

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367721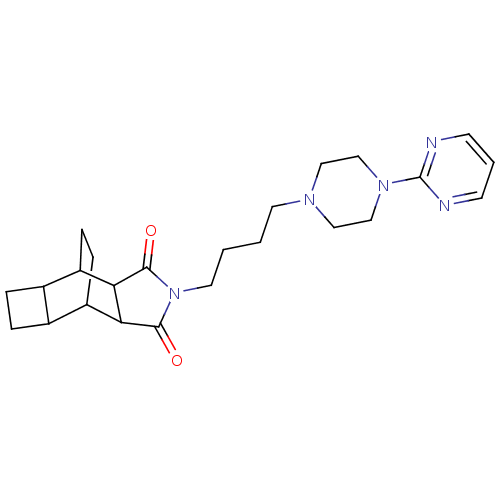 (CHEMBL1202512) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367726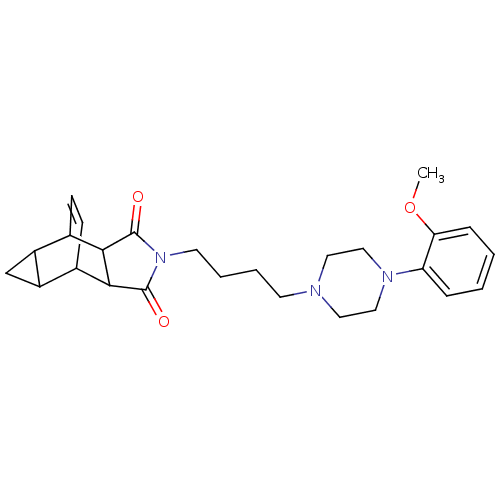 (CHEMBL1202507) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367719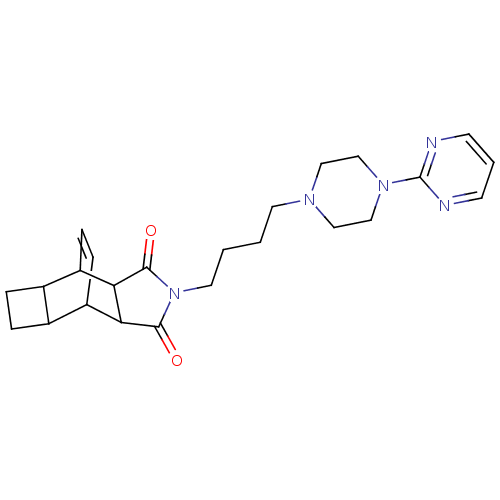 (CHEMBL1202513) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367735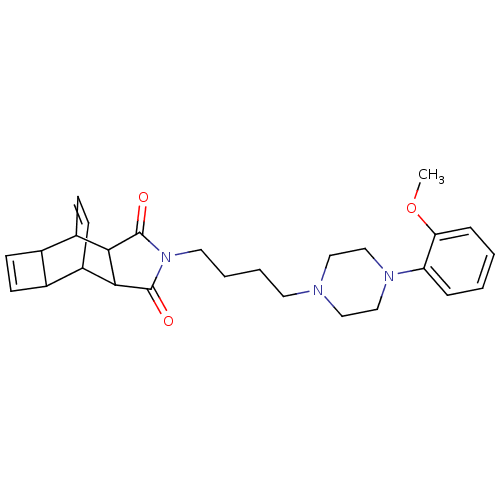 (CHEMBL1204093) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367733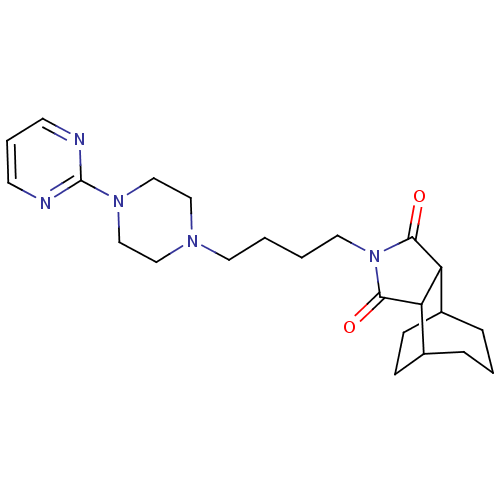 (CHEMBL1202527) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367736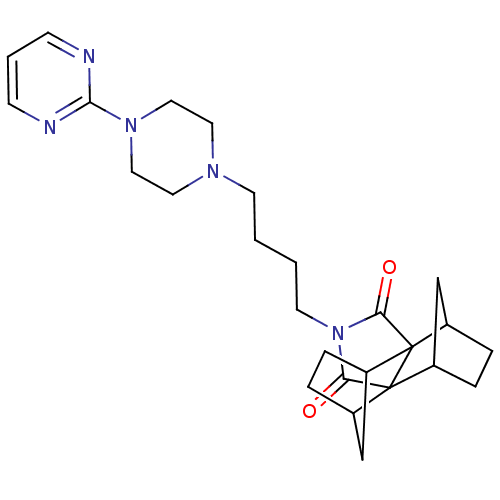 (CHEMBL1202530) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020115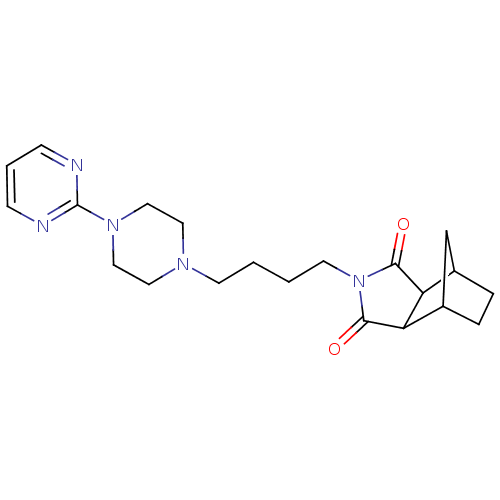 (4-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)-butyl]-4-az...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005127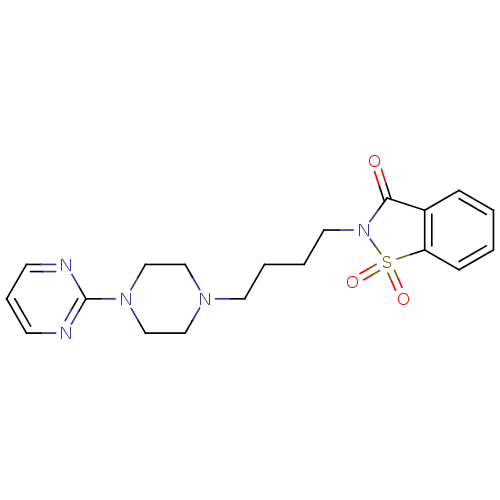 (1,1-Dioxo-2-[4-(4-pyrimidin-2-yl-piperazin-1-yl)-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50001859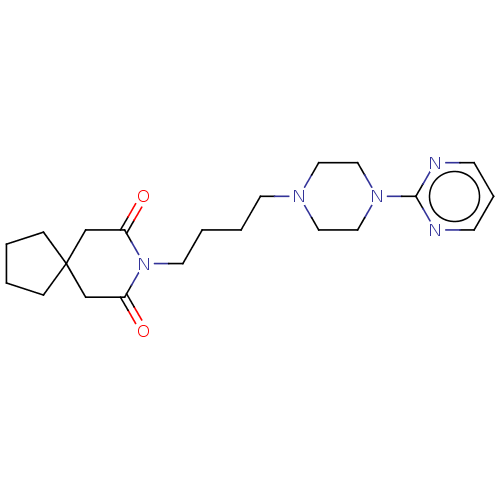 ((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367731 (CHEMBL1202506) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367723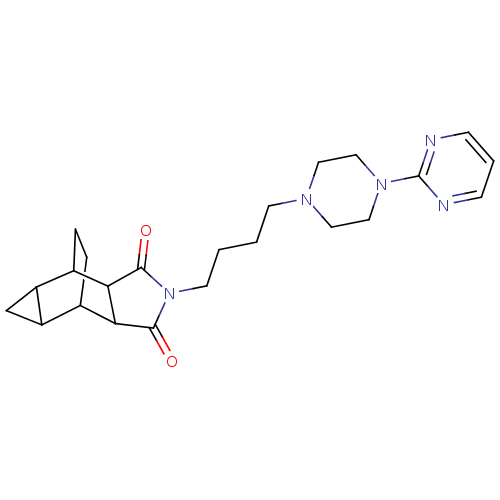 (CHEMBL1202494) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367725 (CHEMBL1202520) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020142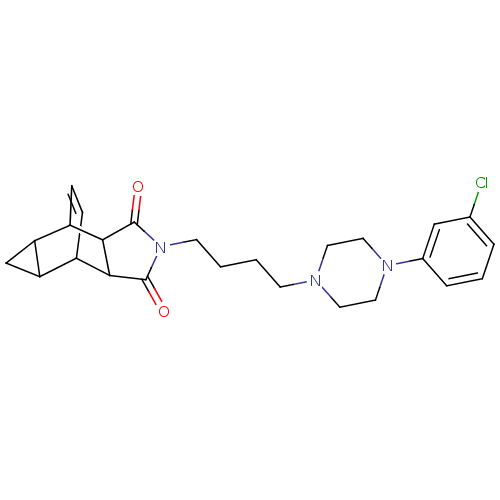 (4-{4-[4-(3-chlorophenyl)hexahydro-1-pyrazinyl]buty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367726 (CHEMBL1202507) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367734 (CHEMBL1202523) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367729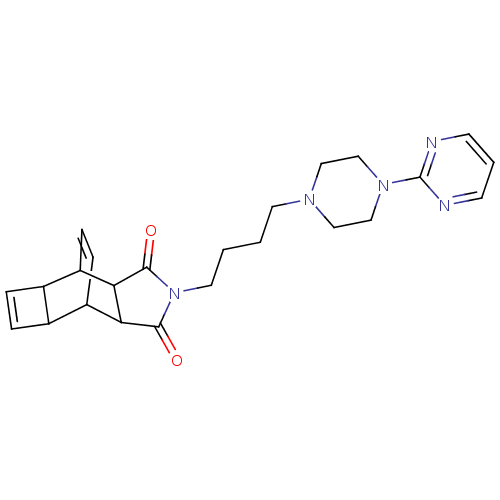 (CHEMBL1204094) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020126 (4-{4-[4-(3-chlorophenyl)hexahydro-1-pyrazinyl]buty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367732 (CHEMBL1202493) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367728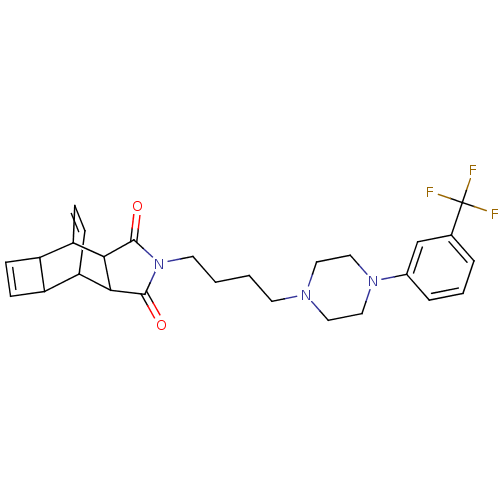 (CHEMBL1202515) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020145 (4-{4-[4-(3-chloro-2-pyrazinyl)hexahydro-1-pyraziny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367730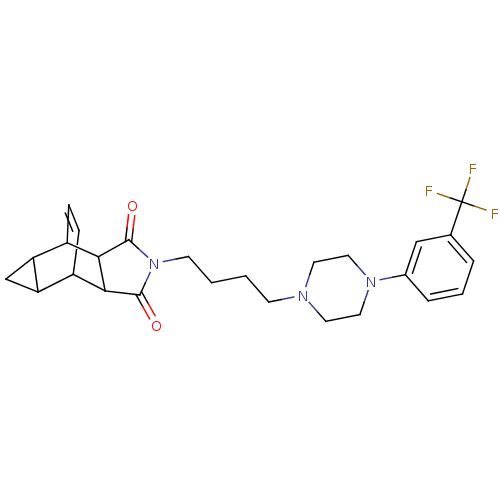 (CHEMBL1202508) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020121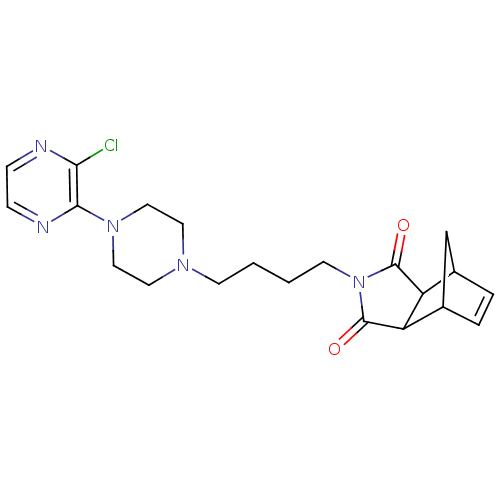 (4-[4-(3'-Chloro-2,3,5,6-tetrahydro-[1,2']bipyrazin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367720 (CHEMBL1202504) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020131 (4-{4-[4-(3-chlorophenyl)hexahydro-1-pyrazinyl]buty...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367722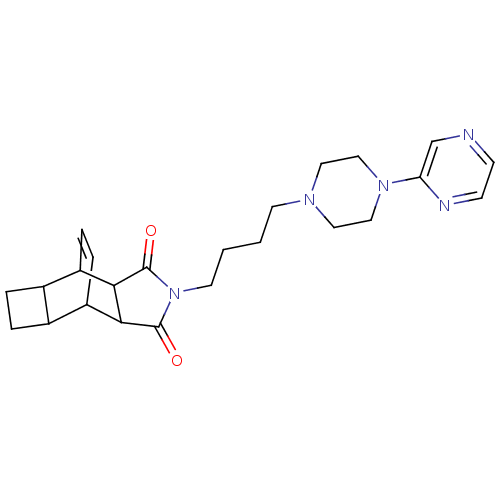 (CHEMBL1202511) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020123 (4-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)-butyl]-4-az...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020122 (4-{4-[4-(6-chloro-2-pyrazinyl)hexahydro-1-pyraziny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367721 (CHEMBL1202512) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367724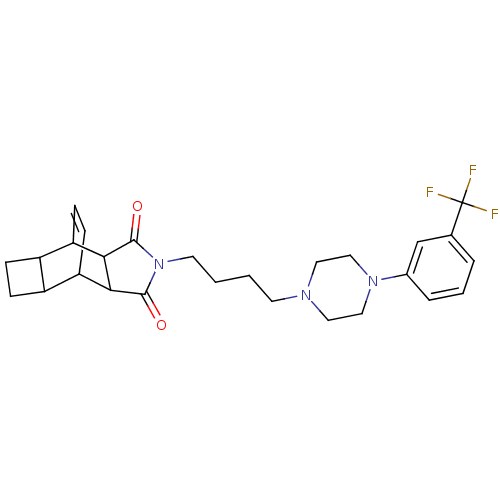 (CHEMBL1202516) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50020122 (4-{4-[4-(6-chloro-2-pyrazinyl)hexahydro-1-pyraziny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50020137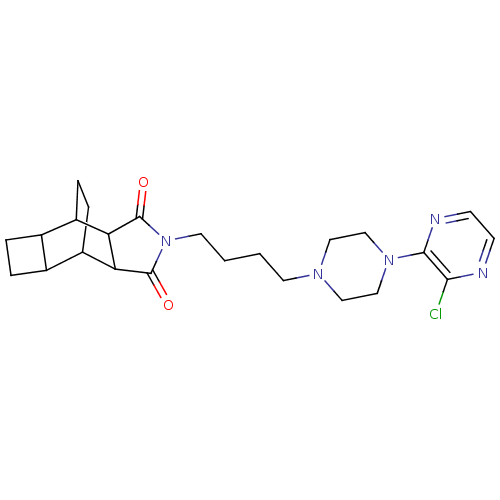 (4-{4-[4-(3-chloro-2-pyrazinyl)hexahydro-1-pyraziny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367719 (CHEMBL1202513) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020130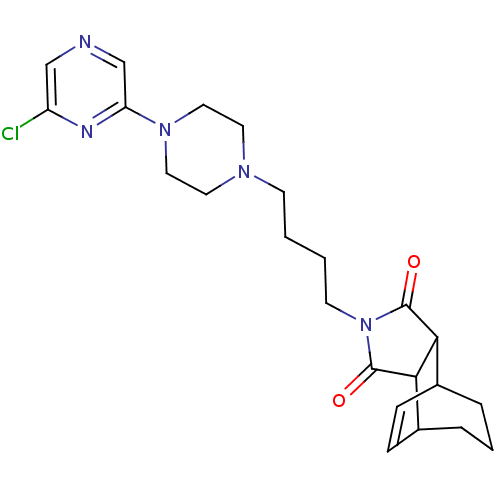 (4-[4-(6'-Chloro-2,3,5,6-tetrahydro-[1,2']bipyrazin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001859 ((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020116 (4-[4-(6'-Chloro-2,3,5,6-tetrahydro-[1,2']bipyrazin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50367727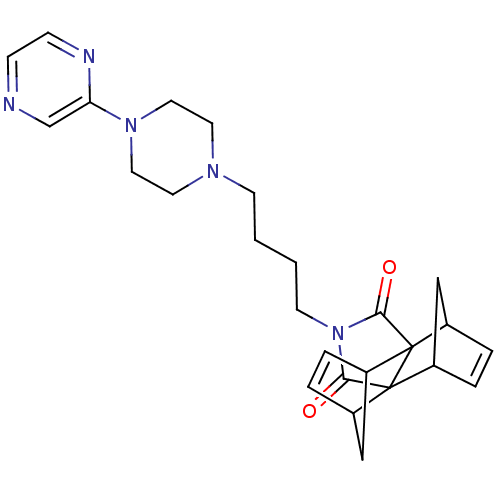 (CHEMBL1202502) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367723 (CHEMBL1202494) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020138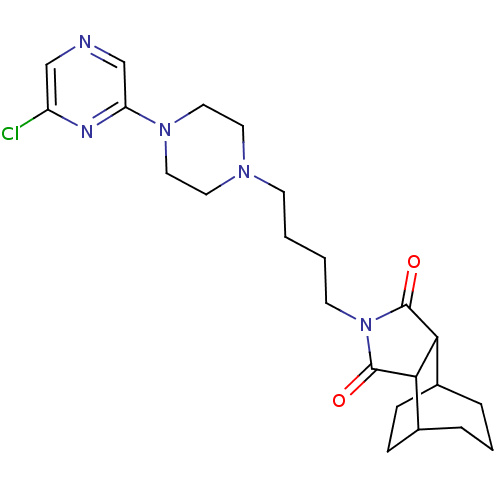 (4-[4-(6'-Chloro-2,3,5,6-tetrahydro-[1,2']bipyrazin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020124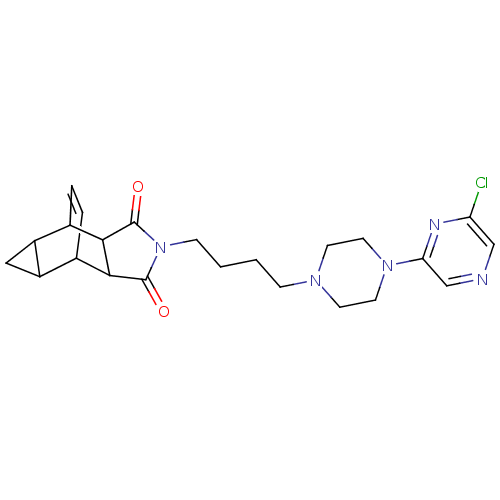 (4-{4-[4-(6-chloro-2-pyrazinyl)hexahydro-1-pyraziny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50020113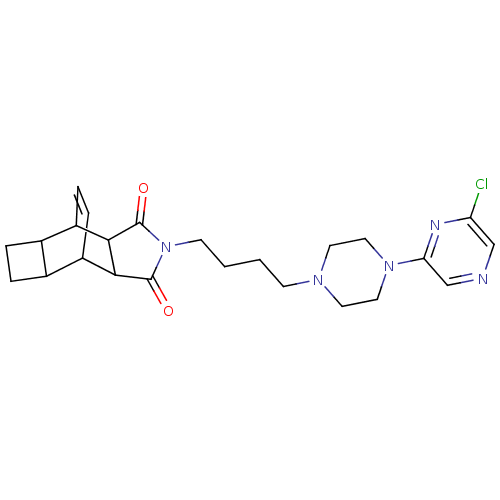 (4-{4-[4-(6-chloro-2-pyrazinyl)hexahydro-1-pyraziny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to 5-hydroxytryptamine 1A receptor from rat hippocampal tissue. | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50020123 (4-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)-butyl]-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367729 (CHEMBL1204094) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 345 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50020116 (4-[4-(6'-Chloro-2,3,5,6-tetrahydro-[1,2']bipyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 345 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50020144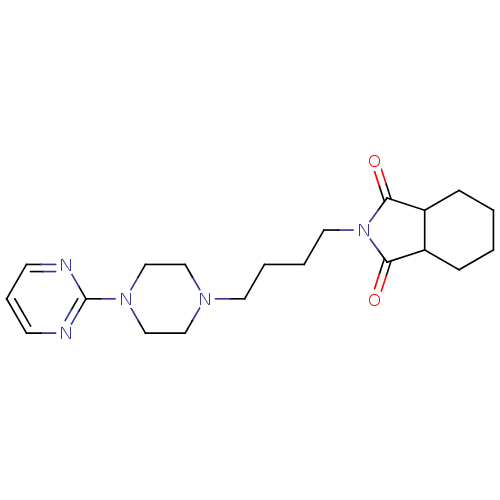 (2-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)-butyl]-hexa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 374 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50020113 (4-{4-[4-(6-chloro-2-pyrazinyl)hexahydro-1-pyraziny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 413 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50367720 (CHEMBL1202504) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 488 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50020124 (4-{4-[4-(6-chloro-2-pyrazinyl)hexahydro-1-pyraziny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50020136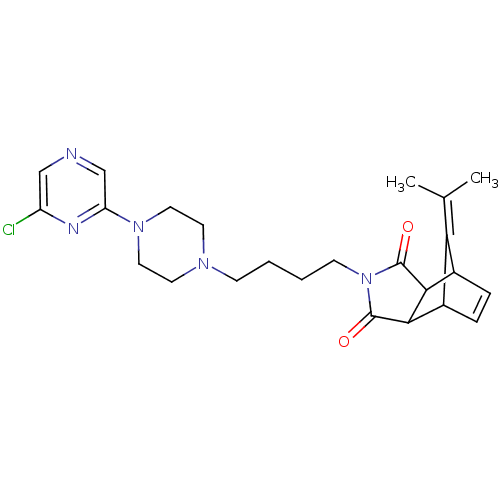 (4-[4-(6'-Chloro-2,3,5,6-tetrahydro-[1,2']bipyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 968 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50020141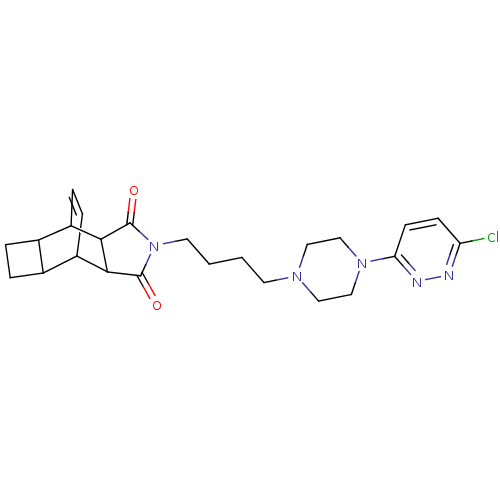 (4-{4-[4-(6-chloro-3-pyridazinyl)hexahydro-1-pyrazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50020125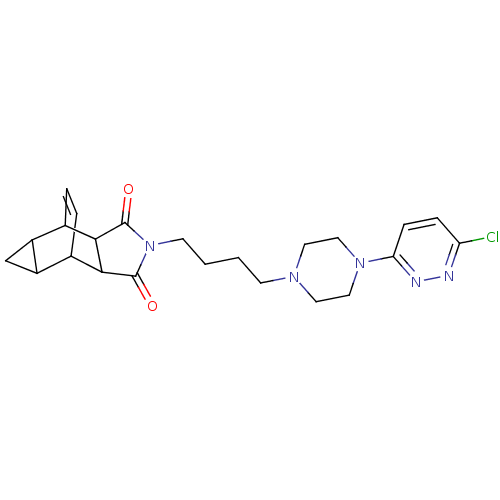 (4-{4-[4-(6-chloro-3-pyridazinyl)hexahydro-1-pyrazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]spiperone binding to Dopamine receptor D2 from rat brain limbic tissue | J Med Chem 31: 1382-92 (1988) BindingDB Entry DOI: 10.7270/Q2PC32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||