

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249132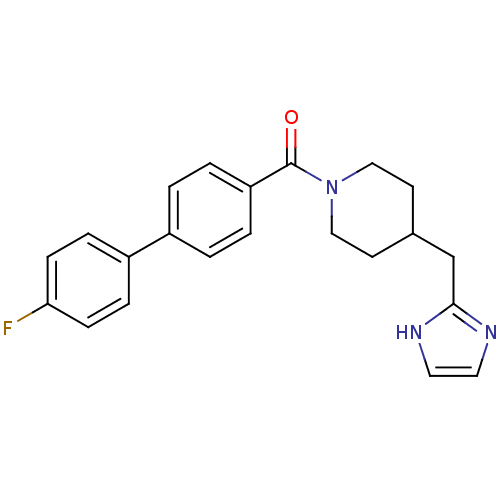 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(4'-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50587589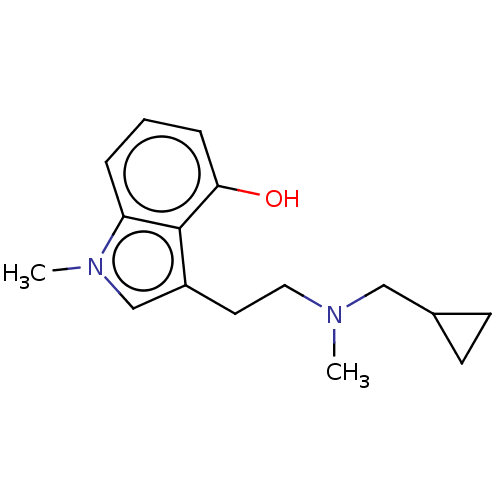 (CHEMBL5081637) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.245 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of human 5HT2B expressed in HEK293T cells co-transfected with Galphaq-RLuc8, Ggamma1-GFP2 and Gbeta1 assessed as dissociation of Galphaq f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00578 BindingDB Entry DOI: 10.7270/Q2BR8X3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249483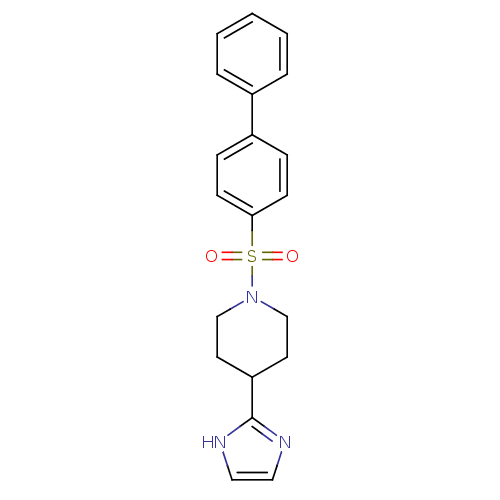 (1-(biphenyl-4-ylsulfonyl)-4-(1H-imidazol-2-yl)pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249464 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249131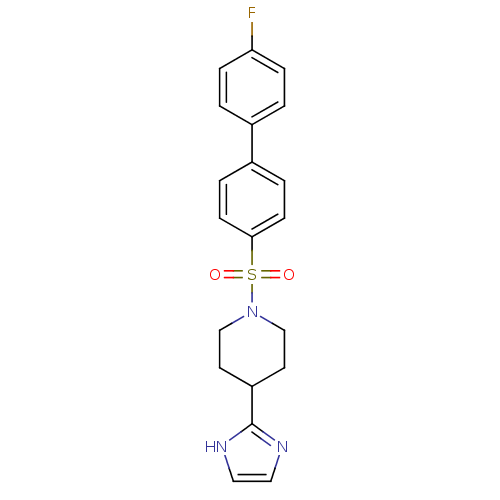 (1-(4'-fluorobiphenyl-4-ylsulfonyl)-4-(1H-imidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249134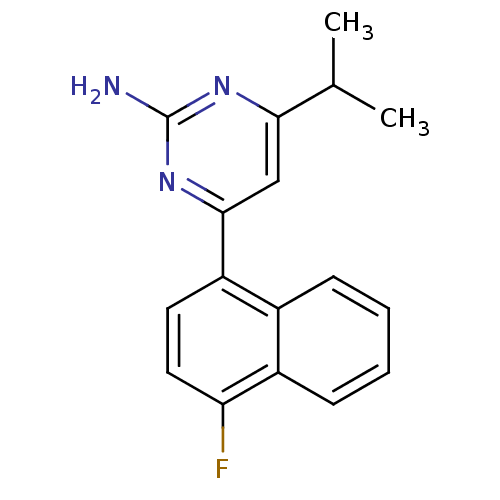 (4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249486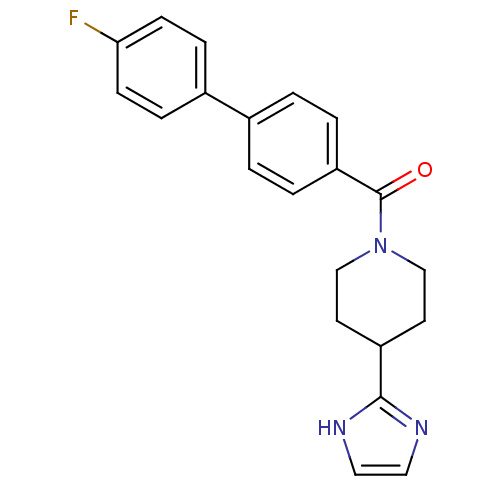 ((4-(1H-imidazol-2-yl)piperidin-1-yl)(4'-fluorobiph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50081701 (3-[2-(dimethylamino)ethyl]-1H-indol-4-ol | 4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of human 5HT2B expressed in HEK293T cells co-transfected with Galphaq-RLuc8, Ggamma1-GFP2 and Gbeta1 assessed as dissociation of Galphaq f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00578 BindingDB Entry DOI: 10.7270/Q2BR8X3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191171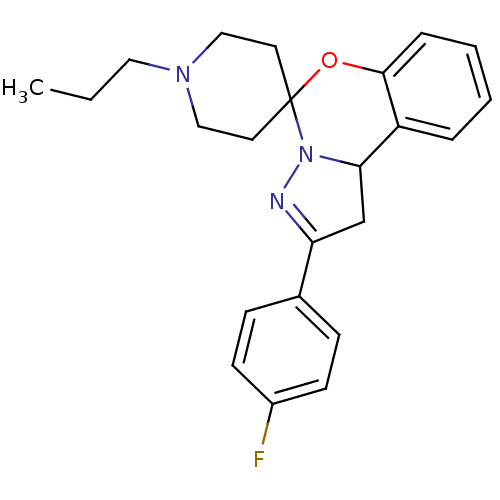 (2-(4-flurophenyl)-1,10b-dihydro-benzo[e]pyrazolo[1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249176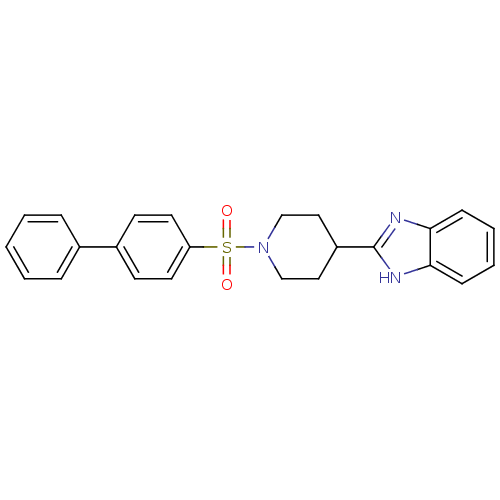 (2-(1-(biphenyl-4-ylsulfonyl)piperidin-4-yl)-1H-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249482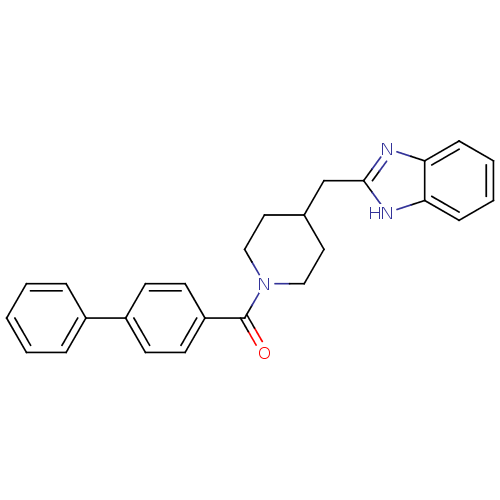 ((4-((1H-benzo[d]imidazol-2-yl)methyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249126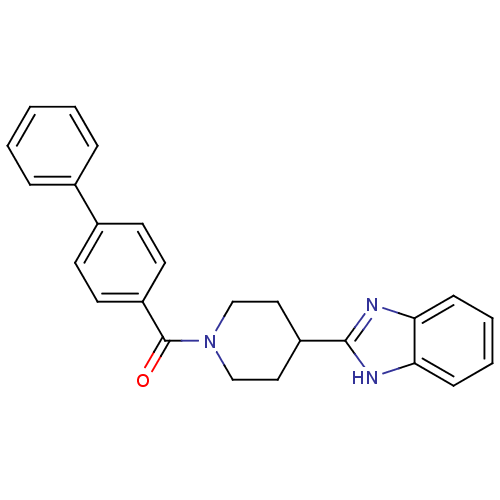 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249462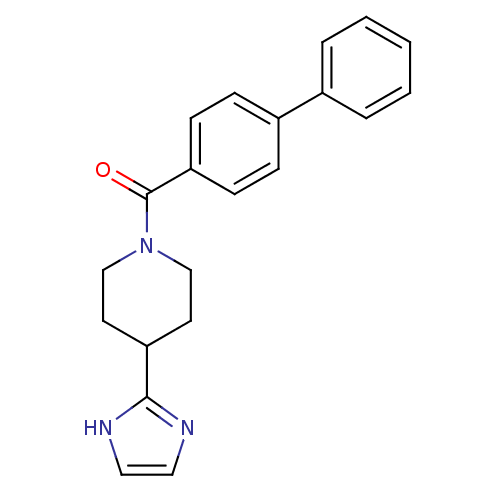 ((4-(1H-imidazol-2-yl)piperidin-1-yl)(biphenyl-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM28582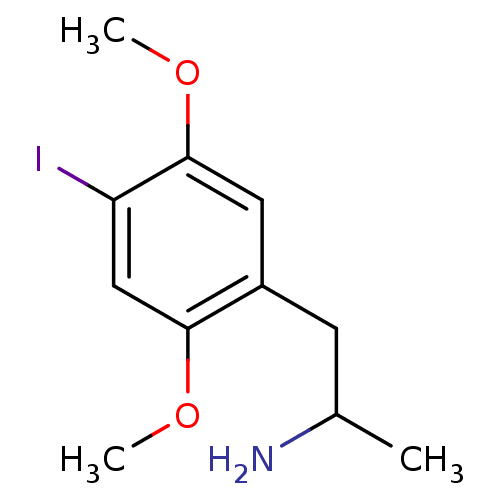 (1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]DOI from human recombinant 5HT2B receptor expressed in CHO cells measured after 60 mins by scintillation counting method | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM28582 (1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I](+/-)DOI from human recombinant 5-HT2B receptor expressed in CHO cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249128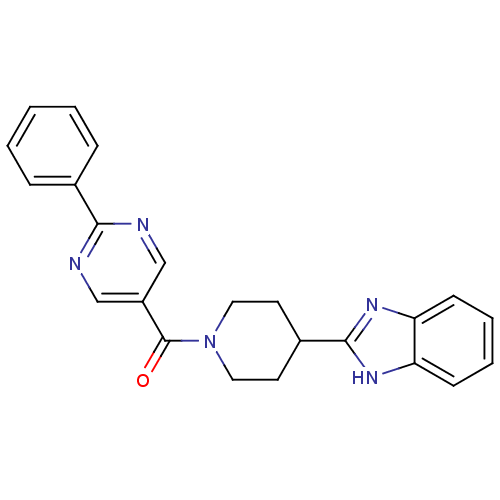 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(2-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50010594 (2-Chloro-11-(4-methyl-piperazin-1-yl)-5H-dibenzo[b...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Göteborg Curated by ChEMBL | Assay Description Binding affinity towards 5-HT2C receptor from rat using [3H]-mesulergine as radioligand | J Med Chem 42: 2235-44 (1999) Article DOI: 10.1021/jm991005d BindingDB Entry DOI: 10.7270/Q2KH0MH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50010594 (2-Chloro-11-(4-methyl-piperazin-1-yl)-5H-dibenzo[b...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of [3H]-mesulergine binding to 5-hydroxytryptamine 2C receptor in rat brain membranes | J Med Chem 40: 4146-53 (1998) Article DOI: 10.1021/jm9704457 BindingDB Entry DOI: 10.7270/Q2DV1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ACADIA Pharmaceuticals Inc. | Assay Description R-SAT assays were performed as described previously (Weiner et al., 2001), with the following modifications. In brief, NIH-3T3 cells were grown to 80... | J Pharmacol Exp Ther 317: 910-8 (2006) Article DOI: 10.1124/jpet.105.097006 BindingDB Entry DOI: 10.7270/Q269728N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50060681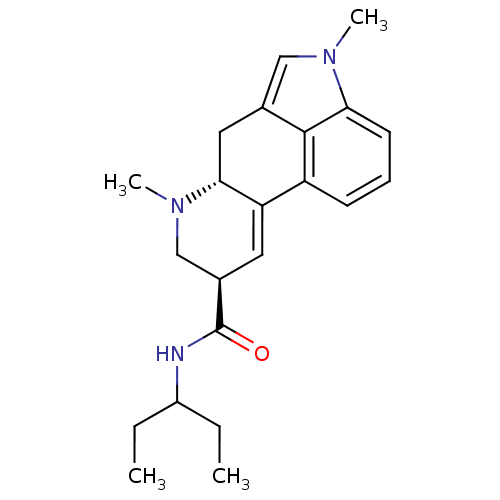 ((6aR,9R)-4,7-Dimethyl-4,6,6a,7,8,9-hexahydro-indol...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of radiolabeled [3H]-Ketanserin ligand binding to 5-hydroxytryptamine 2C receptor | J Med Chem 40: 3670-8 (1997) Article DOI: 10.1021/jm970376w BindingDB Entry DOI: 10.7270/Q24Q7VP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM28582 (1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Displacement of [125I](+/-)DOI from human recombinant 5-HT2B receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 60: 349-361 (2017) Article DOI: 10.1021/acs.jmedchem.6b01422 BindingDB Entry DOI: 10.7270/Q27M0B6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Displacement of [125I]+/-DOI from human recombinant 5-HT2B receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249427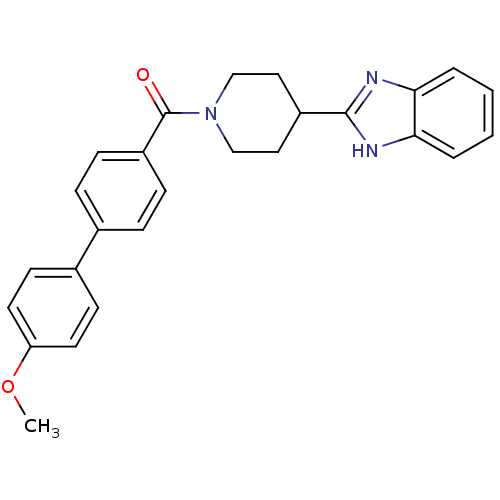 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4'-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249180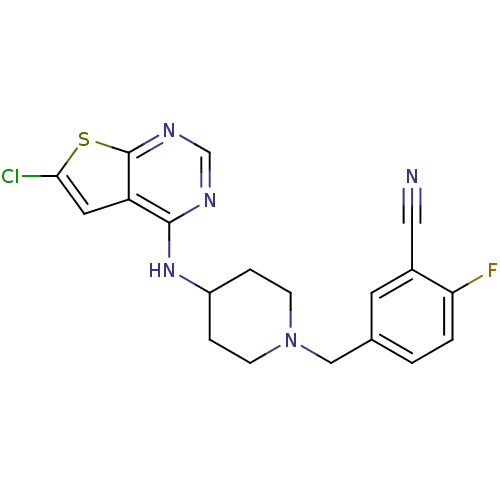 (5-((4-(6-chlorothieno[2,3-d]pyrimidin-4-ylamino)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249129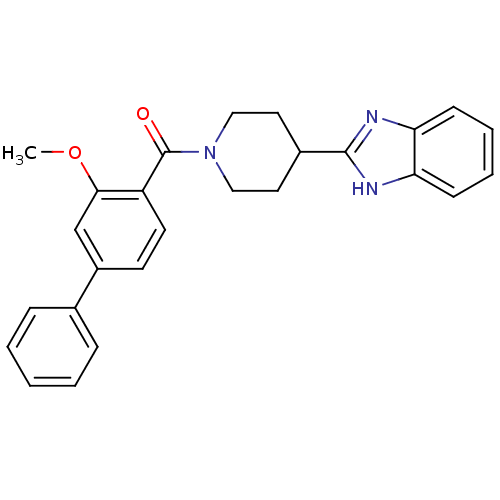 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(3-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50024204 (1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Galway Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5-HT2B receptor in CHOK1 cells measured after 30 mins by HTRF assay | Eur J Med Chem 176: 292-309 (2019) Article DOI: 10.1016/j.ejmech.2019.04.064 BindingDB Entry DOI: 10.7270/Q2NP27V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249130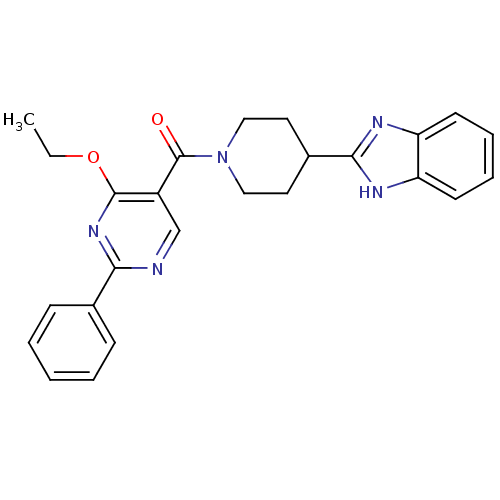 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249484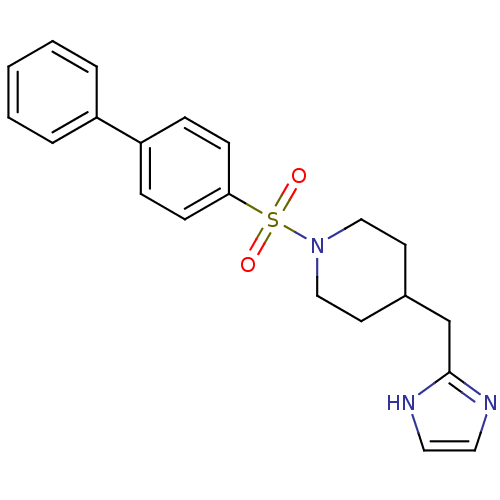 (4-((1H-imidazol-2-yl)methyl)-1-(biphenyl-4-ylsulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50130841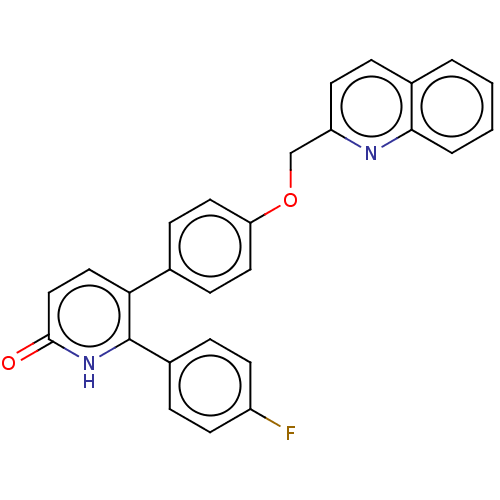 (CHEMBL3634745) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glenmark Research Centre Curated by ChEMBL | Assay Description Binding affinity to human 5HT2b | J Med Chem 58: 8292-308 (2015) Article DOI: 10.1021/acs.jmedchem.5b01240 BindingDB Entry DOI: 10.7270/Q2833TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249132 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(4'-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50170191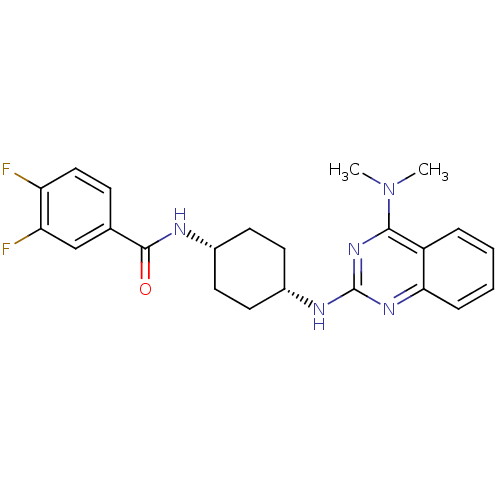 (CHEMBL182150 | N-((cis)-4-(4-(dimethylamino)quinaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]LSD from human 5HT2B receptor | Bioorg Med Chem Lett 19: 6166-71 (2009) Article DOI: 10.1016/j.bmcl.2009.09.003 BindingDB Entry DOI: 10.7270/Q2KK9BVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of [3H]-mesulergine binding to 5-hydroxytryptamine 2C receptor in rat brain membranes | J Med Chem 40: 4146-53 (1998) Article DOI: 10.1021/jm9704457 BindingDB Entry DOI: 10.7270/Q2DV1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Göteborg Curated by ChEMBL | Assay Description Binding affinity towards 5-HT2C receptor from rat using [3H]-mesulergine as radioligand | J Med Chem 42: 2235-44 (1999) Article DOI: 10.1021/jm991005d BindingDB Entry DOI: 10.7270/Q2KH0MH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249459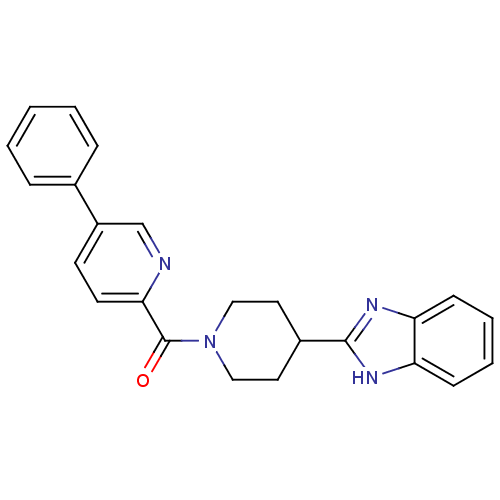 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(5-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50426499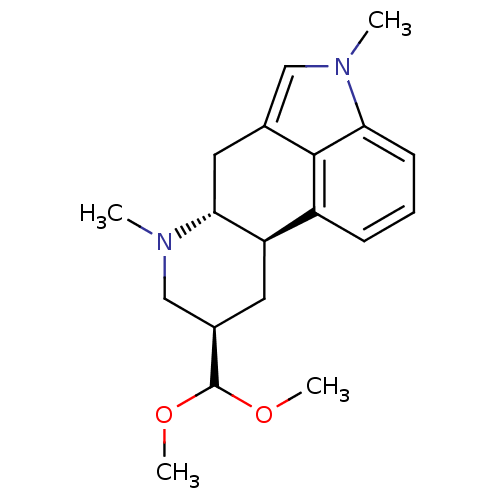 (CHEMBL2323581) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT2B receptor transfected in CHO cell assessed as decrease in 5-HT-induced IP1 production preincubated for 5 mins befo... | ACS Med Chem Lett 4: 254-258 (2013) Article DOI: 10.1021/ml3003814 BindingDB Entry DOI: 10.7270/Q2S183T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50078063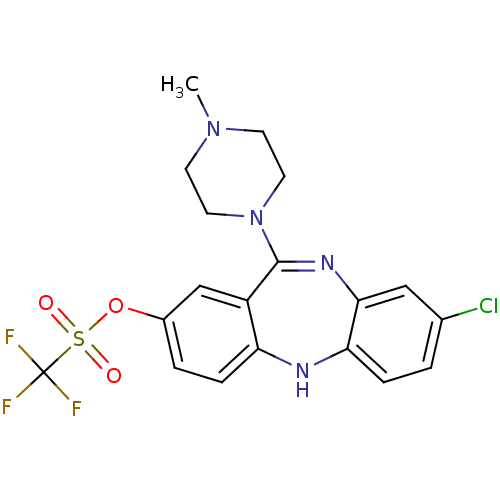 (CHEMBL70319 | Trifluoro-methanesulfonic acid 8-chl...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Göteborg Curated by ChEMBL | Assay Description Binding affinity towards 5-HT2C receptor from rat using [3H]-mesulergine as radioligand | J Med Chem 42: 2235-44 (1999) Article DOI: 10.1021/jm991005d BindingDB Entry DOI: 10.7270/Q2KH0MH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249464 ((4-((1H-imidazol-2-yl)methyl)piperidin-1-yl)(biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191182 (1-propyl-4'-(pyridin-4-yl)-8'-oxa-5',6'-diazaspiro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249173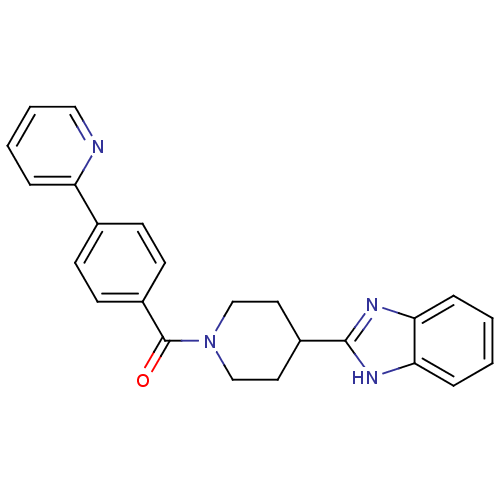 ((4-(1H-benzo[d]imidazol-2-yl)piperidin-1-yl)(4-(py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191179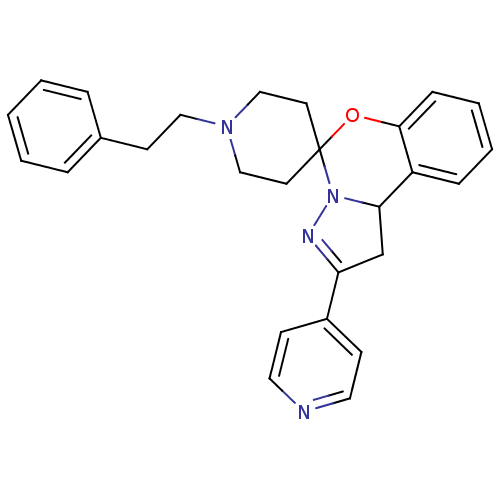 (1-(2-phenylethyl)-4'-(pyridin-4-yl)-8'-oxa-5',6'-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50148838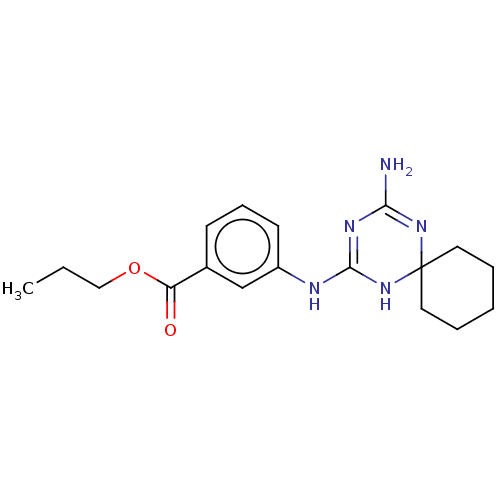 (CHEMBL3770512) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences, Beijing Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHO-K1 cells assessed as calcium flux after 60 mins by FLIPR assay | J Med Chem 59: 707-20 (2016) Article DOI: 10.1021/acs.jmedchem.5b01631 BindingDB Entry DOI: 10.7270/Q2D50PT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249134 (4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191173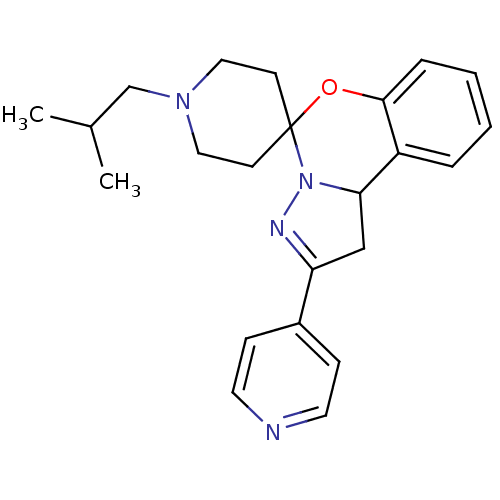 (1-(2-methylpropyl)-4'-(pyridin-4-yl)-8'-oxa-5',6'-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50061558 (2-Methoxy-11-(4-methyl-piperazin-1-yl)-5H-dibenzo[...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of [3H]-mesulergine binding to 5-hydroxytryptamine 2C receptor in rat brain membranes | J Med Chem 40: 4146-53 (1998) Article DOI: 10.1021/jm9704457 BindingDB Entry DOI: 10.7270/Q2DV1J1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50061558 (2-Methoxy-11-(4-methyl-piperazin-1-yl)-5H-dibenzo[...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Göteborg Curated by ChEMBL | Assay Description Binding affinity towards 5-HT2C receptor from rat using [3H]-mesulergine as radioligand | J Med Chem 42: 2235-44 (1999) Article DOI: 10.1021/jm991005d BindingDB Entry DOI: 10.7270/Q2KH0MH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249175 (3-(4-(1H-benzo[d]imidazol-2-yl)piperidine-1-carbon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical Curated by ChEMBL | Assay Description Antagonist activity at 5-HT2B receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of serotonin-induced intracellular Ca2+ flux ... | Bioorg Med Chem Lett 19: 2206-10 (2009) Article DOI: 10.1016/j.bmcl.2009.02.126 BindingDB Entry DOI: 10.7270/Q20Z735D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50426498 (1,6-Dimethylcabergoline | CHEMBL2323579) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Antagonist activity at human 5-HT2B receptor transfected in CHO cell assessed as decrease in 5-HT-induced IP1 production preincubated for 5 mins befo... | ACS Med Chem Lett 4: 254-258 (2013) Article DOI: 10.1021/ml3003814 BindingDB Entry DOI: 10.7270/Q2S183T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50467971 (CHEMBL4290245) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Biological Sciences Curated by ChEMBL | Assay Description Antagonist activity at 5HT2B (unknown origin) expressed in CHOK1 cells assessed as inhibition of agonist-induced effect preincubated for 60 mins at 3... | ACS Med Chem Lett 9: 1019-1024 (2018) Article DOI: 10.1021/acsmedchemlett.8b00300 BindingDB Entry DOI: 10.7270/Q2DZ0C0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 25.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science & Technology; NHWA Pharma. Corporation US Patent | Assay Description (1) The prepared membrane was first applied with appropriate amount of homogenized liquid, and homogenizer was used for evenly dispersing. 15 tubes w... | US Patent US9018213 (2015) BindingDB Entry DOI: 10.7270/Q27M06N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50191181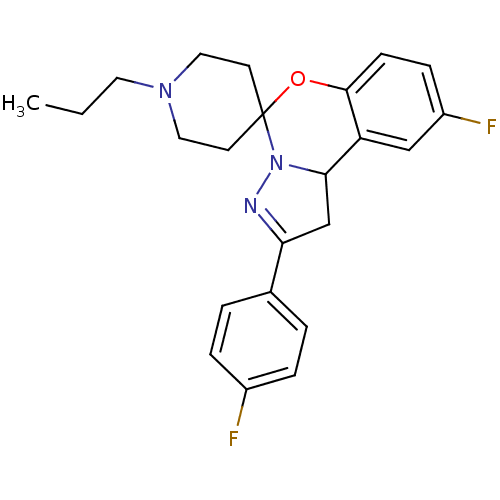 (12'-fluoro-4'-(4-fluorophenyl)-1-propyl-8'-oxa-5',...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Hopital Saint-Louis Curated by ChEMBL | Assay Description Activity at 5HT2B receptor expressed in CHO cell assessed as inhibition of alpha-methyl-5HT-stimulated calcium release | Bioorg Med Chem Lett 16: 4830-3 (2006) Article DOI: 10.1016/j.bmcl.2006.06.068 BindingDB Entry DOI: 10.7270/Q2BV7G7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 299 total ) | Next | Last >> |