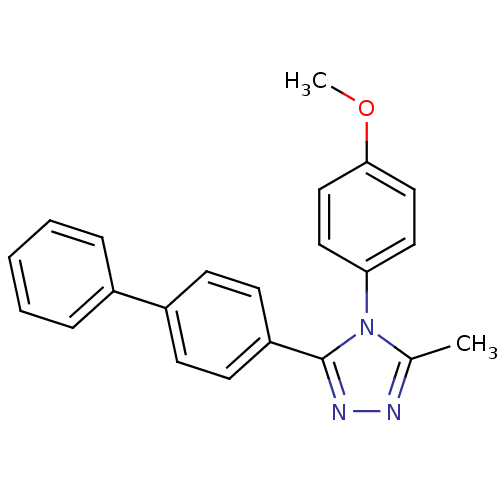 (3-Biphenyl-4-yl-4-(4-methoxy-phenyl)-5-methyl-4H-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50262207 (2-Methoxy-5-[3-(2'-methoxy-biphenyl-4-yl)-5-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262328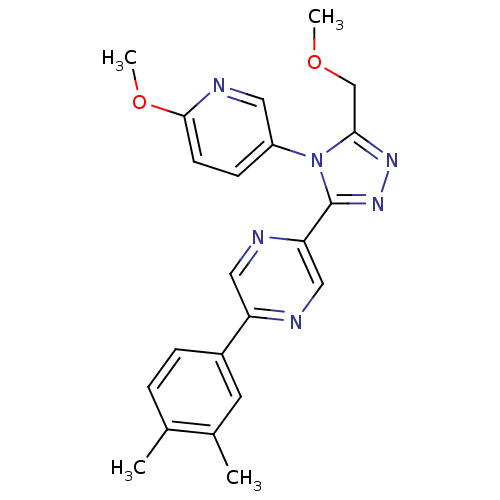 (2-(3,4-dimethylphenyl)-5-(5-(methoxymethyl)-4-(6-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262271 (2-(2-chlorophenyl)-5-(4-(6-methoxypyridin-3-yl)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262272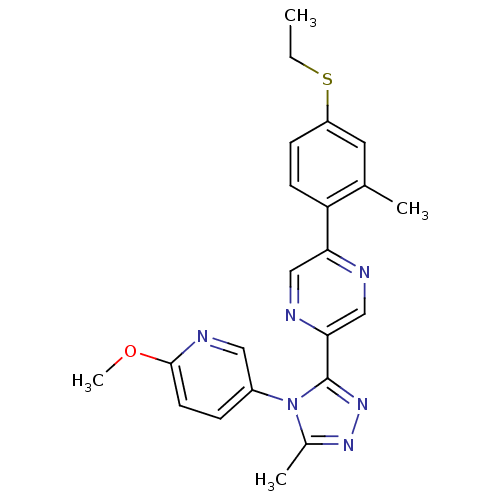 (2-(4-(ethylthio)-2-methylphenyl)-5-(4-(6-methoxypy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262207 (2-Methoxy-5-[3-(2'-methoxy-biphenyl-4-yl)-5-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262153 (4-{2-[2-(3-Biphenyl-4-yl-5-methyl-[1,2,4]triazol-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 304 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262270 (2-(4-fluoro-2-methylphenyl)-5-(4-(6-methoxypyridin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 388 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262328 (2-(3,4-dimethylphenyl)-5-(5-(methoxymethyl)-4-(6-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 432 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50262206 (3-(2'-Methoxy-biphenyl-4-yl)-4-(4-methoxy-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262206 (3-(2'-Methoxy-biphenyl-4-yl)-4-(4-methoxy-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50262273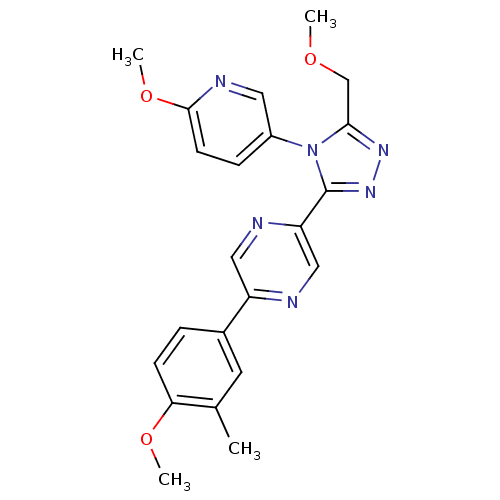 (2-(4-methoxy-3-methylphenyl)-5-(5-(methoxymethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human cloned oxytocin receptor by cell based beta lactamase reporter assay | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262329 (2-(2-chloro-3-fluorophenyl)-5-(4-(6-methoxypyridin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 732 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262210 (2-(4-fluoro-3-methylphenyl)-5-(5-(methoxymethyl)-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262273 (2-(4-methoxy-3-methylphenyl)-5-(5-(methoxymethyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262269 (3-(5-(5-(methoxymethyl)-4-(pyridin-3-yl)-4H-1,2,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50262327 (4-(5-(5-(methoxymethyl)-4-(6-methoxypyridin-3-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V1a receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50262208 (2-(5-(methoxymethyl)-4-(6-methoxypyridin-3-yl)-4H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50262269 (3-(5-(5-(methoxymethyl)-4-(pyridin-3-yl)-4H-1,2,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50262270 (2-(4-fluoro-2-methylphenyl)-5-(4-(6-methoxypyridin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50262272 (2-(4-(ethylthio)-2-methylphenyl)-5-(4-(6-methoxypy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50262273 (2-(4-methoxy-3-methylphenyl)-5-(5-(methoxymethyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50262327 (4-(5-(5-(methoxymethyl)-4-(6-methoxypyridin-3-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50262328 (2-(3,4-dimethylphenyl)-5-(5-(methoxymethyl)-4-(6-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50262210 (2-(4-fluoro-3-methylphenyl)-5-(5-(methoxymethyl)-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Antagonist activity at vassopressin V2 receptor (unknown origin) | Bioorg Med Chem Lett 18: 4278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.06.098 BindingDB Entry DOI: 10.7270/Q28W3D4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||