 Found 50 hits Enz. Inhib. hit(s) with all data for entry = 50037244
Found 50 hits Enz. Inhib. hit(s) with all data for entry = 50037244 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM2681
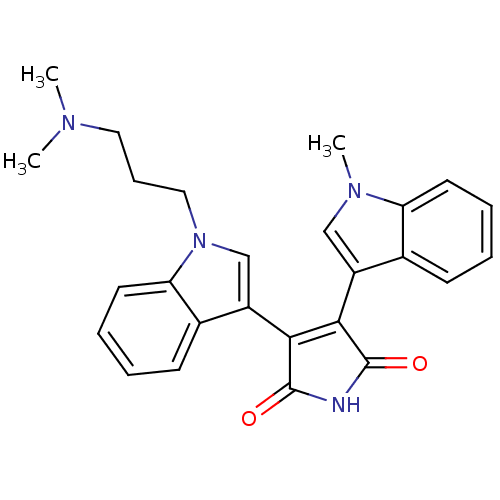
(3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C26H26N4O2/c1-28(2)13-8-14-30-16-20(18-10-5-7-12-22(18)30)24-23(25(31)27-26(24)32)19-15-29(3)21-11-6-4-9-17(19)21/h4-7,9-12,15-16H,8,13-14H2,1-3H3,(H,27,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM3175

(3-[1-[3-(Amidinothio)propyl]-3-indolyl]-4-(1-methy...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(CCCSC(N)=N)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C25H23N5O2S/c1-29-13-17(15-7-2-4-9-19(15)29)21-22(24(32)28-23(21)31)18-14-30(11-6-12-33-25(26)27)20-10-5-3-8-16(18)20/h2-5,7-10,13-14H,6,11-12H2,1H3,(H3,26,27)(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM13336
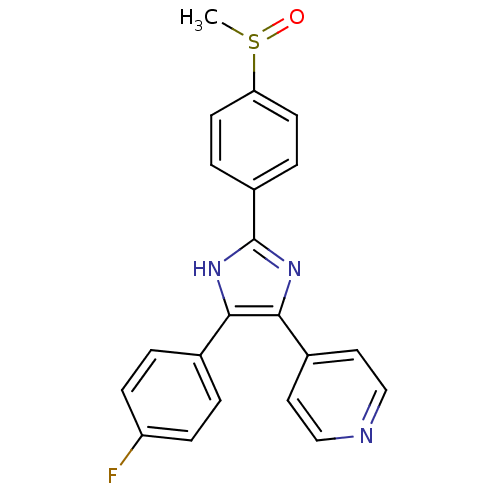
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase N2
(Homo sapiens (Human)) | BDBM14029
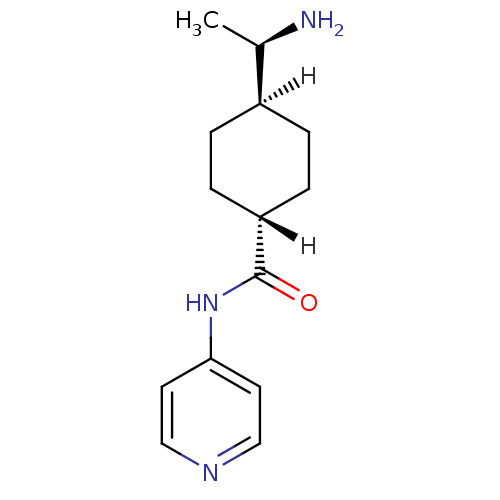
((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...)Show SMILES [H][C@@]1(CC[C@@]([H])(CC1)C(=O)Nc1ccncc1)[C@@H](C)N |r,wU:4.4,1.18,17.20,wD:4.8,1.0,(1.92,.41,;1.06,-.86,;-.27,-1.63,;-1.61,-.86,;-1.61,.68,;-1.61,2.22,;-.27,1.45,;1.06,.68,;-2.94,1.45,;-2.94,2.99,;-4.27,.68,;-5.61,1.45,;-5.61,2.99,;-6.94,3.76,;-8.28,2.99,;-8.28,1.45,;-6.94,.68,;2.6,-.86,;3.37,.47,;3.37,-2.2,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18)/t10-,11-,12-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C related kinase 2 (PRK2) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM14029

((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...)Show SMILES [H][C@@]1(CC[C@@]([H])(CC1)C(=O)Nc1ccncc1)[C@@H](C)N |r,wU:4.4,1.18,17.20,wD:4.8,1.0,(1.92,.41,;1.06,-.86,;-.27,-1.63,;-1.61,-.86,;-1.61,.68,;-1.61,2.22,;-.27,1.45,;1.06,.68,;-2.94,1.45,;-2.94,2.99,;-4.27,.68,;-5.61,1.45,;-5.61,2.99,;-6.94,3.76,;-8.28,2.99,;-8.28,1.45,;-6.94,.68,;2.6,-.86,;3.37,.47,;3.37,-2.2,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18)/t10-,11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase ROCK2 (ROCKII) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Malate dehydrogenase
(Thermus thermophilus) | BDBM50126829
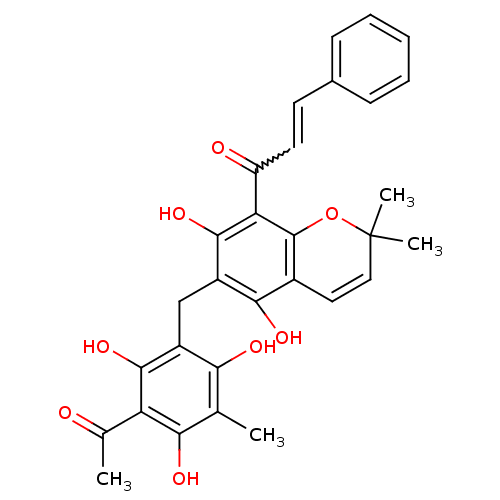
((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...)Show SMILES CC(=O)c1c(O)c(C)c(O)c(Cc2c(O)c3C=CC(C)(C)Oc3c(C(=O)C=Cc3ccccc3)c2O)c1O |w:26.26,c:16| Show InChI InChI=1S/C30H28O8/c1-15-24(33)19(27(36)22(16(2)31)25(15)34)14-20-26(35)18-12-13-30(3,4)38-29(18)23(28(20)37)21(32)11-10-17-8-6-5-7-9-17/h5-13,33-37H,14H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50108771

(2'-amino-3'-methoxyflavone | 2-(2-Amino-3-methoxy-...)Show InChI InChI=1S/C16H13NO3/c1-19-14-8-4-6-11(16(14)17)15-9-12(18)10-5-2-3-7-13(10)20-15/h2-9H,17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Prostaglandin G/H synthase 1 (COX-1) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50336799
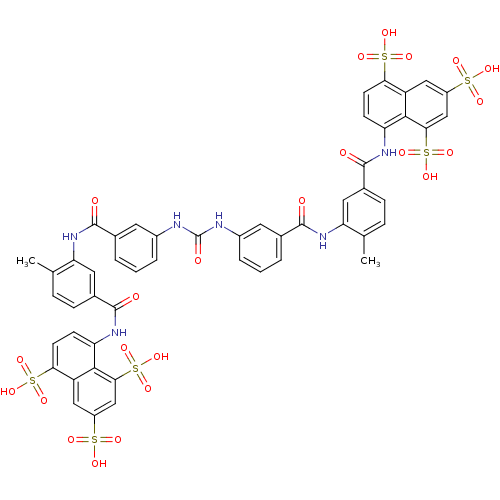
(5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against DNA-DNA dependent protein kinase (DNA-PK) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase N1
(Homo sapiens (Human)) | BDBM14027
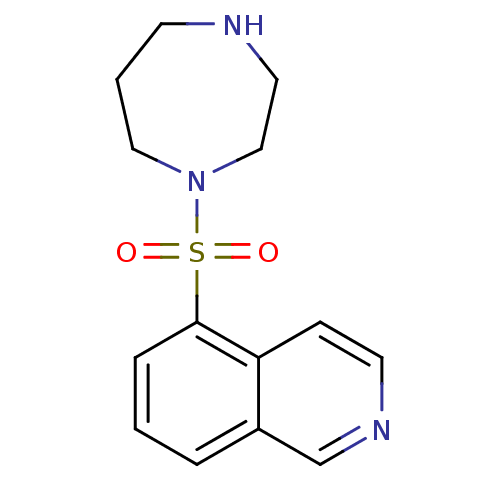
(5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...)Show InChI InChI=1S/C14H17N3O2S/c18-20(19,17-9-2-6-15-8-10-17)14-4-1-3-12-11-16-7-5-13(12)14/h1,3-5,7,11,15H,2,6,8-10H2 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C related kinase 1 (PRK1) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Myosin light chain kinase, smooth muscle
(Homo sapiens (Human)) | BDBM15234
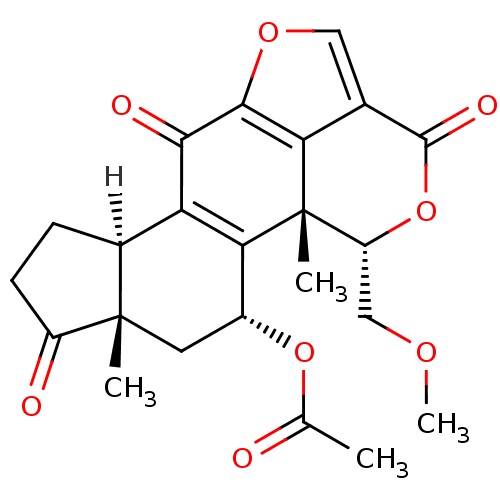
((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)C[C@@H](OC(C)=O)C1=C2C(=O)c2occ3c2[C@]1(C)[C@@H](COC)OC3=O |r,c:15| Show InChI InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Smooth muscle myosin light chain kinase (mMLCK) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 5
(Homo sapiens (Human)) | BDBM50126829

((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...)Show SMILES CC(=O)c1c(O)c(C)c(O)c(Cc2c(O)c3C=CC(C)(C)Oc3c(C(=O)C=Cc3ccccc3)c2O)c1O |w:26.26,c:16| Show InChI InChI=1S/C30H28O8/c1-15-24(33)19(27(36)22(16(2)31)25(15)34)14-20-26(35)18-12-13-30(3,4)38-29(18)23(28(20)37)21(32)11-10-17-8-6-5-7-9-17/h5-13,33-37H,14H2,1-4H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of p38-regulated activated kinase (PRAK) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM14027

(5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...)Show InChI InChI=1S/C14H17N3O2S/c18-20(19,17-9-2-6-15-8-10-17)14-4-1-3-12-11-16-7-5-13(12)14/h1,3-5,7,11,15H,2,6,8-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase ROCK2 (ROCKII) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50108771

(2'-amino-3'-methoxyflavone | 2-(2-Amino-3-methoxy-...)Show InChI InChI=1S/C16H13NO3/c1-19-14-8-4-6-11(16(14)17)15-9-12(18)10-5-2-3-7-13(10)20-15/h2-9H,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen activated protein kinase kinase 1 (MKK1) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM13336

(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Prostaglandin G/H synthase 1 (COX-1) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50023867
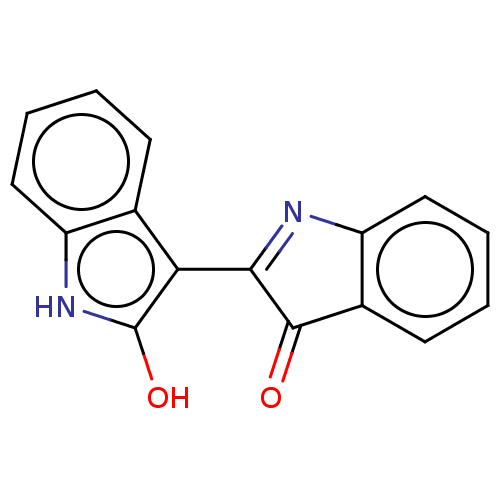
(Indirubin)Show InChI InChI=1S/C16H10N2O2/c19-15-10-6-2-4-8-12(10)17-14(15)13-9-5-1-3-7-11(9)18-16(13)20/h1-8,18,20H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 2 (CDK2) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2581

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NCc2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O/c24-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)23-19/h1-8,22-23H,9H2,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50126829

((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...)Show SMILES CC(=O)c1c(O)c(C)c(O)c(Cc2c(O)c3C=CC(C)(C)Oc3c(C(=O)C=Cc3ccccc3)c2O)c1O |w:26.26,c:16| Show InChI InChI=1S/C30H28O8/c1-15-24(33)19(27(36)22(16(2)31)25(15)34)14-20-26(35)18-12-13-30(3,4)38-29(18)23(28(20)37)21(32)11-10-17-8-6-5-7-9-17/h5-13,33-37H,14H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C delta (PKCdelta) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50027095

(CHEMBL36450 | SC-68376)Show InChI InChI=1S/C15H12N2O/c1-11-17-14(12-5-3-2-4-6-12)15(18-11)13-7-9-16-10-8-13/h2-10H,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM2581

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NCc2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O/c24-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)23-19/h1-8,22-23H,9H2,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM2581

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NCc2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O/c24-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)23-19/h1-8,22-23H,9H2,(H,21,24) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50111589
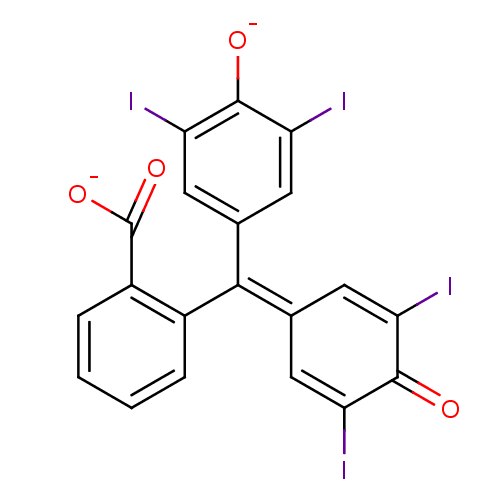
(2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...)Show SMILES [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1 |c:18,t:12| Show InChI InChI=1S/C20H10I4O4/c21-13-5-9(6-14(22)18(13)25)17(10-7-15(23)19(26)16(24)8-10)11-3-1-2-4-12(11)20(27)28/h1-8,25H,(H,27,28)/p-2 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50072147
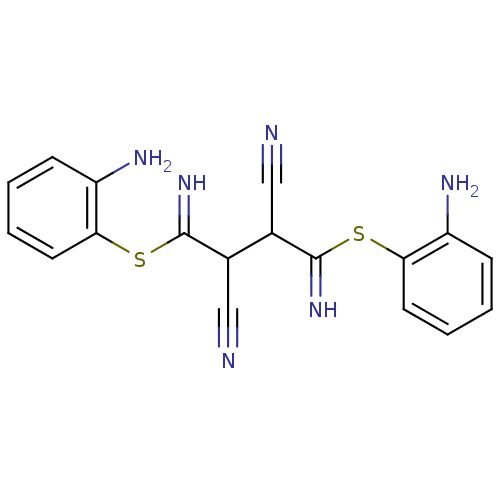
((2Z,3Z)-2,3-bis(amino(2-aminophenylthio)methylene)...)Show InChI InChI=1S/C18H16N6S2/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22/h1-8,11-12,23-24H,21-22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen activated protein kinase kinase 1 (MKK1) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50111604
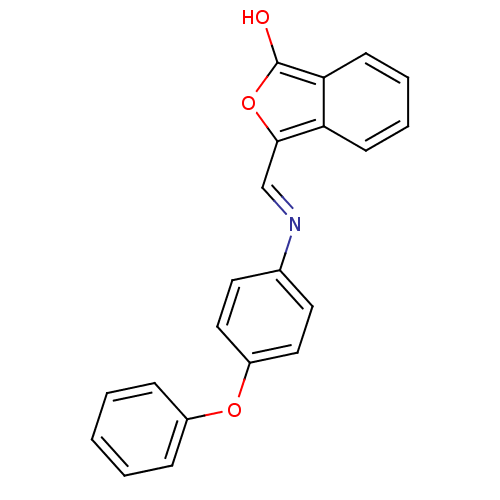
(3-[(4-Phenoxy-phenylamino)-methylene]-3H-isobenzof...)Show InChI InChI=1S/C21H15NO3/c23-21-19-9-5-4-8-18(19)20(25-21)14-22-15-10-12-17(13-11-15)24-16-6-2-1-3-7-16/h1-14,23H/b22-14+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50023867

(Indirubin)Show InChI InChI=1S/C16H10N2O2/c19-15-10-6-2-4-8-12(10)17-14(15)13-9-5-1-3-7-11(9)18-16(13)20/h1-8,18,20H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Src tyrosine kinase |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM3175

(3-[1-[3-(Amidinothio)propyl]-3-indolyl]-4-(1-methy...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(CCCSC(N)=N)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C25H23N5O2S/c1-29-13-17(15-7-2-4-9-19(15)29)21-22(24(32)28-23(21)31)18-14-30(11-6-12-33-25(26)27)20-10-5-3-8-16(18)20/h2-5,7-10,13-14H,6,11-12H2,1H3,(H3,26,27)(H,28,31,32) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50126829

((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...)Show SMILES CC(=O)c1c(O)c(C)c(O)c(Cc2c(O)c3C=CC(C)(C)Oc3c(C(=O)C=Cc3ccccc3)c2O)c1O |w:26.26,c:16| Show InChI InChI=1S/C30H28O8/c1-15-24(33)19(27(36)22(16(2)31)25(15)34)14-20-26(35)18-12-13-30(3,4)38-29(18)23(28(20)37)21(32)11-10-17-8-6-5-7-9-17/h5-13,33-37H,14H2,1-4H3 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50111585

(4-(4-Bromo-phenylazo)-phenol | 4-bromophenylazophe...)Show InChI InChI=1S/C12H9BrN2O/c13-9-1-3-10(4-2-9)14-15-11-5-7-12(16)8-6-11/h1-8,16H | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM50111591
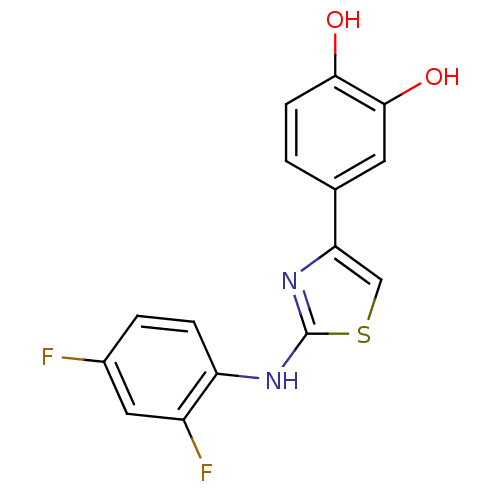
(4-[2-(2,4-Difluoro-phenylamino)-thiazol-4-yl]-benz...)Show InChI InChI=1S/C15H10F2N2O2S/c16-9-2-3-11(10(17)6-9)18-15-19-12(7-22-15)8-1-4-13(20)14(21)5-8/h1-7,20-21H,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM50111589

(2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...)Show SMILES [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1 |c:18,t:12| Show InChI InChI=1S/C20H10I4O4/c21-13-5-9(6-14(22)18(13)25)17(10-7-15(23)19(26)16(24)8-10)11-3-1-2-4-12(11)20(27)28/h1-8,25H,(H,27,28)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma
(Homo sapiens (Human)) | BDBM2581

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NCc2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O/c24-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)23-19/h1-8,22-23H,9H2,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase A |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50310357
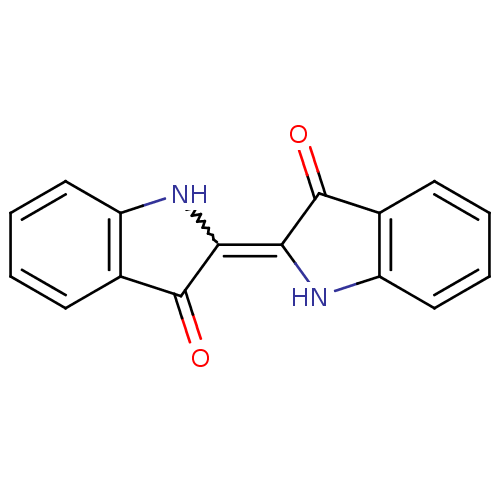
(CHEMBL599552 | indigo)Show InChI InChI=1S/C16H10N2O2/c19-15-9-5-1-3-7-11(9)17-13(15)14-16(20)10-6-2-4-8-12(10)18-14/h1-8,17-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Src tyrosine kinase |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50336799

(5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C (PKC) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM50023867

(Indirubin)Show InChI InChI=1S/C16H10N2O2/c19-15-10-6-2-4-8-12(10)17-14(15)13-9-5-1-3-7-11(9)18-16(13)20/h1-8,18,20H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM50111585

(4-(4-Bromo-phenylazo)-phenol | 4-bromophenylazophe...)Show InChI InChI=1S/C12H9BrN2O/c13-9-1-3-10(4-2-9)14-15-11-5-7-12(16)8-6-11/h1-8,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM3175

(3-[1-[3-(Amidinothio)propyl]-3-indolyl]-4-(1-methy...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(CCCSC(N)=N)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C25H23N5O2S/c1-29-13-17(15-7-2-4-9-19(15)29)21-22(24(32)28-23(21)31)18-14-30(11-6-12-33-25(26)27)20-10-5-3-8-16(18)20/h2-5,7-10,13-14H,6,11-12H2,1H3,(H3,26,27)(H,28,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 5
(Homo sapiens (Human)) | BDBM50072147

((2Z,3Z)-2,3-bis(amino(2-aminophenylthio)methylene)...)Show InChI InChI=1S/C18H16N6S2/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22/h1-8,11-12,23-24H,21-22H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of p38-regulated activated kinase (Protein kinase PRAK) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM50126827

(4-(1-Hydroxy-naphthalen-2-ylazo)-naphthalene-1-sul...)Show SMILES Oc1c(ccc2ccccc12)N=Nc1ccc(c2ccccc12)S([O-])(=O)=O |w:11.12| Show InChI InChI=1S/C20H14N2O4S/c23-20-14-6-2-1-5-13(14)9-10-18(20)22-21-17-11-12-19(27(24,25)26)16-8-4-3-7-15(16)17/h1-12,23H,(H,24,25,26)/p-1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM50310357

(CHEMBL599552 | indigo)Show InChI InChI=1S/C16H10N2O2/c19-15-9-5-1-3-7-11(9)17-13(15)14-16(20)10-6-2-4-8-12(10)18-14/h1-8,17-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50310357

(CHEMBL599552 | indigo)Show InChI InChI=1S/C16H10N2O2/c19-15-9-5-1-3-7-11(9)17-13(15)14-16(20)10-6-2-4-8-12(10)18-14/h1-8,17-18H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 2 (CDK2) |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50126827

(4-(1-Hydroxy-naphthalen-2-ylazo)-naphthalene-1-sul...)Show SMILES Oc1c(ccc2ccccc12)N=Nc1ccc(c2ccccc12)S([O-])(=O)=O |w:11.12| Show InChI InChI=1S/C20H14N2O4S/c23-20-14-6-2-1-5-13(14)9-10-18(20)22-21-17-11-12-19(27(24,25)26)16-8-4-3-7-15(16)17/h1-12,23H,(H,24,25,26)/p-1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50310357

(CHEMBL599552 | indigo)Show InChI InChI=1S/C16H10N2O2/c19-15-9-5-1-3-7-11(9)17-13(15)14-16(20)10-6-2-4-8-12(10)18-14/h1-8,17-18H | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50111591

(4-[2-(2,4-Difluoro-phenylamino)-thiazol-4-yl]-benz...)Show InChI InChI=1S/C15H10F2N2O2S/c16-9-2-3-11(10(17)6-9)18-15-19-12(7-22-15)8-1-4-13(20)14(21)5-8/h1-7,20-21H,(H,18,19) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50023867

(Indirubin)Show InChI InChI=1S/C16H10N2O2/c19-15-10-6-2-4-8-12(10)17-14(15)13-9-5-1-3-7-11(9)18-16(13)20/h1-8,18,20H | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM50072147

((2Z,3Z)-2,3-bis(amino(2-aminophenylthio)methylene)...)Show InChI InChI=1S/C18H16N6S2/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22/h1-8,11-12,23-24H,21-22H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50072147

((2Z,3Z)-2,3-bis(amino(2-aminophenylthio)methylene)...)Show InChI InChI=1S/C18H16N6S2/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22/h1-8,11-12,23-24H,21-22H2 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM50111604

(3-[(4-Phenoxy-phenylamino)-methylene]-3H-isobenzof...)Show InChI InChI=1S/C21H15NO3/c23-21-19-9-5-4-8-18(19)20(25-21)14-22-15-10-12-17(13-11-15)24-16-6-2-1-3-7-16/h1-14,23H/b22-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM2681

(3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C26H26N4O2/c1-28(2)13-8-14-30-16-20(18-10-5-7-12-22(18)30)24-23(25(31)27-26(24)32)19-15-29(3)21-11-6-4-9-17(19)21/h4-7,9-12,15-16H,8,13-14H2,1-3H3,(H,27,31,32) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Chymotrypsinogen from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase
(Thermus thermophilus) | BDBM2681

(3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12 |t:9| Show InChI InChI=1S/C26H26N4O2/c1-28(2)13-8-14-30-16-20(18-10-5-7-12-22(18)30)24-23(25(31)27-26(24)32)19-15-29(3)21-11-6-4-9-17(19)21/h4-7,9-12,15-16H,8,13-14H2,1-3H3,(H,27,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus |
J Med Chem 46: 1478-83 (2003)
Article DOI: 10.1021/jm020427b
BindingDB Entry DOI: 10.7270/Q2R78FZT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data