

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189445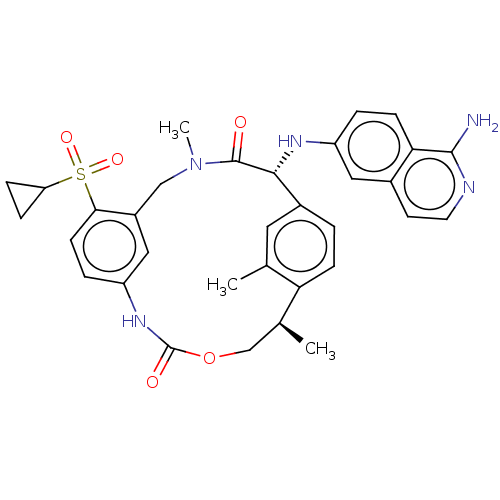 (US9174974, Example 31) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189441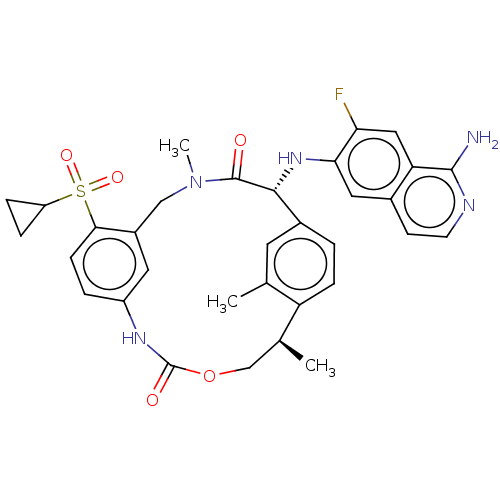 (US9174974, Example 26) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50191346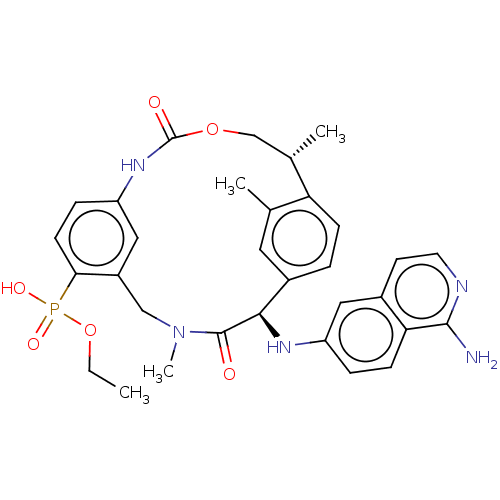 (CHEMBL3978562) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189434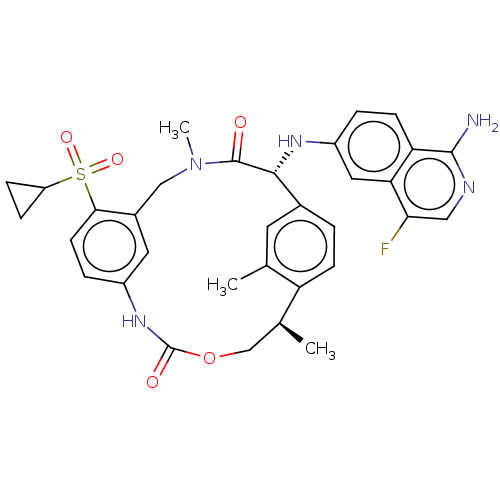 (US9174974, Example 19) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189429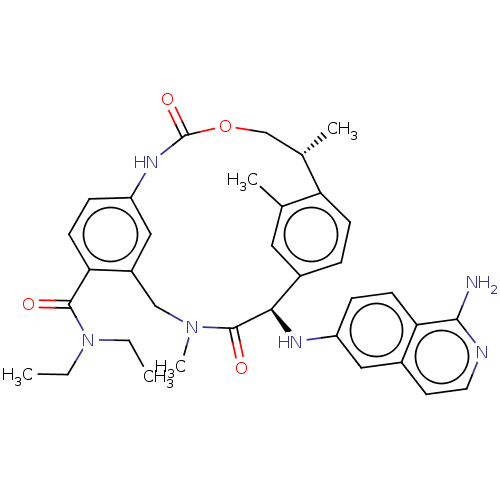 (US9174974, Example 14) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189427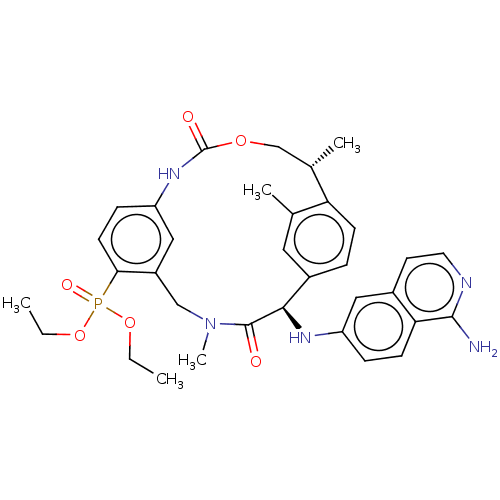 (US9174974, Example 12) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50191351 (CHEMBL3909009) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50191344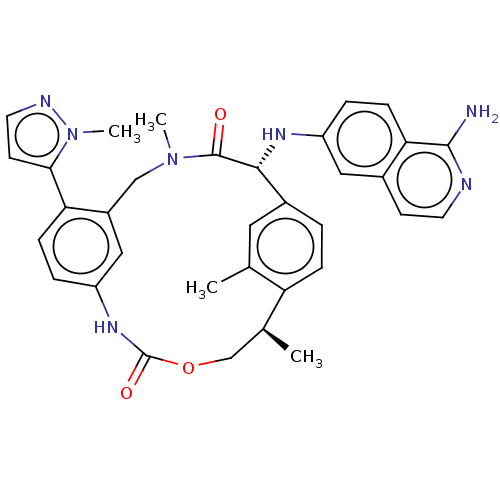 (CHEMBL3951940) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM189416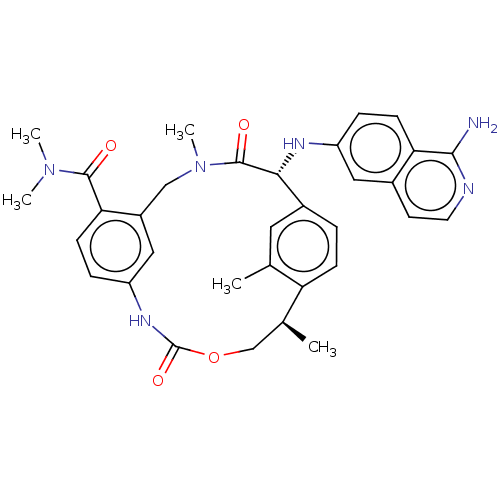 (US9174974, Example 1) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50191350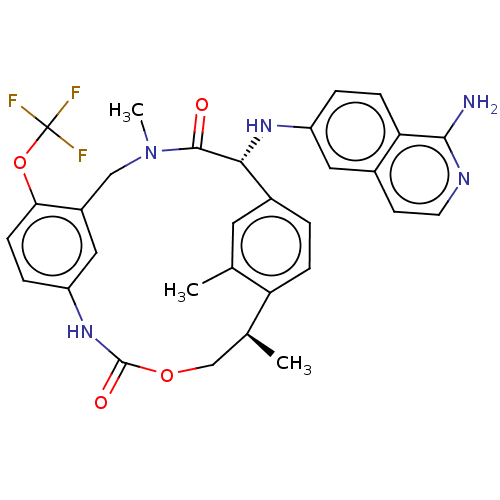 (CHEMBL3935309) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50191345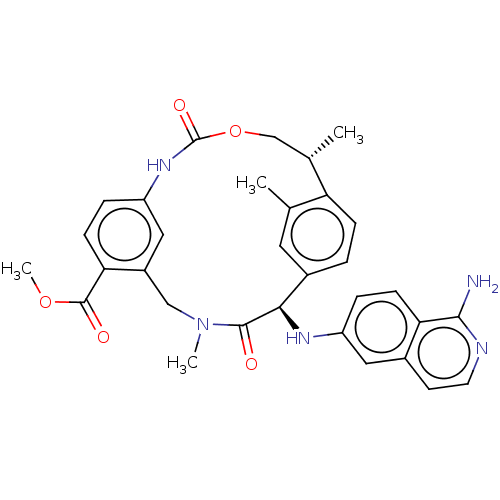 (CHEMBL3942944) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50191349 (CHEMBL3926417) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII/Tissue factor (Homo sapiens (Human)) | BDBM50191347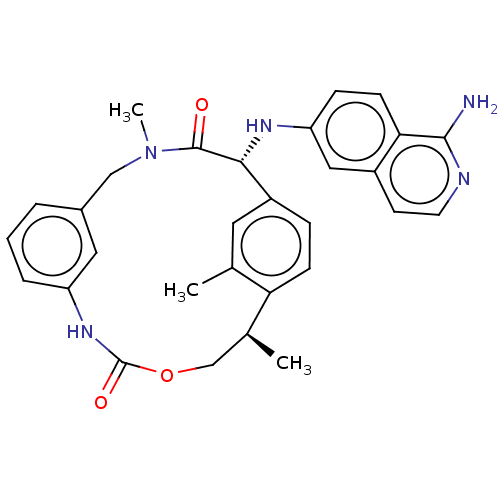 (CHEMBL3907376) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50191347 (CHEMBL3907376) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50191350 (CHEMBL3935309) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50191351 (CHEMBL3909009) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM189434 (US9174974, Example 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human activated protein C using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric ... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50191344 (CHEMBL3951940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50191345 (CHEMBL3942944) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM189445 (US9174974, Example 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM189427 (US9174974, Example 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM189434 (US9174974, Example 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-(D)-Pro-Phe-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric ... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50191349 (CHEMBL3926417) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM189429 (US9174974, Example 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM189434 (US9174974, Example 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM189416 (US9174974, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM189441 (US9174974, Example 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50191350 (CHEMBL3935309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectroph... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50191346 (CHEMBL3978562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM189434 (US9174974, Example 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-(D)-Val-Leu-LyspNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM189434 (US9174974, Example 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 9a using methylsulfonyl-D-cyclohexylglycyl-Gly-Arg-AMC as substrate assessed as release of AMC after 10 to 120 mins by spe... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM189445 (US9174974, Example 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectroph... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50191344 (CHEMBL3951940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectroph... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM189445 (US9174974, Example 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50191345 (CHEMBL3942944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectroph... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-1 (Homo sapiens (Human)) | BDBM50191346 (CHEMBL3978562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human HK1 using H-D-Val-Leu-Arg-AFC as substrate assessed as release of AFC after 10 to 120 mins by spectrofluorimetric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM189427 (US9174974, Example 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50191344 (CHEMBL3951940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM189434 (US9174974, Example 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM189445 (US9174974, Example 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of alpha-thrombin (unknown origin) using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotom... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM189427 (US9174974, Example 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectroph... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM189434 (US9174974, Example 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectroph... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM189429 (US9174974, Example 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectroph... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50191346 (CHEMBL3978562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectroph... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM189429 (US9174974, Example 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM189416 (US9174974, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectroph... | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50191349 (CHEMBL3926417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50191345 (CHEMBL3942944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM189434 (US9174974, Example 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 12a using H-(D)-CHT-Gly-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50191350 (CHEMBL3935309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyroGlu-Pro-Arg-pNA as substrate assessed as release of pNA after 10 to 120 mins by spectrophotometric method | J Med Chem 59: 7125-37 (2016) Article DOI: 10.1021/acs.jmedchem.6b00469 BindingDB Entry DOI: 10.7270/Q2TM7D37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 66 total ) | Next | Last >> |