 Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50031302
Found 38 hits Enz. Inhib. hit(s) with all data for entry = 50031302 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310767

(Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES O=C(N[C@H](CN(C[C@H](Cc1ccccc1)NC(=O)OCc1nccs1)Cc1ccncc1)Cc1ccccc1)OCc1cncs1 |r| Show InChI InChI=1S/C34H36N6O4S2/c41-33(43-23-31-19-36-25-46-31)38-29(17-26-7-3-1-4-8-26)21-40(20-28-11-13-35-14-12-28)22-30(18-27-9-5-2-6-10-27)39-34(42)44-24-32-37-15-16-45-32/h1-16,19,25,29-30H,17-18,20-24H2,(H,38,41)(H,39,42)/t29-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310765
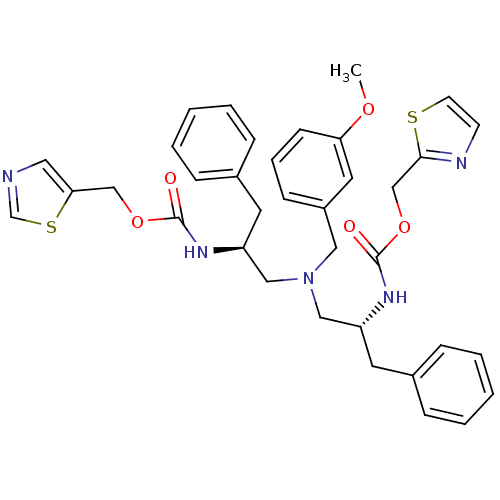
(CHEMBL1077937 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...)Show SMILES COc1cccc(CN(C[C@H](Cc2ccccc2)NC(=O)OCc2cncs2)C[C@@H](Cc2ccccc2)NC(=O)OCc2nccs2)c1 |r| Show InChI InChI=1S/C36H39N5O5S2/c1-44-32-14-8-13-29(19-32)21-41(22-30(17-27-9-4-2-5-10-27)39-35(42)45-24-33-20-37-26-48-33)23-31(18-28-11-6-3-7-12-28)40-36(43)46-25-34-38-15-16-47-34/h2-16,19-20,26,30-31H,17-18,21-25H2,1H3,(H,39,42)(H,40,43)/t30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM520

(1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1csc(n1)C(C)C)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310761
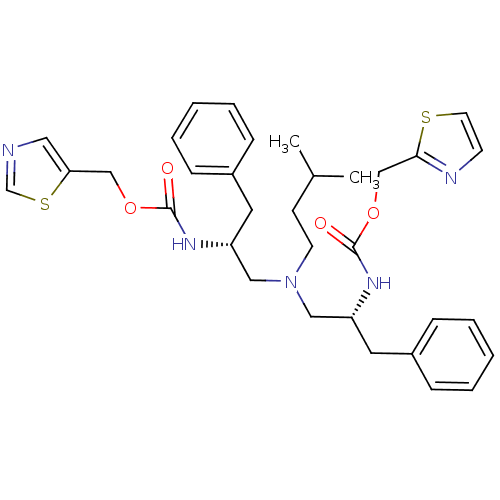
(Bis-N-[(R)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES CC(C)CCN(C[C@@H](Cc1ccccc1)NC(=O)OCc1cncs1)C[C@@H](Cc1ccccc1)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C33H41N5O4S2/c1-25(2)13-15-38(20-28(17-26-9-5-3-6-10-26)36-32(39)41-22-30-19-34-24-44-30)21-29(18-27-11-7-4-8-12-27)37-33(40)42-23-31-35-14-16-43-31/h3-12,14,16,19,24-25,28-29H,13,15,17-18,20-23H2,1-2H3,(H,36,39)(H,37,40)/t28-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310760

(Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES O=C(N[C@H](CN(C[C@H](Cc1ccccc1)NC(=O)OCc1nccs1)CC1CC1)Cc1ccccc1)OCc1cncs1 |r| Show InChI InChI=1S/C32H37N5O4S2/c38-31(40-21-29-17-33-23-43-29)35-27(15-24-7-3-1-4-8-24)19-37(18-26-11-12-26)20-28(16-25-9-5-2-6-10-25)36-32(39)41-22-30-34-13-14-42-30/h1-10,13-14,17,23,26-28H,11-12,15-16,18-22H2,(H,35,38)(H,36,39)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310764

(Bis-N-[(R)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES O=C(N[C@@H](CN(C[C@@H](Cc1ccccc1)NC(=O)OCc1nccs1)Cc1ccccc1)Cc1ccccc1)OCc1cncs1 |r| Show InChI InChI=1S/C35H37N5O4S2/c41-34(43-24-32-20-36-26-46-32)38-30(18-27-10-4-1-5-11-27)22-40(21-29-14-8-3-9-15-29)23-31(19-28-12-6-2-7-13-28)39-35(42)44-25-33-37-16-17-45-33/h1-17,20,26,30-31H,18-19,21-25H2,(H,38,41)(H,39,42)/t30-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310751

(CHEMBL1079228 | Tris-N-[2-(thiazol-5-ylmethoxycarb...)Show SMILES O=C(NC(CN(CC(Cc1ccccc1)NC(=O)OCc1cncs1)CC(Cc1ccccc1)NC(=O)OCc1nccs1)Cc1ccccc1)OCc1cncs1 Show InChI InChI=1S/C42H45N7O6S3/c50-40(53-26-37-21-43-29-57-37)46-34(18-31-10-4-1-5-11-31)23-49(24-35(19-32-12-6-2-7-13-32)47-41(51)54-27-38-22-44-30-58-38)25-36(20-33-14-8-3-9-15-33)48-42(52)55-28-39-45-16-17-56-39/h1-17,21-22,29-30,34-36H,18-20,23-28H2,(H,46,50)(H,47,51)(H,48,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310768

(Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES O=C(N[C@H](CN(C[C@H](Cc1ccccc1)NC(=O)OCc1nccs1)Cc1cnc[nH]1)Cc1ccccc1)OCc1cncs1 |r| Show InChI InChI=1S/C32H35N7O4S2/c40-31(42-20-29-16-34-23-45-29)37-26(13-24-7-3-1-4-8-24)17-39(19-28-15-33-22-36-28)18-27(14-25-9-5-2-6-10-25)38-32(41)43-21-30-35-11-12-44-30/h1-12,15-16,22-23,26-27H,13-14,17-21H2,(H,33,36)(H,37,40)(H,38,41)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310776
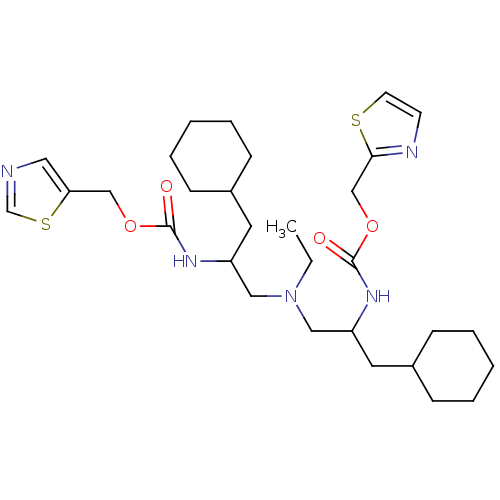
(Bis-N-[2-(thiazol-5-ylmethoxycarbonylamino)-3-cycl...)Show SMILES CCN(CC(CC1CCCCC1)NC(=O)OCc1cncs1)CC(CC1CCCCC1)NC(=O)OCc1nccs1 Show InChI InChI=1S/C30H47N5O4S2/c1-2-35(18-25(15-23-9-5-3-6-10-23)33-29(36)38-20-27-17-31-22-41-27)19-26(16-24-11-7-4-8-12-24)34-30(37)39-21-28-32-13-14-40-28/h13-14,17,22-26H,2-12,15-16,18-21H2,1H3,(H,33,36)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310766

(Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES O=C(N[C@H](CN(C[C@H](Cc1ccccc1)NC(=O)OCc1nccs1)Cc1cccnc1)Cc1ccccc1)OCc1cncs1 |r| Show InChI InChI=1S/C34H36N6O4S2/c41-33(43-23-31-19-36-25-46-31)38-29(16-26-8-3-1-4-9-26)21-40(20-28-12-7-13-35-18-28)22-30(17-27-10-5-2-6-11-27)39-34(42)44-24-32-37-14-15-45-32/h1-15,18-19,25,29-30H,16-17,20-24H2,(H,38,41)(H,39,42)/t29-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310759

(Bis-N-[(R)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES CC(C)CN(C[C@@H](Cc1ccccc1)NC(=O)OCc1cncs1)C[C@@H](Cc1ccccc1)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C32H39N5O4S2/c1-24(2)18-37(19-27(15-25-9-5-3-6-10-25)35-31(38)40-21-29-17-33-23-43-29)20-28(16-26-11-7-4-8-12-26)36-32(39)41-22-30-34-13-14-42-30/h3-14,17,23-24,27-28H,15-16,18-22H2,1-2H3,(H,35,38)(H,36,39)/t27-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310769
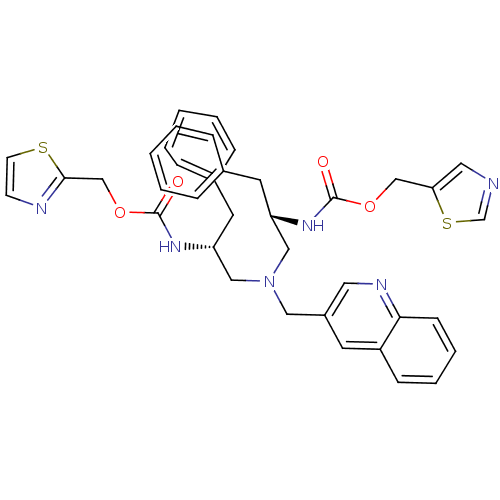
(CHEMBL1077941 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...)Show SMILES O=C(N[C@H](CN(C[C@@H](Cc1ccccc1)NC(=O)OCc1nccs1)Cc1cnc2ccccc2c1)Cc1ccccc1)OCc1cncs1 |r| Show InChI InChI=1S/C38H38N6O4S2/c45-37(47-25-34-21-39-27-50-34)42-32(18-28-9-3-1-4-10-28)23-44(22-30-17-31-13-7-8-14-35(31)41-20-30)24-33(19-29-11-5-2-6-12-29)43-38(46)48-26-36-40-15-16-49-36/h1-17,20-21,27,32-33H,18-19,22-26H2,(H,42,45)(H,43,46)/t32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310763
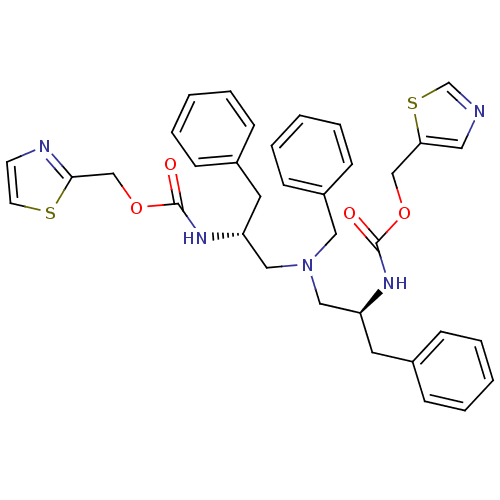
(CHEMBL1077935 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...)Show SMILES O=C(N[C@H](CN(C[C@@H](Cc1ccccc1)NC(=O)OCc1nccs1)Cc1ccccc1)Cc1ccccc1)OCc1cncs1 |r| Show InChI InChI=1S/C35H37N5O4S2/c41-34(43-24-32-20-36-26-46-32)38-30(18-27-10-4-1-5-11-27)22-40(21-29-14-8-3-9-15-29)23-31(19-28-12-6-2-7-13-28)39-35(42)44-25-33-37-16-17-45-33/h1-17,20,26,30-31H,18-19,21-25H2,(H,38,41)(H,39,42)/t30-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310775
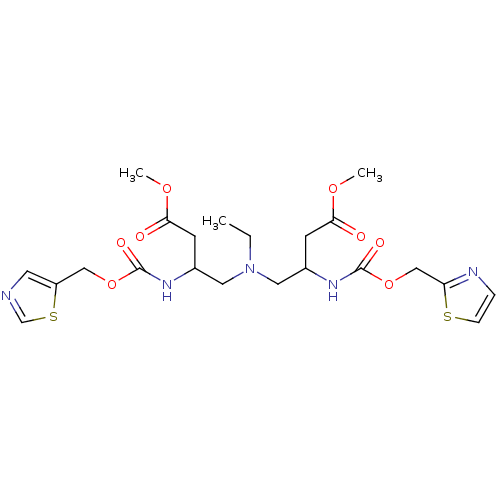
(Bis-N-[2-(thiazol-5-ylmethoxycarbonylamino)-3-meth...)Show SMILES CCN(CC(CC(=O)OC)NC(=O)OCc1cncs1)CC(CC(=O)OC)NC(=O)OCc1nccs1 Show InChI InChI=1S/C22H31N5O8S2/c1-4-27(10-15(7-19(28)32-2)25-21(30)34-12-17-9-23-14-37-17)11-16(8-20(29)33-3)26-22(31)35-13-18-24-5-6-36-18/h5-6,9,14-16H,4,7-8,10-13H2,1-3H3,(H,25,30)(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310749

(CHEMBL1079896 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...)Show SMILES O=C(N[C@H](CNC[C@@H](Cc1ccccc1)NC(=O)OCc1nccs1)Cc1ccccc1)OCc1cncs1 |r| Show InChI InChI=1S/C28H31N5O4S2/c34-27(36-18-25-17-30-20-39-25)32-23(13-21-7-3-1-4-8-21)15-29-16-24(14-22-9-5-2-6-10-22)33-28(35)37-19-26-31-11-12-38-26/h1-12,17,20,23-24,29H,13-16,18-19H2,(H,32,34)(H,33,35)/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310758

(CHEMBL1077930 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...)Show SMILES CC(C)CN(C[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)C[C@@H](Cc1ccccc1)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C32H39N5O4S2/c1-24(2)18-37(19-27(15-25-9-5-3-6-10-25)35-31(38)40-21-29-17-33-23-43-29)20-28(16-26-11-7-4-8-12-26)36-32(39)41-22-30-34-13-14-42-30/h3-14,17,23-24,27-28H,15-16,18-22H2,1-2H3,(H,35,38)(H,36,39)/t27-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310778

(CHEMBL1078122 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...)Show SMILES CCN(CC(Cc1ccc(O)cc1)NC(=O)OCc1cncs1)CC(Cc1ccc(O)cc1)NC(=O)OCc1nccs1 Show InChI InChI=1S/C30H35N5O6S2/c1-2-35(16-23(13-21-3-7-25(36)8-4-21)33-29(38)40-18-27-15-31-20-43-27)17-24(14-22-5-9-26(37)10-6-22)34-30(39)41-19-28-32-11-12-42-28/h3-12,15,20,23-24,36-37H,2,13-14,16-19H2,1H3,(H,33,38)(H,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310750
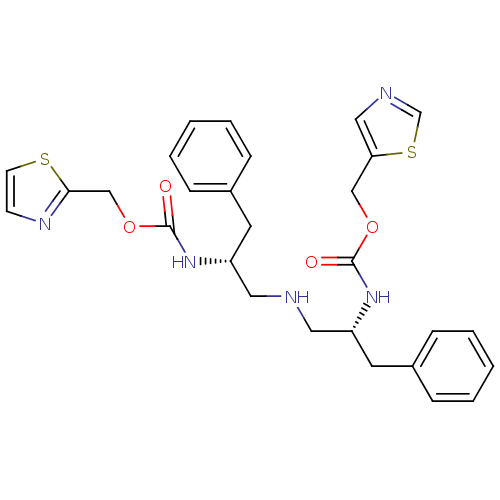
(Bis-N-[(R)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES O=C(N[C@@H](CNC[C@@H](Cc1ccccc1)NC(=O)OCc1nccs1)Cc1ccccc1)OCc1cncs1 |r| Show InChI InChI=1S/C28H31N5O4S2/c34-27(36-18-25-17-30-20-39-25)32-23(13-21-7-3-1-4-8-21)15-29-16-24(14-22-9-5-2-6-10-22)33-28(35)37-19-26-31-11-12-38-26/h1-12,17,20,23-24,29H,13-16,18-19H2,(H,32,34)(H,33,35)/t23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310753

(CHEMBL1079230 | N-[(S)-2-thiazol-5-ylmethoxycarbon...)Show SMILES CC(C)(C)CN(C[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)C[C@@H](Cc1ccccc1)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C33H41N5O4S2/c1-33(2,3)23-38(19-27(16-25-10-6-4-7-11-25)36-31(39)41-21-29-18-34-24-44-29)20-28(17-26-12-8-5-9-13-26)37-32(40)42-22-30-35-14-15-43-30/h4-15,18,24,27-28H,16-17,19-23H2,1-3H3,(H,36,39)(H,37,40)/t27-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310755
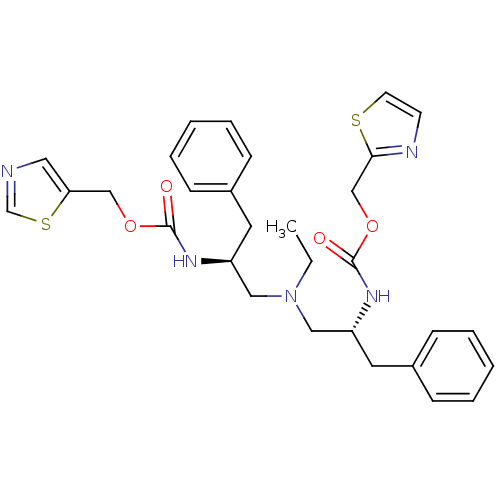
(CHEMBL1081147 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...)Show SMILES CCN(C[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)C[C@@H](Cc1ccccc1)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C30H35N5O4S2/c1-2-35(18-25(15-23-9-5-3-6-10-23)33-29(36)38-20-27-17-31-22-41-27)19-26(16-24-11-7-4-8-12-24)34-30(37)39-21-28-32-13-14-40-28/h3-14,17,22,25-26H,2,15-16,18-21H2,1H3,(H,33,36)(H,34,37)/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310752
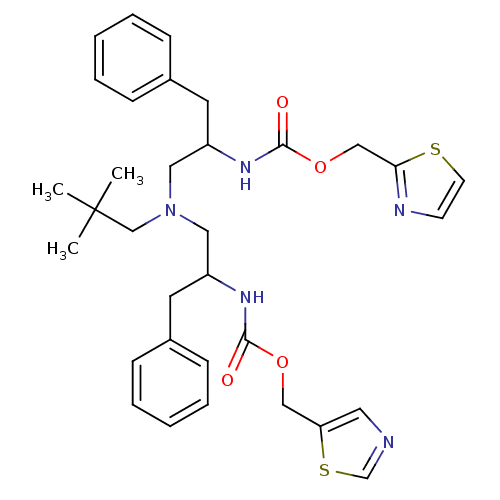
(Bis-N-[2-(thiazol-5-ylmethoxycarbonylamino)-3-phen...)Show SMILES CC(C)(C)CN(CC(Cc1ccccc1)NC(=O)OCc1cncs1)CC(Cc1ccccc1)NC(=O)OCc1nccs1 Show InChI InChI=1S/C33H41N5O4S2/c1-33(2,3)23-38(19-27(16-25-10-6-4-7-11-25)36-31(39)41-21-29-18-34-24-44-29)20-28(17-26-12-8-5-9-13-26)37-32(40)42-22-30-35-14-15-43-30/h4-15,18,24,27-28H,16-17,19-23H2,1-3H3,(H,36,39)(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310756

(Bis-N-[(R)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES CCN(C[C@@H](Cc1ccccc1)NC(=O)OCc1cncs1)C[C@@H](Cc1ccccc1)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C30H35N5O4S2/c1-2-35(18-25(15-23-9-5-3-6-10-23)33-29(36)38-20-27-17-31-22-41-27)19-26(16-24-11-7-4-8-12-24)34-30(37)39-21-28-32-13-14-40-28/h3-14,17,22,25-26H,2,15-16,18-21H2,1H3,(H,33,36)(H,34,37)/t25-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310757
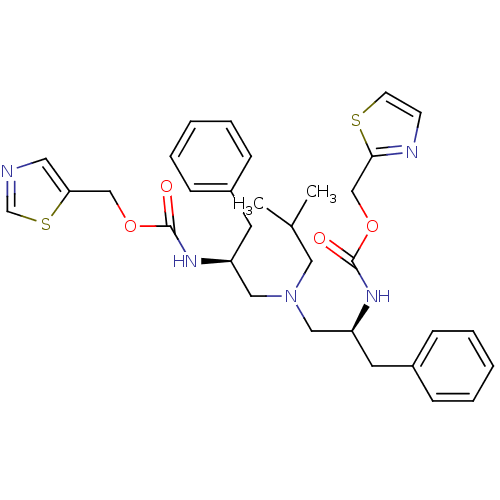
(Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES CC(C)CN(C[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)C[C@H](Cc1ccccc1)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C32H39N5O4S2/c1-24(2)18-37(19-27(15-25-9-5-3-6-10-25)35-31(38)40-21-29-17-33-23-43-29)20-28(16-26-11-7-4-8-12-26)36-32(39)41-22-30-34-13-14-42-30/h3-14,17,23-24,27-28H,15-16,18-22H2,1-2H3,(H,35,38)(H,36,39)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310762
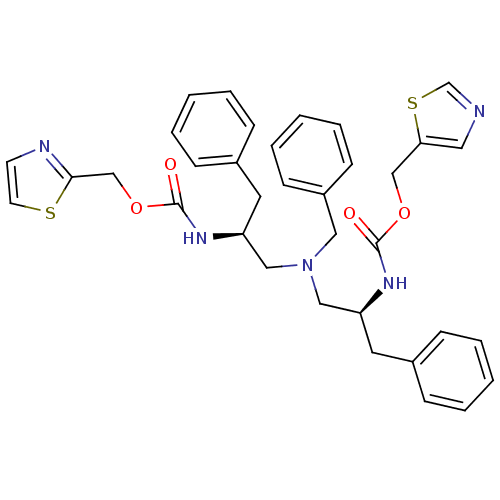
(Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES O=C(N[C@H](CN(C[C@H](Cc1ccccc1)NC(=O)OCc1nccs1)Cc1ccccc1)Cc1ccccc1)OCc1cncs1 |r| Show InChI InChI=1S/C35H37N5O4S2/c41-34(43-24-32-20-36-26-46-32)38-30(18-27-10-4-1-5-11-27)22-40(21-29-14-8-3-9-15-29)23-31(19-28-12-6-2-7-13-28)39-35(42)44-25-33-37-16-17-45-33/h1-17,20,26,30-31H,18-19,21-25H2,(H,38,41)(H,39,42)/t30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310777
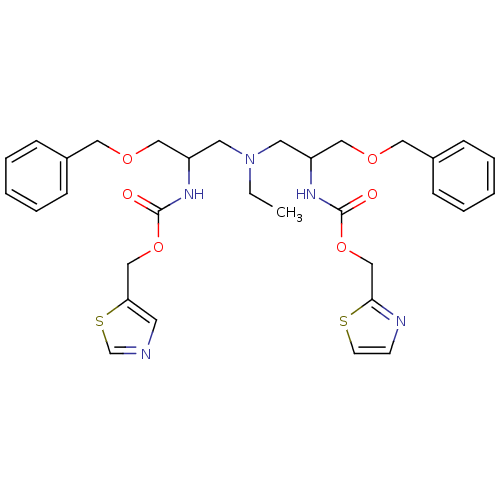
(CHEMBL1078121 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...)Show SMILES CCN(CC(COCc1ccccc1)NC(=O)OCc1cncs1)CC(COCc1ccccc1)NC(=O)OCc1nccs1 Show InChI InChI=1S/C32H39N5O6S2/c1-2-37(16-27(20-40-18-25-9-5-3-6-10-25)35-31(38)42-22-29-15-33-24-45-29)17-28(21-41-19-26-11-7-4-8-12-26)36-32(39)43-23-30-34-13-14-44-30/h3-15,24,27-28H,2,16-23H2,1H3,(H,35,38)(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310774
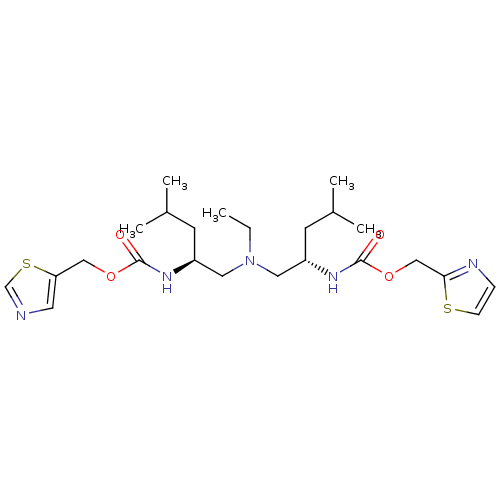
(Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-4-...)Show SMILES CCN(C[C@H](CC(C)C)NC(=O)OCc1cncs1)C[C@H](CC(C)C)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C24H39N5O4S2/c1-6-29(12-19(9-17(2)3)27-23(30)32-14-21-11-25-16-35-21)13-20(10-18(4)5)28-24(31)33-15-22-26-7-8-34-22/h7-8,11,16-20H,6,9-10,12-15H2,1-5H3,(H,27,30)(H,28,31)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310748
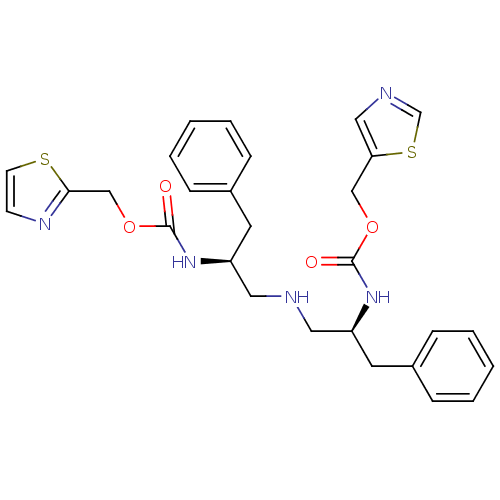
(Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES O=C(N[C@H](CNC[C@H](Cc1ccccc1)NC(=O)OCc1nccs1)Cc1ccccc1)OCc1cncs1 |r| Show InChI InChI=1S/C28H31N5O4S2/c34-27(36-18-25-17-30-20-39-25)32-23(13-21-7-3-1-4-8-21)15-29-16-24(14-22-9-5-2-6-10-22)33-28(35)37-19-26-31-11-12-38-26/h1-12,17,20,23-24,29H,13-16,18-19H2,(H,32,34)(H,33,35)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310754
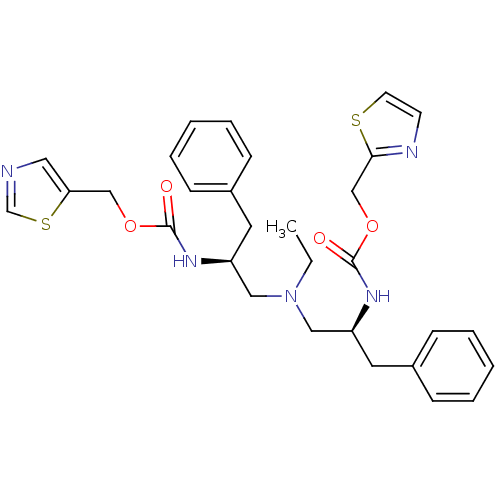
(Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)-3-...)Show SMILES CCN(C[C@H](Cc1ccccc1)NC(=O)OCc1cncs1)C[C@H](Cc1ccccc1)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C30H35N5O4S2/c1-2-35(18-25(15-23-9-5-3-6-10-23)33-29(36)38-20-27-17-31-22-41-27)19-26(16-24-11-7-4-8-12-24)34-30(37)39-21-28-32-13-14-40-28/h3-14,17,22,25-26H,2,15-16,18-21H2,1H3,(H,33,36)(H,34,37)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310780
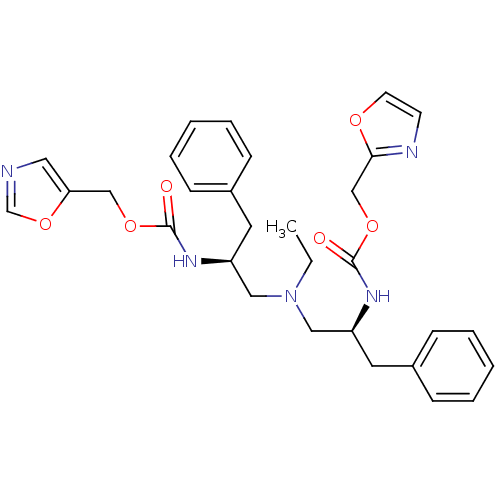
(Bis-N-[(S)-2-(oxazol-5-ylmethoxycarbonylamino)-3-p...)Show SMILES CCN(C[C@H](Cc1ccccc1)NC(=O)OCc1cnco1)C[C@H](Cc1ccccc1)NC(=O)OCc1ncco1 |r| Show InChI InChI=1S/C30H35N5O6/c1-2-35(18-25(15-23-9-5-3-6-10-23)33-29(36)39-20-27-17-31-22-41-27)19-26(16-24-11-7-4-8-12-24)34-30(37)40-21-28-32-13-14-38-28/h3-14,17,22,25-26H,2,15-16,18-21H2,1H3,(H,33,36)(H,34,37)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 274 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310779

(CHEMBL1078123 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...)Show SMILES CCN(CC(Cc1ccc(OCc2ccccc2)cc1)NC(=O)OCc1cncs1)CC(Cc1ccc(OCc2ccccc2)cc1)NC(=O)OCc1nccs1 Show InChI InChI=1S/C44H47N5O6S2/c1-2-49(26-37(47-43(50)54-30-41-25-45-32-57-41)23-33-13-17-39(18-14-33)52-28-35-9-5-3-6-10-35)27-38(48-44(51)55-31-42-46-21-22-56-42)24-34-15-19-40(20-16-34)53-29-36-11-7-4-8-12-36/h3-22,25,32,37-38H,2,23-24,26-31H2,1H3,(H,47,50)(H,48,51) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310784
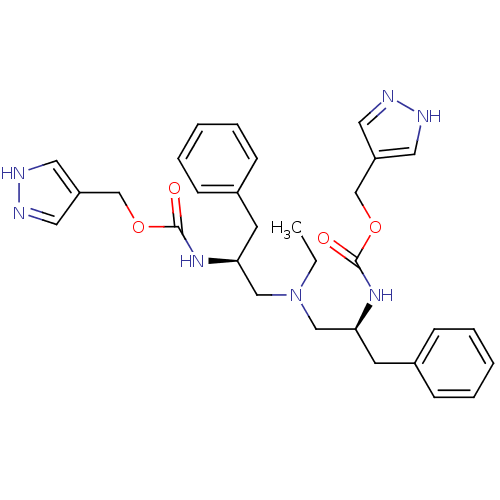
(Bis-N-[(S)-2-(pyrazol-4-ylmethoxycarbonylamino)-3-...)Show SMILES CCN(C[C@H](Cc1ccccc1)NC(=O)OCc1cn[nH]c1)C[C@H](Cc1ccccc1)NC(=O)OCc1cn[nH]c1 |r| Show InChI InChI=1S/C30H37N7O4/c1-2-37(19-27(13-23-9-5-3-6-10-23)35-29(38)40-21-25-15-31-32-16-25)20-28(14-24-11-7-4-8-12-24)36-30(39)41-22-26-17-33-34-18-26/h3-12,15-18,27-28H,2,13-14,19-22H2,1H3,(H,31,32)(H,33,34)(H,35,38)(H,36,39)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310781
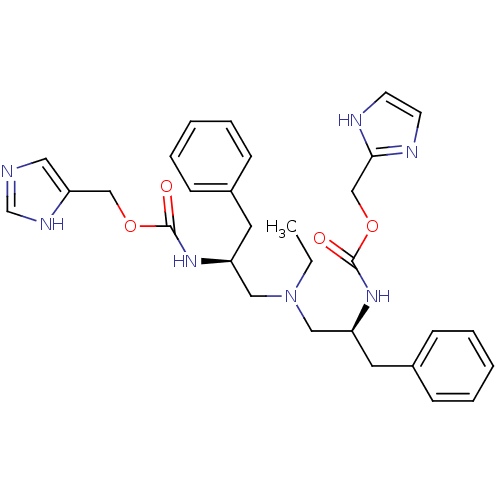
(Bis-N-[(S)-2-(imidazol-4-ylmethoxycarbonylamino)-3...)Show SMILES CCN(C[C@H](Cc1ccccc1)NC(=O)OCc1cnc[nH]1)C[C@H](Cc1ccccc1)NC(=O)OCc1ncc[nH]1 |r| Show InChI InChI=1S/C30H37N7O4/c1-2-37(18-25(15-23-9-5-3-6-10-23)35-29(38)40-20-27-17-31-22-34-27)19-26(16-24-11-7-4-8-12-24)36-30(39)41-21-28-32-13-14-33-28/h3-14,17,22,25-26H,2,15-16,18-21H2,1H3,(H,31,34)(H,32,33)(H,35,38)(H,36,39)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310782

(Bis-N-[(S)-2-(thien-2-ylmethoxycarbonylamino)-3-ph...)Show SMILES CCN(C[C@H](Cc1ccccc1)NC(=O)OCc1cccs1)C[C@H](Cc1ccccc1)NC(=O)OCc1cccs1 |r| Show InChI InChI=1S/C32H37N3O4S2/c1-2-35(21-27(19-25-11-5-3-6-12-25)33-31(36)38-23-29-15-9-17-40-29)22-28(20-26-13-7-4-8-14-26)34-32(37)39-24-30-16-10-18-41-30/h3-18,27-28H,2,19-24H2,1H3,(H,33,36)(H,34,37)/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 741 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310773

(Bis-N-[(R)-2-(thiazol-5-ylmethoxycarbonylamino)pro...)Show SMILES CCN(C[C@@H](C)NC(=O)OCc1cncs1)C[C@@H](C)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C18H27N5O4S2/c1-4-23(8-13(2)21-17(24)26-10-15-7-19-12-29-15)9-14(3)22-18(25)27-11-16-20-5-6-28-16/h5-7,12-14H,4,8-11H2,1-3H3,(H,21,24)(H,22,25)/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310772
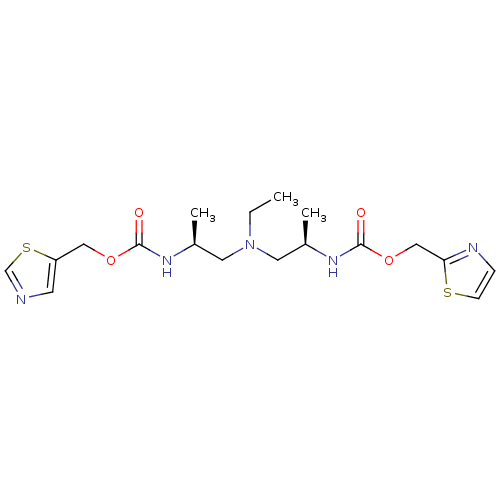
(CHEMBL1080417 | N-[(S)-2-(thiazol-5-ylmethoxycarbo...)Show SMILES CCN(C[C@H](C)NC(=O)OCc1cncs1)C[C@@H](C)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C18H27N5O4S2/c1-4-23(8-13(2)21-17(24)26-10-15-7-19-12-29-15)9-14(3)22-18(25)27-11-16-20-5-6-28-16/h5-7,12-14H,4,8-11H2,1-3H3,(H,21,24)(H,22,25)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310783
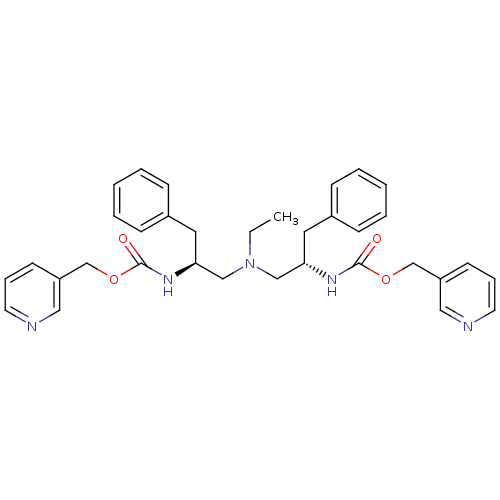
(Bis-N-[(S)-2-(pyridin-3-ylmethoxycarbonylamino)-3-...)Show SMILES CCN(C[C@H](Cc1ccccc1)NC(=O)OCc1cccnc1)C[C@H](Cc1ccccc1)NC(=O)OCc1cccnc1 |r| Show InChI InChI=1S/C34H39N5O4/c1-2-39(23-31(19-27-11-5-3-6-12-27)37-33(40)42-25-29-15-9-17-35-21-29)24-32(20-28-13-7-4-8-14-28)38-34(41)43-26-30-16-10-18-36-22-30/h3-18,21-22,31-32H,2,19-20,23-26H2,1H3,(H,37,40)(H,38,41)/t31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310771
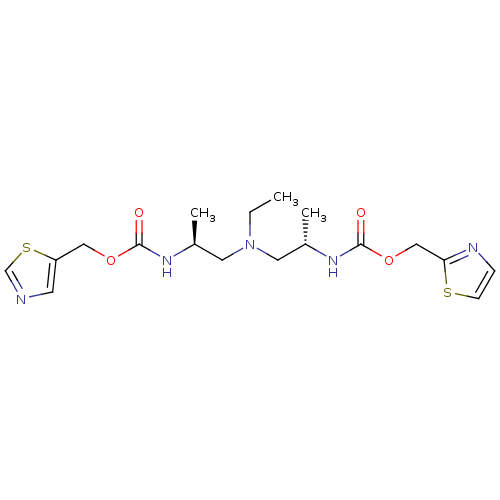
(Bis-N-[(S)-2-(thiazol-5-ylmethoxycarbonylamino)pro...)Show SMILES CCN(C[C@H](C)NC(=O)OCc1cncs1)C[C@H](C)NC(=O)OCc1nccs1 |r| Show InChI InChI=1S/C18H27N5O4S2/c1-4-23(8-13(2)21-17(24)26-10-15-7-19-12-29-15)9-14(3)22-18(25)27-11-16-20-5-6-28-16/h5-7,12-14H,4,8-11H2,1-3H3,(H,21,24)(H,22,25)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50310770
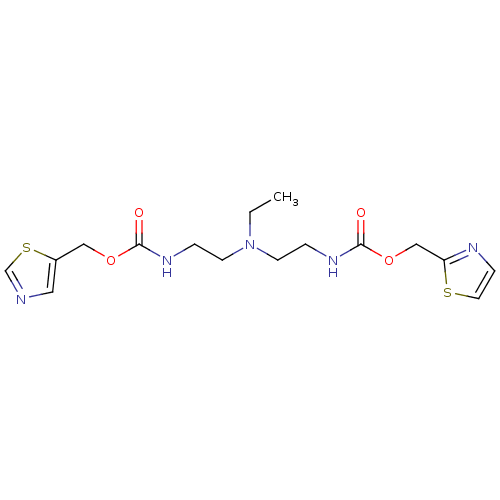
(Bis-N-[2-(thiazol-5-ylmethoxycarbonylamino)ethyl]e...)Show InChI InChI=1S/C16H23N5O4S2/c1-2-21(6-3-19-15(22)24-10-13-9-17-12-27-13)7-4-20-16(23)25-11-14-18-5-8-26-14/h5,8-9,12H,2-4,6-7,10-11H2,1H3,(H,19,22)(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP3A4-mediated oxidation of 7-benzyloxyquinoline |
Bioorg Med Chem Lett 19: 5444-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.118
BindingDB Entry DOI: 10.7270/Q21R6QN0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data