

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314628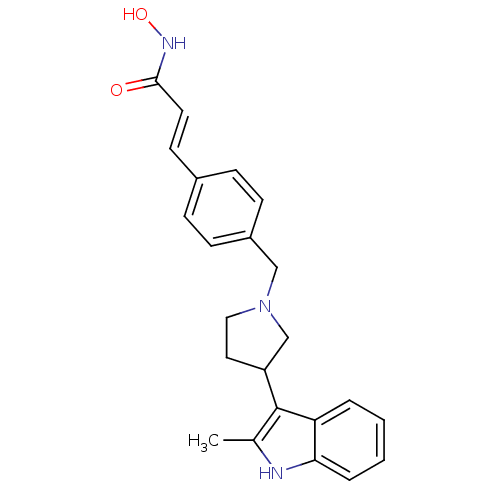 ((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314630 ((E)-N-Hydroxy-3-(4-{1-[2-(2-methyl-1H-indol-3-yl)e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314637 ((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314627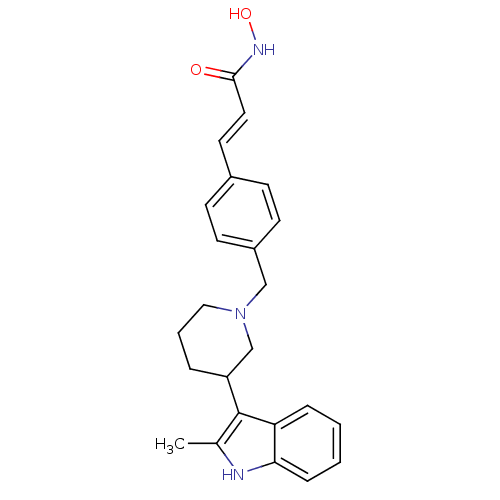 ((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314642 ((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50314642 ((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC3 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19428 ((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314640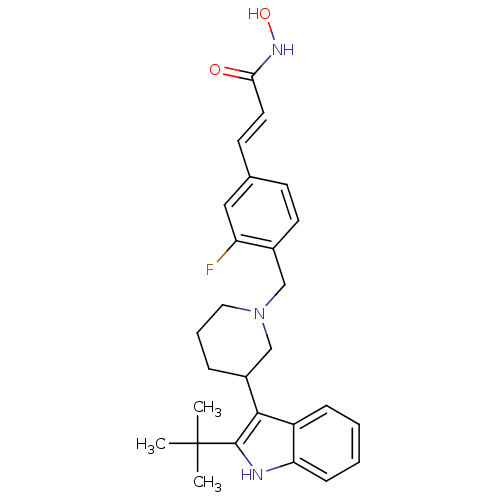 ((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314636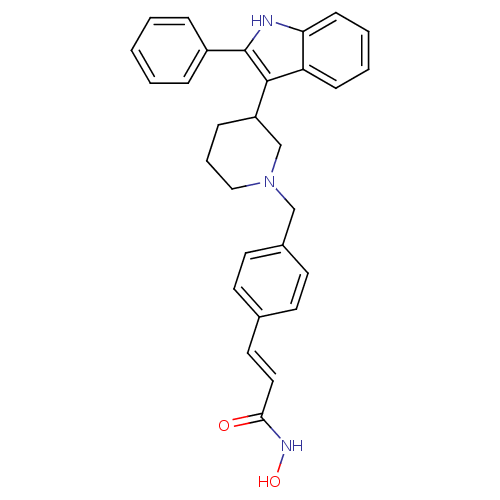 ((E)-N-Hydroxy-3-{4-[3-(2-phenyl-1H-indol-3-yl)pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314635 ((E)-N-Hydroxy-3-{4-[3-(1H-indol-3-yl)piperidin-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314633 (CHEMBL1093362 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314643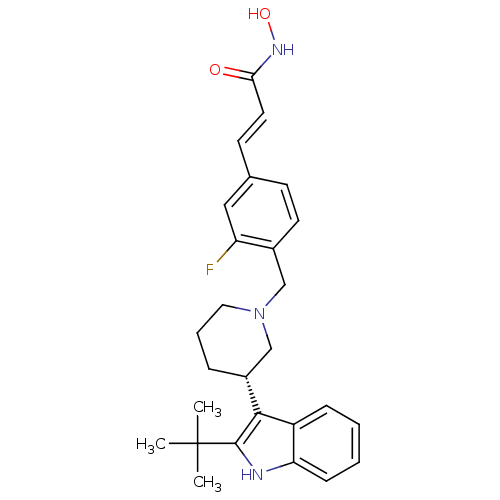 ((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314639 ((E)-3-{3-Fluoro-4-[3-(2-phenyl-1H-indol-3-yl)piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314641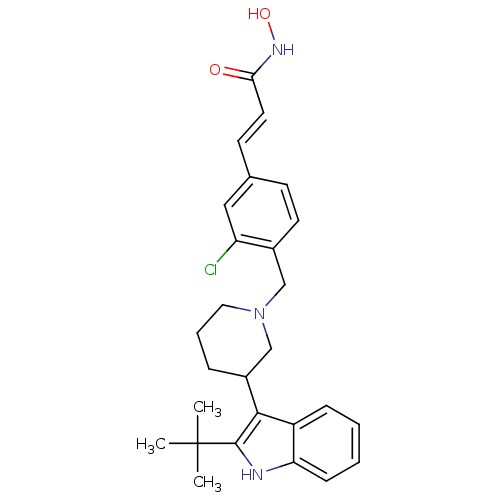 ((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50314643 ((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC3 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314629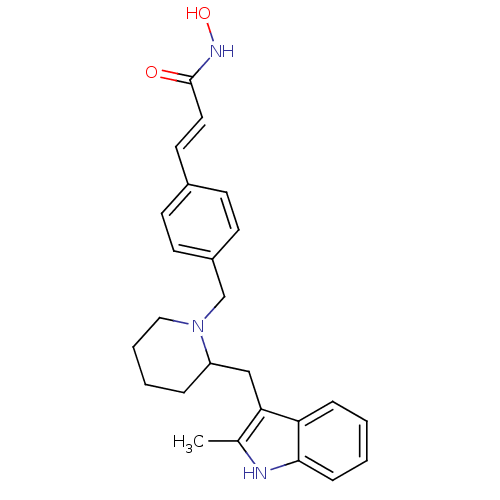 ((E)-N-Hydroxy-3-{4-[2-(2-methyl-1H-indol-3-ylmethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314631 (CHEMBL1088949 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50314642 ((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC4 expressed in baculovirus system by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50314642 ((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC2 expressed in baculovirus system by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50314643 ((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC4 expressed in baculovirus system by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314632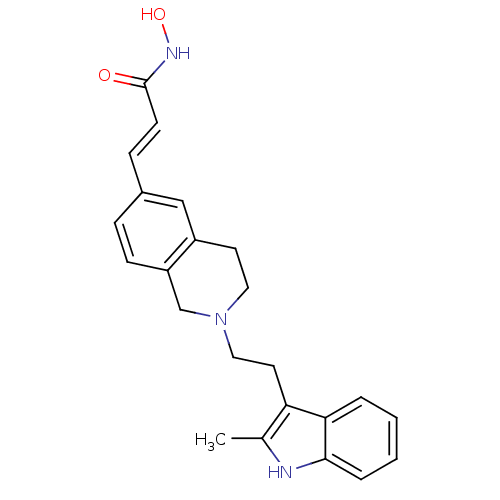 (CHEMBL1089701 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50314643 ((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC2 expressed in baculovirus system by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314634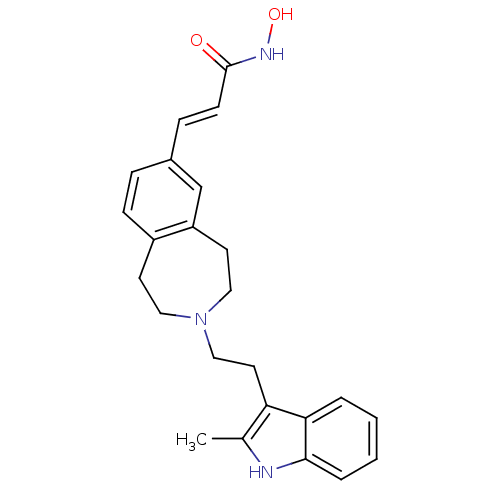 (CHEMBL1089301 | N-hydroxy-3-(3-(2-(2-methyl-1H-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50314643 ((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC6 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50314642 ((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 118 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC6 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314638 ((E)-3-{3-Fluoro-4-[3-(1H-indol-3-yl)piperidin-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50314642 ((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in baculovirus system by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50314643 ((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 364 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC8 expressed in baculovirus system by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314633 (CHEMBL1093362 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314634 (CHEMBL1089301 | N-hydroxy-3-(3-(2-(2-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314631 (CHEMBL1088949 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314629 ((E)-N-Hydroxy-3-{4-[2-(2-methyl-1H-indol-3-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314641 ((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM19428 ((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314628 ((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314637 ((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314632 (CHEMBL1089701 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314638 ((E)-3-{3-Fluoro-4-[3-(1H-indol-3-yl)piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314639 ((E)-3-{3-Fluoro-4-[3-(2-phenyl-1H-indol-3-yl)piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314630 ((E)-N-Hydroxy-3-(4-{1-[2-(2-methyl-1H-indol-3-yl)e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314627 ((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314636 ((E)-N-Hydroxy-3-{4-[3-(2-phenyl-1H-indol-3-yl)pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314635 ((E)-N-Hydroxy-3-{4-[3-(1H-indol-3-yl)piperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314640 ((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||