 Found 96 hits Enz. Inhib. hit(s) with all data for entry = 50046704
Found 96 hits Enz. Inhib. hit(s) with all data for entry = 50046704 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126976
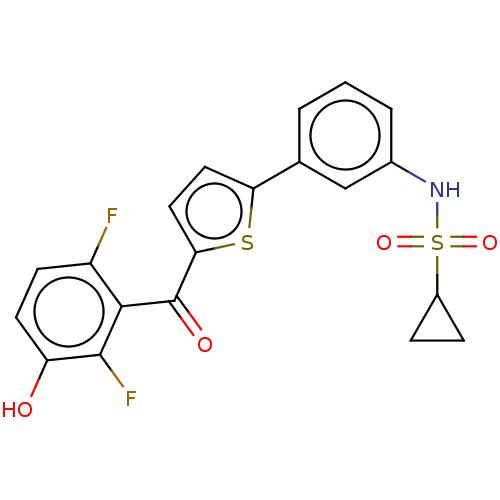
(CHEMBL3629590)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)C3CC3)c2)c1F Show InChI InChI=1S/C20H15F2NO4S2/c21-14-6-7-15(24)19(22)18(14)20(25)17-9-8-16(28-17)11-2-1-3-12(10-11)23-29(26,27)13-4-5-13/h1-3,6-10,13,23-24H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126971

(CHEMBL3629585)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)c3ccc(Br)cc3OC(F)(F)F)c2)c1F Show InChI InChI=1S/C24H13BrF5NO5S2/c25-13-4-9-20(17(11-13)36-24(28,29)30)38(34,35)31-14-3-1-2-12(10-14)18-7-8-19(37-18)23(33)21-15(26)5-6-16(32)22(21)27/h1-11,31-32H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126973
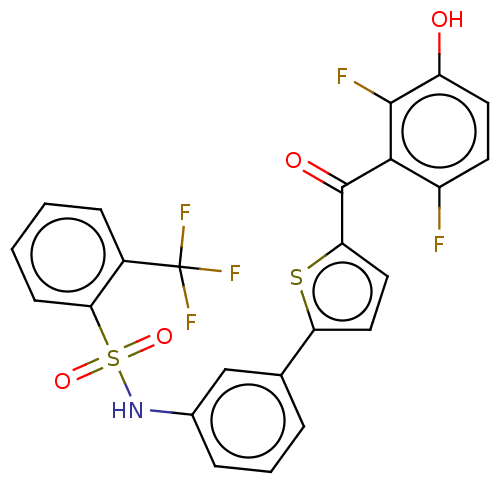
(CHEMBL3629587)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)c3ccccc3C(F)(F)F)c2)c1F Show InChI InChI=1S/C24H14F5NO4S2/c25-16-8-9-17(31)22(26)21(16)23(32)19-11-10-18(35-19)13-4-3-5-14(12-13)30-36(33,34)20-7-2-1-6-15(20)24(27,28)29/h1-12,30-31H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50126975
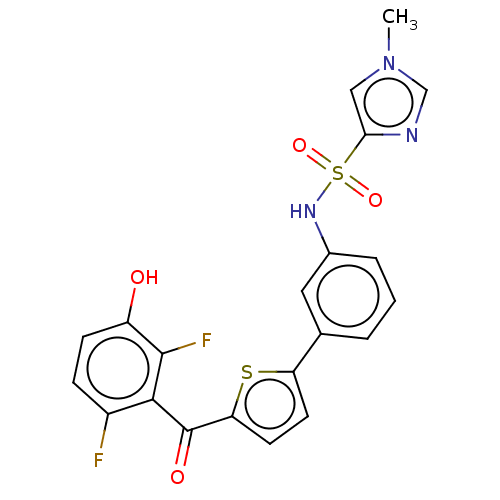
(CHEMBL3629589)Show SMILES Cn1cnc(c1)S(=O)(=O)Nc1cccc(c1)-c1ccc(s1)C(=O)c1c(F)ccc(O)c1F Show InChI InChI=1S/C21H15F2N3O4S2/c1-26-10-18(24-11-26)32(29,30)25-13-4-2-3-12(9-13)16-7-8-17(31-16)21(28)19-14(22)5-6-15(27)20(19)23/h2-11,25,27H,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysi... |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126972
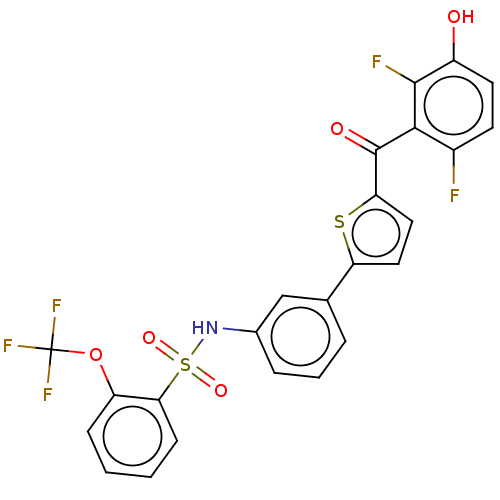
(CHEMBL3629586)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)c3ccccc3OC(F)(F)F)c2)c1F Show InChI InChI=1S/C24H14F5NO5S2/c25-15-8-9-16(31)22(26)21(15)23(32)19-11-10-18(36-19)13-4-3-5-14(12-13)30-37(33,34)20-7-2-1-6-17(20)35-24(27,28)29/h1-12,30-31H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50126974
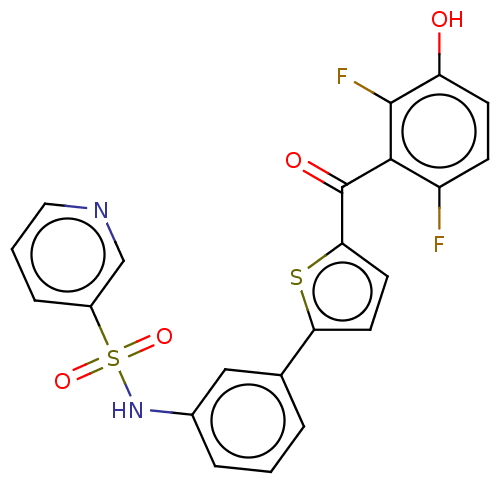
(CHEMBL3629588)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)c3cccnc3)c2)c1F Show InChI InChI=1S/C22H14F2N2O4S2/c23-16-6-7-17(27)21(24)20(16)22(28)19-9-8-18(31-19)13-3-1-4-14(11-13)26-32(29,30)15-5-2-10-25-12-15/h1-12,26-27H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysi... |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126974

(CHEMBL3629588)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)c3cccnc3)c2)c1F Show InChI InChI=1S/C22H14F2N2O4S2/c23-16-6-7-17(27)21(24)20(16)22(28)19-9-8-18(31-19)13-3-1-4-14(11-13)26-32(29,30)15-5-2-10-25-12-15/h1-12,26-27H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126978
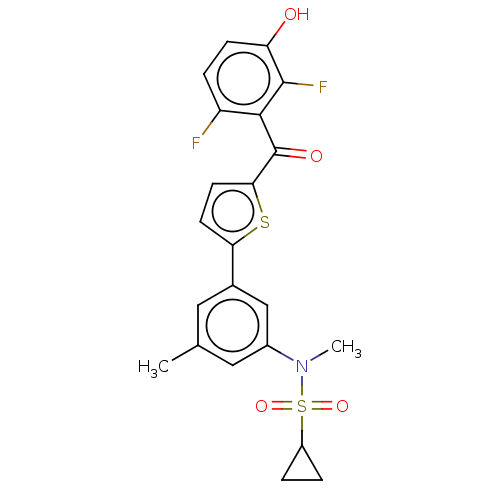
(CHEMBL3629592)Show SMILES CN(c1cc(C)cc(c1)-c1ccc(s1)C(=O)c1c(F)ccc(O)c1F)S(=O)(=O)C1CC1 Show InChI InChI=1S/C22H19F2NO4S2/c1-12-9-13(11-14(10-12)25(2)31(28,29)15-3-4-15)18-7-8-19(30-18)22(27)20-16(23)5-6-17(26)21(20)24/h5-11,15,26H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126977
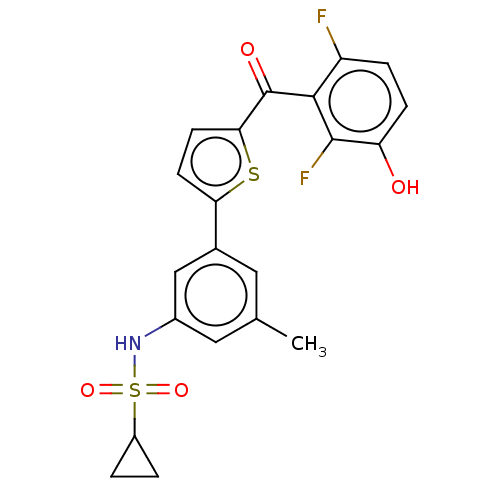
(CHEMBL3629591)Show SMILES Cc1cc(NS(=O)(=O)C2CC2)cc(c1)-c1ccc(s1)C(=O)c1c(F)ccc(O)c1F Show InChI InChI=1S/C21H17F2NO4S2/c1-11-8-12(10-13(9-11)24-30(27,28)14-2-3-14)17-6-7-18(29-17)21(26)19-15(22)4-5-16(25)20(19)23/h4-10,14,24-25H,2-3H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126975

(CHEMBL3629589)Show SMILES Cn1cnc(c1)S(=O)(=O)Nc1cccc(c1)-c1ccc(s1)C(=O)c1c(F)ccc(O)c1F Show InChI InChI=1S/C21H15F2N3O4S2/c1-26-10-18(24-11-26)32(29,30)25-13-4-2-3-12(9-13)16-7-8-17(31-16)21(28)19-14(22)5-6-15(27)20(19)23/h2-11,25,27H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50126973

(CHEMBL3629587)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)c3ccccc3C(F)(F)F)c2)c1F Show InChI InChI=1S/C24H14F5NO4S2/c25-16-8-9-17(31)22(26)21(16)23(32)19-11-10-18(35-19)13-4-3-5-14(12-13)30-36(33,34)20-7-2-1-6-15(20)24(27,28)29/h1-12,30-31H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysi... |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50126976

(CHEMBL3629590)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)C3CC3)c2)c1F Show InChI InChI=1S/C20H15F2NO4S2/c21-14-6-7-15(24)19(22)18(14)20(25)17-9-8-16(28-17)11-2-1-3-12(10-11)23-29(26,27)13-4-5-13/h1-3,6-10,13,23-24H,4-5H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysi... |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50299643
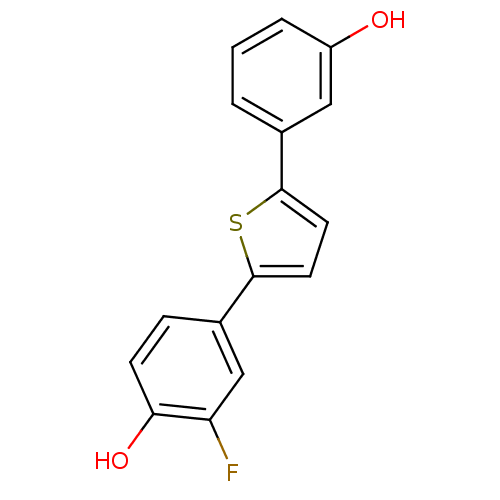
(2-Fluoro-4-[5-(3-hydroxyphenyl)-2-thienyl]phenol |...)Show InChI InChI=1S/C16H11FO2S/c17-13-9-11(4-5-14(13)19)16-7-6-15(20-16)10-2-1-3-12(18)8-10/h1-9,18-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50126977

(CHEMBL3629591)Show SMILES Cc1cc(NS(=O)(=O)C2CC2)cc(c1)-c1ccc(s1)C(=O)c1c(F)ccc(O)c1F Show InChI InChI=1S/C21H17F2NO4S2/c1-11-8-12(10-13(9-11)24-30(27,28)14-2-3-14)17-6-7-18(29-17)21(26)19-15(22)4-5-16(25)20(19)23/h4-10,14,24-25H,2-3H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysi... |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126983

(CHEMBL3629439)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2ccc(Br)cc2OC(F)(F)F)c1 Show InChI InChI=1S/C24H15BrF3NO5S2/c25-16-7-10-22(19(13-16)34-24(26,27)28)36(32,33)29-17-5-1-3-14(11-17)20-8-9-21(35-20)23(31)15-4-2-6-18(30)12-15/h1-13,29-30H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126970
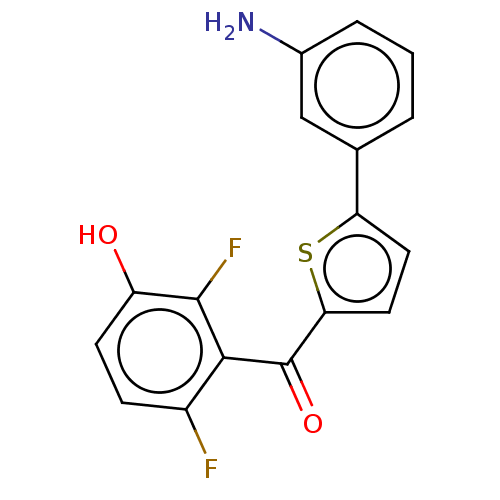
(CHEMBL3629584)Show InChI InChI=1S/C17H11F2NO2S/c18-11-4-5-12(21)16(19)15(11)17(22)14-7-6-13(23-14)9-2-1-3-10(20)8-9/h1-8,21H,20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126999
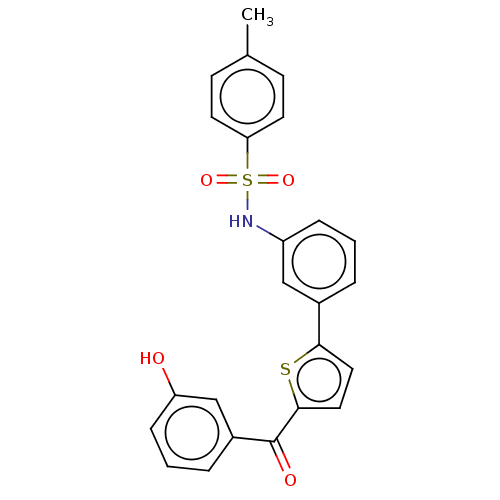
(CHEMBL3629593)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1cccc(c1)-c1ccc(s1)C(=O)c1cccc(O)c1 Show InChI InChI=1S/C24H19NO4S2/c1-16-8-10-21(11-9-16)31(28,29)25-19-6-2-4-17(14-19)22-12-13-23(30-22)24(27)18-5-3-7-20(26)15-18/h2-15,25-26H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50127002
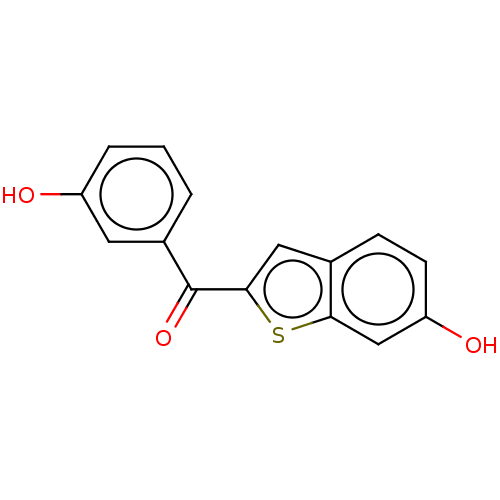
(CHEMBL3629595)Show InChI InChI=1S/C15H10O3S/c16-11-3-1-2-10(6-11)15(18)14-7-9-4-5-12(17)8-13(9)19-14/h1-8,16-17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50396084
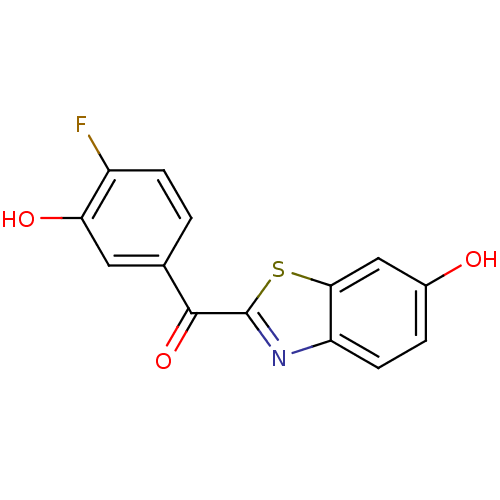
(CHEMBL2170750)Show InChI InChI=1S/C14H8FNO3S/c15-9-3-1-7(5-11(9)18)13(19)14-16-10-4-2-8(17)6-12(10)20-14/h1-6,17-18H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50126972

(CHEMBL3629586)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)c3ccccc3OC(F)(F)F)c2)c1F Show InChI InChI=1S/C24H14F5NO5S2/c25-15-8-9-16(31)22(26)21(15)23(32)19-11-10-18(36-19)13-4-3-5-14(12-13)30-37(33,34)20-7-2-1-6-17(20)35-24(27,28)29/h1-12,30-31H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysi... |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126986
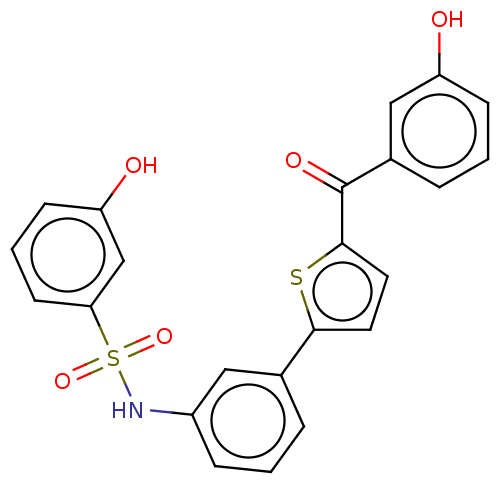
(CHEMBL3629442)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2cccc(O)c2)c1 Show InChI InChI=1S/C23H17NO5S2/c25-18-7-2-5-16(13-18)23(27)22-11-10-21(30-22)15-4-1-6-17(12-15)24-31(28,29)20-9-3-8-19(26)14-20/h1-14,24-26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126966

(CHEMBL3629458)Show SMILES Cc1ccccc1S(=O)(=O)Nc1cccc(c1)-c1ccc(s1)C(=O)c1cccc(O)c1 Show InChI InChI=1S/C24H19NO4S2/c1-16-6-2-3-11-23(16)31(28,29)25-19-9-4-7-17(14-19)21-12-13-22(30-21)24(27)18-8-5-10-20(26)15-18/h2-15,25-26H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50126971

(CHEMBL3629585)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)c3ccc(Br)cc3OC(F)(F)F)c2)c1F Show InChI InChI=1S/C24H13BrF5NO5S2/c25-13-4-9-20(17(11-13)36-24(28,29)30)38(34,35)31-14-3-1-2-12(10-14)18-7-8-19(37-18)23(33)21-15(26)5-6-16(32)22(21)27/h1-11,31-32H | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysi... |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126967
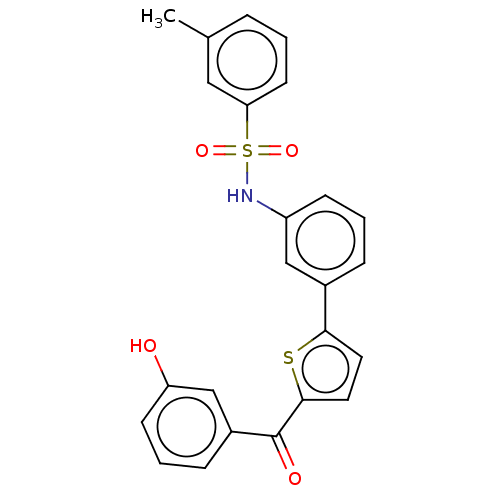
(CHEMBL3629581)Show SMILES Cc1cccc(c1)S(=O)(=O)Nc1cccc(c1)-c1ccc(s1)C(=O)c1cccc(O)c1 Show InChI InChI=1S/C24H19NO4S2/c1-16-5-2-10-21(13-16)31(28,29)25-19-8-3-6-17(14-19)22-11-12-23(30-22)24(27)18-7-4-9-20(26)15-18/h2-15,25-26H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50261942

(6-(3-Hydroxyphenyl)-1-phenyl-2-naphthol | CHEMBL46...)Show InChI InChI=1S/C22H16O2/c23-19-8-4-7-16(14-19)17-9-11-20-18(13-17)10-12-21(24)22(20)15-5-2-1-3-6-15/h1-14,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
Estradiol 17-beta-dehydrogenase 2
(Mus musculus) | BDBM50126973

(CHEMBL3629587)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)c3ccccc3C(F)(F)F)c2)c1F Show InChI InChI=1S/C24H14F5NO4S2/c25-16-8-9-17(31)22(26)21(16)23(32)19-11-10-18(35-19)13-4-3-5-14(12-13)30-36(33,34)20-7-2-1-6-15(20)24(27,28)29/h1-12,30-31H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of mouse liver microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50126978

(CHEMBL3629592)Show SMILES CN(c1cc(C)cc(c1)-c1ccc(s1)C(=O)c1c(F)ccc(O)c1F)S(=O)(=O)C1CC1 Show InChI InChI=1S/C22H19F2NO4S2/c1-12-9-13(11-14(10-12)25(2)31(28,29)15-3-4-15)18-7-8-19(30-18)22(27)20-16(23)5-6-17(26)21(20)24/h5-11,15,26H,3-4H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysi... |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50330993
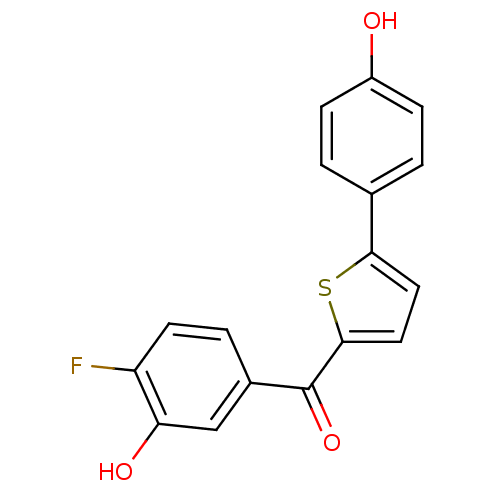
((4-fluoro-3-hydroxyphenyl)(5-(4-hydroxyphenyl)thio...)Show InChI InChI=1S/C17H11FO3S/c18-13-6-3-11(9-14(13)20)17(21)16-8-7-15(22-16)10-1-4-12(19)5-2-10/h1-9,19-20H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126982
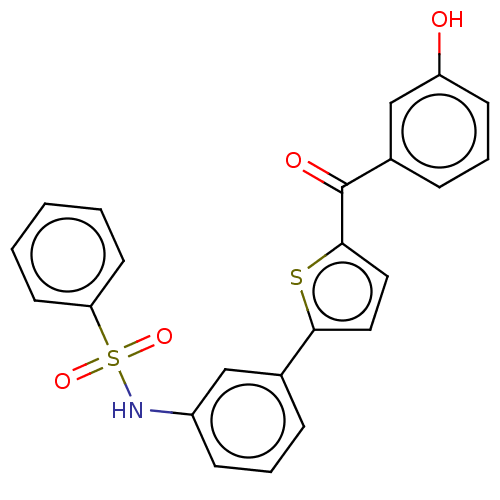
(CHEMBL3629438)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2ccccc2)c1 Show InChI InChI=1S/C23H17NO4S2/c25-19-9-5-7-17(15-19)23(26)22-13-12-21(29-22)16-6-4-8-18(14-16)24-30(27,28)20-10-2-1-3-11-20/h1-15,24-25H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50385880
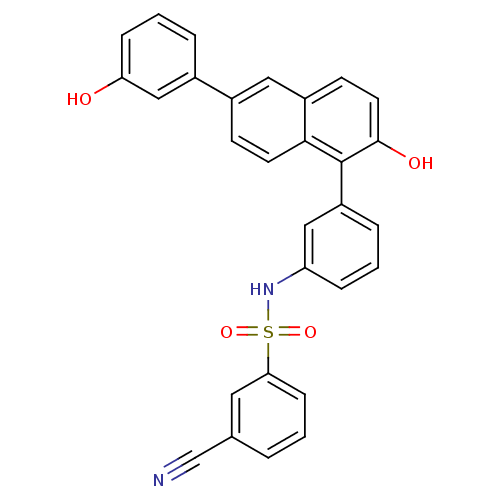
(CHEMBL2041368)Show SMILES Oc1cccc(c1)-c1ccc2c(c(O)ccc2c1)-c1cccc(NS(=O)(=O)c2cccc(c2)C#N)c1 Show InChI InChI=1S/C29H20N2O4S/c30-18-19-4-1-9-26(14-19)36(34,35)31-24-7-2-6-23(16-24)29-27-12-10-21(15-22(27)11-13-28(29)33)20-5-3-8-25(32)17-20/h1-17,31-33H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126984
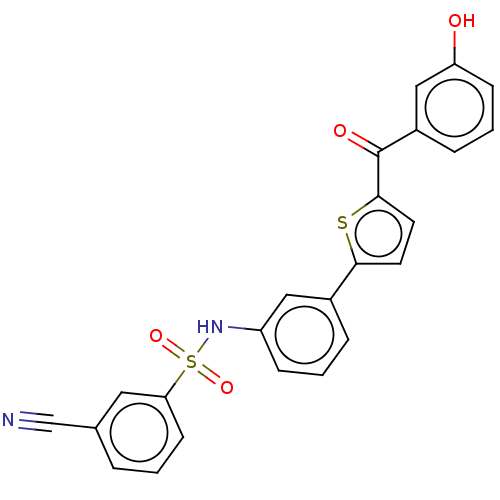
(CHEMBL3629440)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2cccc(c2)C#N)c1 Show InChI InChI=1S/C24H16N2O4S2/c25-15-16-4-1-9-21(12-16)32(29,30)26-19-7-2-5-17(13-19)22-10-11-23(31-22)24(28)18-6-3-8-20(27)14-18/h1-14,26-27H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 2
(Homo sapiens (Human)) | BDBM50126970

(CHEMBL3629584)Show InChI InChI=1S/C17H11F2NO2S/c18-11-4-5-12(21)16(19)15(11)17(22)14-7-6-13(23-14)9-2-1-3-10(20)8-9/h1-8,21H,20H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysi... |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50396080
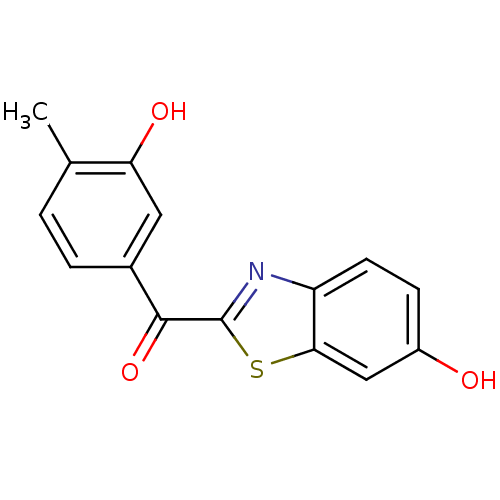
(CHEMBL2170754)Show InChI InChI=1S/C15H11NO3S/c1-8-2-3-9(6-12(8)18)14(19)15-16-11-5-4-10(17)7-13(11)20-15/h2-7,17-18H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126997
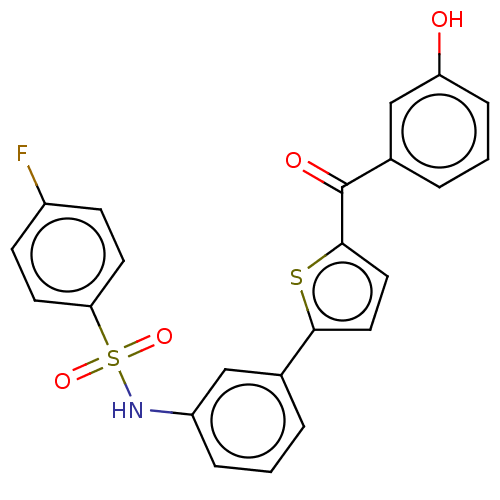
(CHEMBL3629452)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C23H16FNO4S2/c24-17-7-9-20(10-8-17)31(28,29)25-18-5-1-3-15(13-18)21-11-12-22(30-21)23(27)16-4-2-6-19(26)14-16/h1-14,25-26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126991
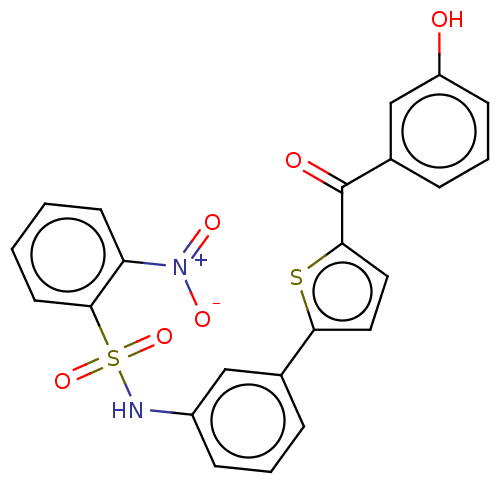
(CHEMBL3629446)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2ccccc2[N+]([O-])=O)c1 Show InChI InChI=1S/C23H16N2O6S2/c26-18-8-4-6-16(14-18)23(27)21-12-11-20(32-21)15-5-3-7-17(13-15)24-33(30,31)22-10-2-1-9-19(22)25(28)29/h1-14,24,26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126988

(CHEMBL3629443)Show SMILES Oc1cccc(c1)C(=O)Nc1cccc(c1)-c1ccc(s1)C(=O)c1cccc(O)c1 Show InChI InChI=1S/C24H17NO4S/c26-19-8-2-5-16(13-19)23(28)22-11-10-21(30-22)15-4-1-7-18(12-15)25-24(29)17-6-3-9-20(27)14-17/h1-14,26-27H,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126964
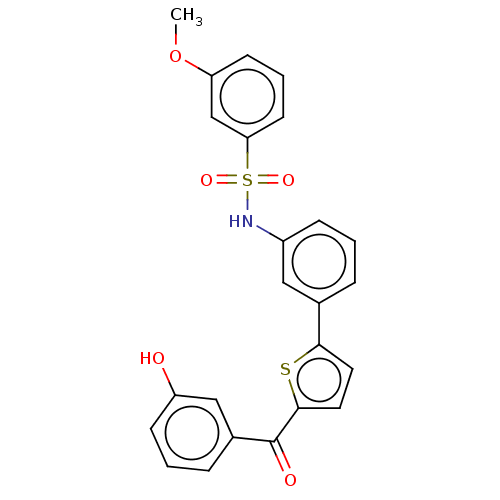
(CHEMBL3629456)Show SMILES COc1cccc(c1)S(=O)(=O)Nc1cccc(c1)-c1ccc(s1)C(=O)c1cccc(O)c1 Show InChI InChI=1S/C24H19NO5S2/c1-30-20-9-4-10-21(15-20)32(28,29)25-18-7-2-5-16(13-18)22-11-12-23(31-22)24(27)17-6-3-8-19(26)14-17/h2-15,25-26H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
Estradiol 17-beta-dehydrogenase 2
(Rattus norvegicus) | BDBM50126972

(CHEMBL3629586)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)c3ccccc3OC(F)(F)F)c2)c1F Show InChI InChI=1S/C24H14F5NO5S2/c25-15-8-9-16(31)22(26)21(15)23(32)19-11-10-18(36-19)13-4-3-5-14(12-13)30-37(33,34)20-7-2-1-6-17(20)35-24(27,28)29/h1-12,30-31H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50127001
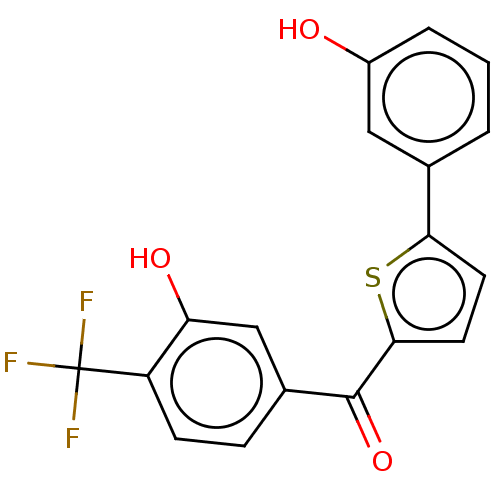
(CHEMBL3629594)Show SMILES Oc1cccc(c1)-c1ccc(s1)C(=O)c1ccc(c(O)c1)C(F)(F)F Show InChI InChI=1S/C18H11F3O3S/c19-18(20,21)13-5-4-11(9-14(13)23)17(24)16-7-6-15(25-16)10-2-1-3-12(22)8-10/h1-9,22-23H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126996
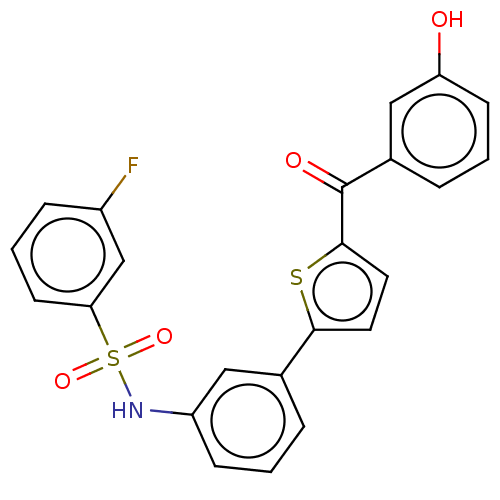
(CHEMBL3629451)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2cccc(F)c2)c1 Show InChI InChI=1S/C23H16FNO4S2/c24-17-6-3-9-20(14-17)31(28,29)25-18-7-1-4-15(12-18)21-10-11-22(30-21)23(27)16-5-2-8-19(26)13-16/h1-14,25-26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126985
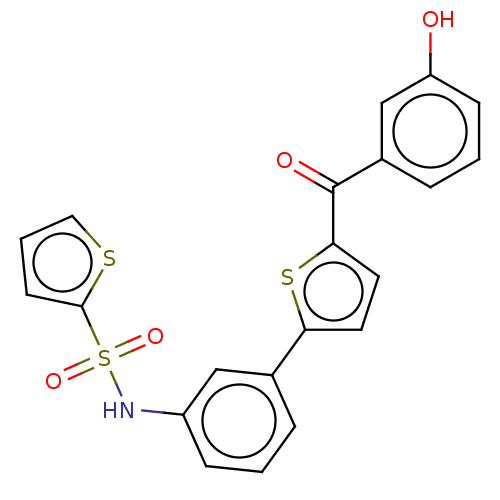
(CHEMBL3629441)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2cccs2)c1 Show InChI InChI=1S/C21H15NO4S3/c23-17-7-2-5-15(13-17)21(24)19-10-9-18(28-19)14-4-1-6-16(12-14)22-29(25,26)20-8-3-11-27-20/h1-13,22-23H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126993
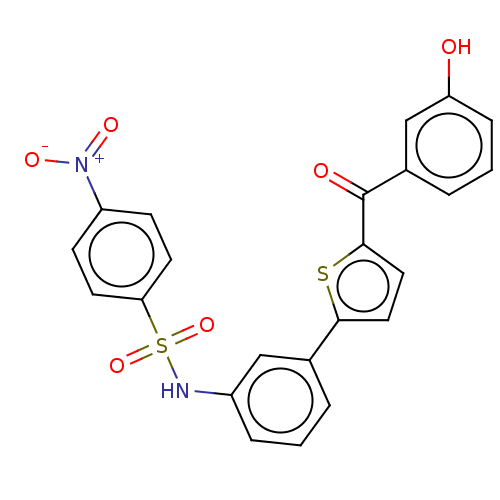
(CHEMBL3629448)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2ccc(cc2)[N+]([O-])=O)c1 Show InChI InChI=1S/C23H16N2O6S2/c26-19-6-2-4-16(14-19)23(27)22-12-11-21(32-22)15-3-1-5-17(13-15)24-33(30,31)20-9-7-18(8-10-20)25(28)29/h1-14,24,26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50127000
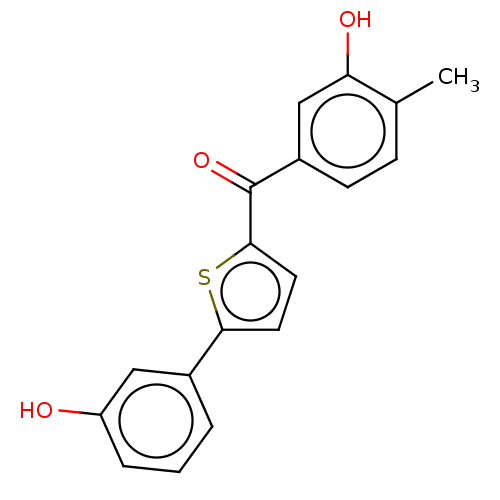
(CHEMBL1277430)Show InChI InChI=1S/C18H14O3S/c1-11-5-6-13(10-15(11)20)18(21)17-8-7-16(22-17)12-3-2-4-14(19)9-12/h2-10,19-20H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126992
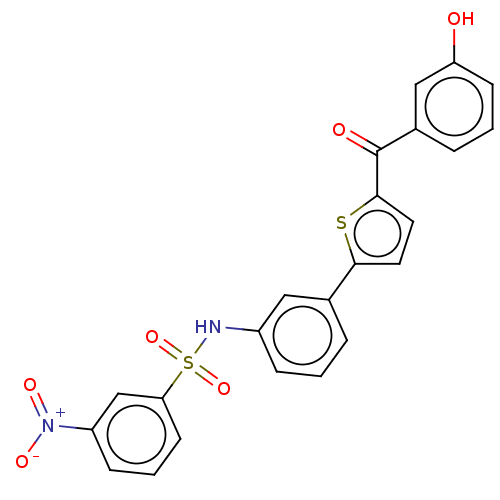
(CHEMBL3629447)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2cccc(c2)[N+]([O-])=O)c1 Show InChI InChI=1S/C23H16N2O6S2/c26-19-8-2-5-16(13-19)23(27)22-11-10-21(32-22)15-4-1-6-17(12-15)24-33(30,31)20-9-3-7-18(14-20)25(28)29/h1-14,24,26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
Estradiol 17-beta-dehydrogenase 2
(Rattus norvegicus) | BDBM50126973

(CHEMBL3629587)Show SMILES Oc1ccc(F)c(C(=O)c2ccc(s2)-c2cccc(NS(=O)(=O)c3ccccc3C(F)(F)F)c2)c1F Show InChI InChI=1S/C24H14F5NO4S2/c25-16-8-9-17(31)22(26)21(16)23(32)19-11-10-18(35-19)13-4-3-5-14(12-13)30-36(33,34)20-7-2-1-6-15(20)24(27,28)29/h1-12,30-31H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126995
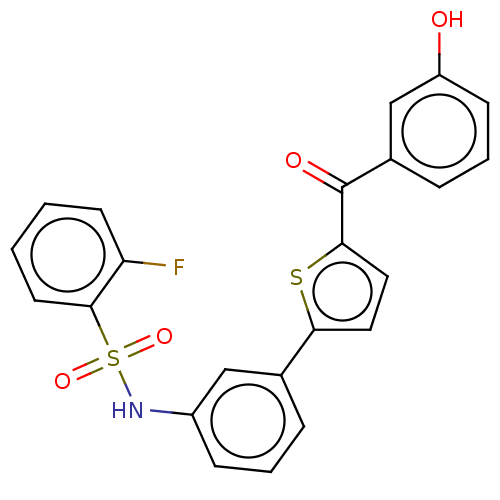
(CHEMBL3629450)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2ccccc2F)c1 Show InChI InChI=1S/C23H16FNO4S2/c24-19-9-1-2-10-22(19)31(28,29)25-17-7-3-5-15(13-17)20-11-12-21(30-20)23(27)16-6-4-8-18(26)14-16/h1-14,25-26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126998
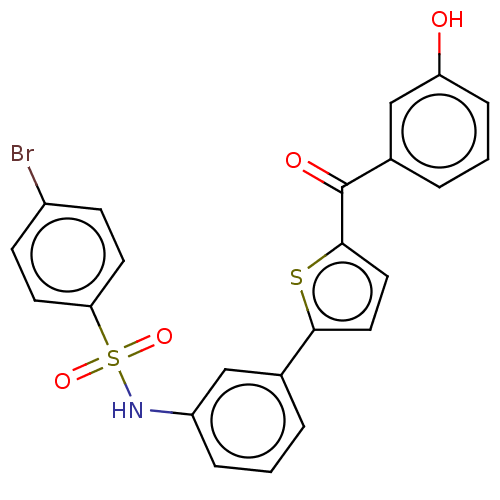
(CHEMBL3629453)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2ccc(Br)cc2)c1 Show InChI InChI=1S/C23H16BrNO4S2/c24-17-7-9-20(10-8-17)31(28,29)25-18-5-1-3-15(13-18)21-11-12-22(30-21)23(27)16-4-2-6-19(26)14-16/h1-14,25-26H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126968

(CHEMBL3629582)Show SMILES COc1ccc(c(F)c1)S(=O)(=O)Nc1cccc(c1)-c1ccc(s1)C(=O)c1cccc(O)c1 Show InChI InChI=1S/C24H18FNO5S2/c1-31-19-8-11-23(20(25)14-19)33(29,30)26-17-6-2-4-15(12-17)21-9-10-22(32-21)24(28)16-5-3-7-18(27)13-16/h2-14,26-27H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
Estradiol 17-beta-dehydrogenase 2
(Mus musculus) | BDBM50126977

(CHEMBL3629591)Show SMILES Cc1cc(NS(=O)(=O)C2CC2)cc(c1)-c1ccc(s1)C(=O)c1c(F)ccc(O)c1F Show InChI InChI=1S/C21H17F2NO4S2/c1-11-8-12(10-13(9-11)24-30(27,28)14-2-3-14)17-6-7-18(29-17)21(26)19-15(22)4-5-16(25)20(19)23/h4-10,14,24-25H,2-3H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of mouse liver microsomal 17beta HSD2 using unlabeled- and labelled [2,4,6,7-3H]-E2 as substrate incubated for 20 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 1
(Homo sapiens (Human)) | BDBM50126990
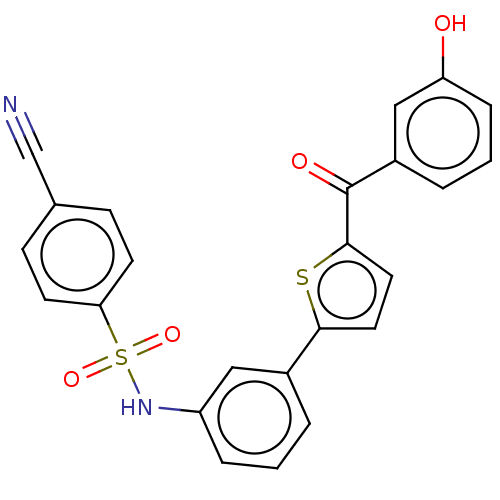
(CHEMBL3629445)Show SMILES Oc1cccc(c1)C(=O)c1ccc(s1)-c1cccc(NS(=O)(=O)c2ccc(cc2)C#N)c1 Show InChI InChI=1S/C24H16N2O4S2/c25-15-16-7-9-21(10-8-16)32(29,30)26-19-5-1-3-17(13-19)22-11-12-23(31-22)24(28)18-4-2-6-20(27)14-18/h1-14,26-27H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human placental cytosolic 17beta HSD1 using unlabeled- and labelled [2,4,6,7-3H]-E1 as substrate incubated for 10 mins by HPLC analysis |
Eur J Med Chem 103: 56-68 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.030
BindingDB Entry DOI: 10.7270/Q22V2HXX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data