 Found 2530 hits Enz. Inhib. hit(s) with Target = 'Phospholipase A2'
Found 2530 hits Enz. Inhib. hit(s) with Target = 'Phospholipase A2' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50226792

(4-(3-(1-benzhydryl-5-chloro-2-(2-((3,4-dichlorophe...)Show SMILES OC(=O)c1ccc(CCCc2c(CCNS(=O)(=O)Cc3ccc(Cl)c(Cl)c3)n(C(c3ccccc3)c3ccccc3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C40H35Cl3N2O4S/c41-32-19-21-37-34(25-32)33(13-7-8-27-14-17-31(18-15-27)40(46)47)38(22-23-44-50(48,49)26-28-16-20-35(42)36(43)24-28)45(37)39(29-9-3-1-4-10-29)30-11-5-2-6-12-30/h1-6,9-12,14-21,24-25,39,44H,7-8,13,22-23,26H2,(H,46,47) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Asubio Pharma Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of cPLA2alpha isolated from human U937 cell cytoplasm assessed as suppression of [14C]arachidonic acid release from L-alpha-1-palmitoyl-2-... |
J Med Chem 57: 7244-62 (2014)
Article DOI: 10.1021/jm500494y
BindingDB Entry DOI: 10.7270/Q2F47QSP |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50185354

(3-methylhydrogen 1-[3-(4-decyloxyphenoxy)-2-oxopro...)Show SMILES CCCCCCCCCCOc1ccc(OCC(=O)Cn2cc(C(=O)OC)c3cc(ccc23)C(O)=O)cc1 Show InChI InChI=1S/C30H37NO7/c1-3-4-5-6-7-8-9-10-17-37-24-12-14-25(15-13-24)38-21-23(32)19-31-20-27(30(35)36-2)26-18-22(29(33)34)11-16-28(26)31/h11-16,18,20H,3-10,17,19,21H2,1-2H3,(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Münster
Curated by ChEMBL
| Assay Description
Inhibition of cPLA2-alpha activity assessed by TPA-induced arachidonic acid release in human platelet |
J Med Chem 49: 2611-20 (2006)
Article DOI: 10.1021/jm051243a
BindingDB Entry DOI: 10.7270/Q2668CSF |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50185348

(3-(methoxycarbonyl)-1-(3-(4-octylphenoxy)-2-oxopro...)Show SMILES CCCCCCCCc1ccc(OCC(=O)Cn2cc(C(=O)OC)c3cc(ccc23)C(O)=O)cc1 Show InChI InChI=1S/C28H33NO6/c1-3-4-5-6-7-8-9-20-10-13-23(14-11-20)35-19-22(30)17-29-18-25(28(33)34-2)24-16-21(27(31)32)12-15-26(24)29/h10-16,18H,3-9,17,19H2,1-2H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Münster
Curated by ChEMBL
| Assay Description
Inhibition of cPLA2-alpha activity assessed by TPA-induced arachidonic acid release in human platelet |
J Med Chem 49: 2611-20 (2006)
Article DOI: 10.1021/jm051243a
BindingDB Entry DOI: 10.7270/Q2668CSF |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053109
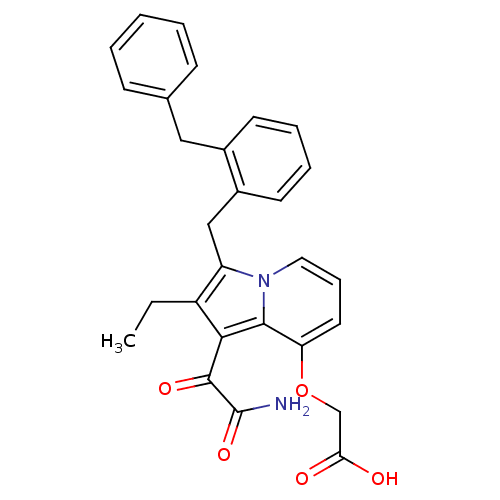
(CHEMBL331755 | [1-Aminooxalyl-3-(2-benzyl-benzyl)-...)Show SMILES CCc1c(Cc2ccccc2Cc2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C28H26N2O5/c1-2-21-22(16-20-12-7-6-11-19(20)15-18-9-4-3-5-10-18)30-14-8-13-23(35-17-24(31)32)26(30)25(21)27(33)28(29)34/h3-14H,2,15-17H2,1H3,(H2,29,34)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50601469
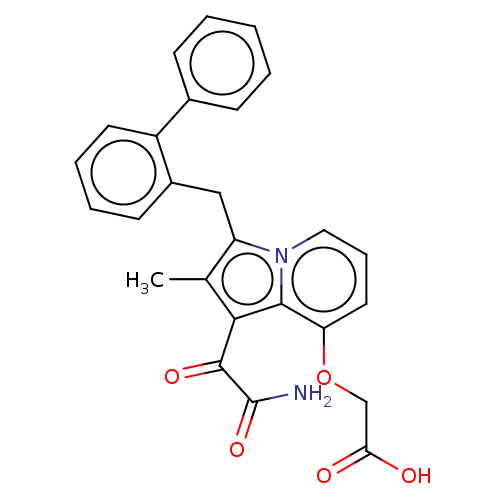
(CHEMBL5172164)Show SMILES Cc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01808
BindingDB Entry DOI: 10.7270/Q2SF316G |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053136
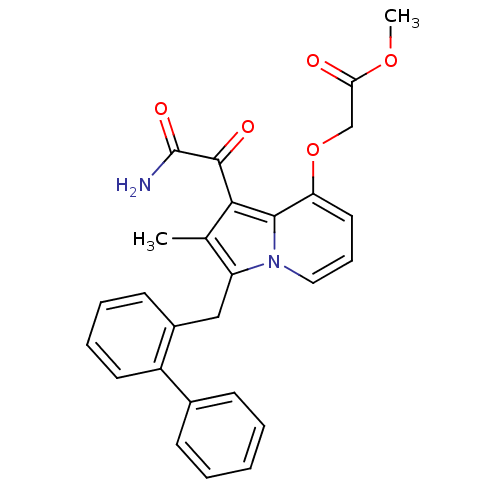
((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-methyl-indo...)Show SMILES COC(=O)COc1cccn2c(Cc3ccccc3-c3ccccc3)c(C)c(C(=O)C(N)=O)c12 Show InChI InChI=1S/C27H24N2O5/c1-17-21(15-19-11-6-7-12-20(19)18-9-4-3-5-10-18)29-14-8-13-22(34-16-23(30)33-2)25(29)24(17)26(31)27(28)32/h3-14H,15-16H2,1-2H3,(H2,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50085993
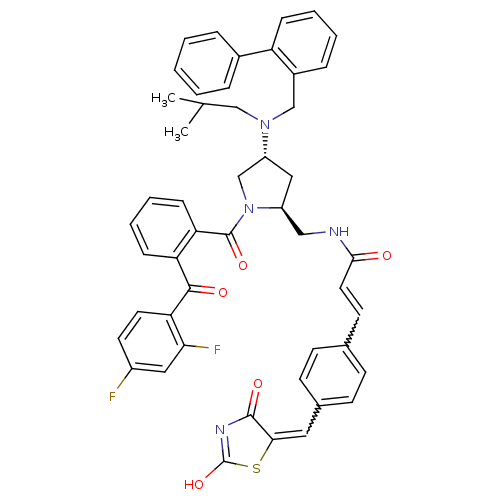
((E)-N-(((2S,4R)-4-((biphenyl-2-ylmethyl)(isobutyl)...)Show SMILES CC(C)CN(Cc1ccccc1-c1ccccc1)[C@@H]1C[C@@H](CNC(=O)C=Cc2ccc(C=C3SC(O)=NC3=O)cc2)N(C1)C(=O)c1ccccc1C(=O)c1ccc(F)cc1F |w:31.32,26.28,c:37| Show InChI InChI=1S/C49H44F2N4O5S/c1-31(2)28-54(29-35-12-6-7-13-39(35)34-10-4-3-5-11-34)38-26-37(27-52-45(56)23-20-32-16-18-33(19-17-32)24-44-47(58)53-49(60)61-44)55(30-38)48(59)41-15-9-8-14-40(41)46(57)42-22-21-36(50)25-43(42)51/h3-25,31,37-38H,26-30H2,1-2H3,(H,52,56)(H,53,58,60)/t37-,38+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) |
J Med Chem 43: 1041-4 (2000)
BindingDB Entry DOI: 10.7270/Q27P8XMZ |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50085984
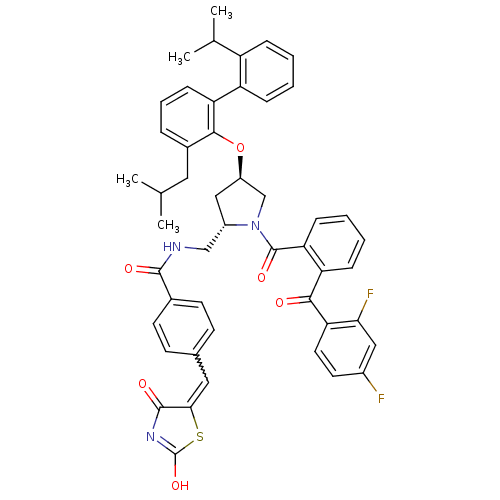
(CHEMBL9161 | N-[1-[2-(2,4-Difluoro-benzoyl)-benzoy...)Show SMILES CC(C)Cc1cccc(c1O[C@@H]1C[C@@H](CNC(=O)c2ccc(C=C3SC(O)=NC3=O)cc2)N(C1)C(=O)c1ccccc1C(=O)c1ccc(F)cc1F)-c1ccccc1C(C)C |w:22.22,c:27| Show InChI InChI=1S/C49H45F2N3O6S/c1-28(2)22-32-10-9-15-39(37-12-6-5-11-36(37)29(3)4)45(32)60-35-25-34(26-52-46(56)31-18-16-30(17-19-31)23-43-47(57)53-49(59)61-43)54(27-35)48(58)40-14-8-7-13-38(40)44(55)41-21-20-33(50)24-42(41)51/h5-21,23-24,28-29,34-35H,22,25-27H2,1-4H3,(H,52,56)(H,53,57,59)/t34-,35+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) |
J Med Chem 43: 1041-4 (2000)
BindingDB Entry DOI: 10.7270/Q27P8XMZ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50053137

((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-ethyl-indol...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C27H24N2O5/c1-2-19-21(15-18-11-6-7-12-20(18)17-9-4-3-5-10-17)29-14-8-13-22(34-16-23(30)31)25(29)24(19)26(32)27(28)33/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01808
BindingDB Entry DOI: 10.7270/Q2SF316G |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161301
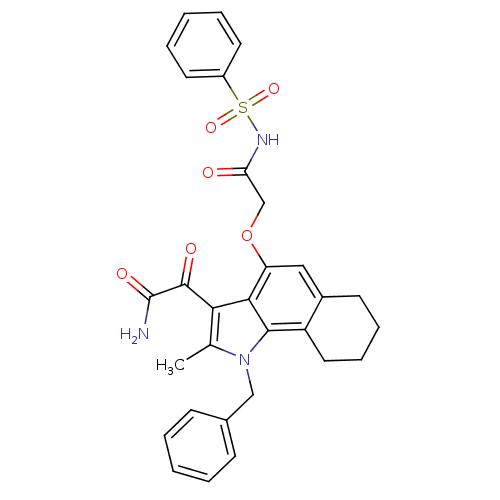
(2-[4-(2-Benzenesulfonylamino-2-oxo-ethoxy)-1-benzy...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cc3CCCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C30H29N3O6S/c1-19-26(29(35)30(31)36)27-24(39-18-25(34)32-40(37,38)22-13-6-3-7-14-22)16-21-12-8-9-15-23(21)28(27)33(19)17-20-10-4-2-5-11-20/h2-7,10-11,13-14,16H,8-9,12,15,17-18H2,1H3,(H2,31,36)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50055367
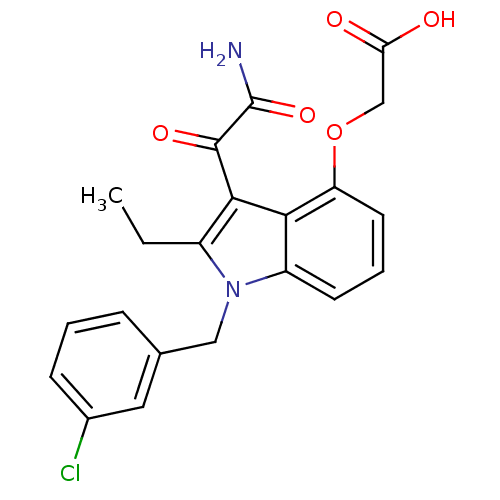
(CHEMBL345986 | [3-Aminooxalyl-1-(3-chloro-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(Cl)c1 Show InChI InChI=1S/C21H19ClN2O5/c1-2-14-19(20(27)21(23)28)18-15(7-4-8-16(18)29-11-17(25)26)24(14)10-12-5-3-6-13(22)9-12/h3-9H,2,10-11H2,1H3,(H2,23,28)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound wastested for inhibition of porcine secreted pancreatic PLA2 |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055371
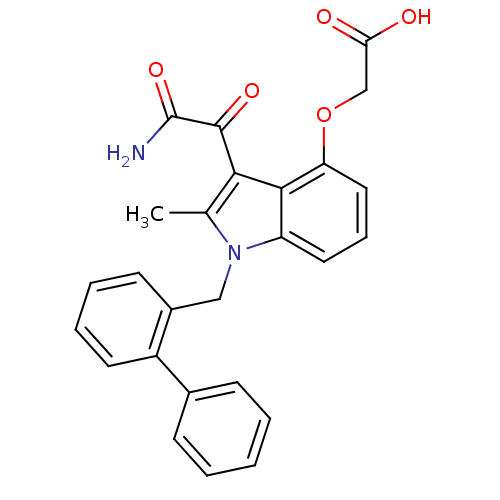
((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053106
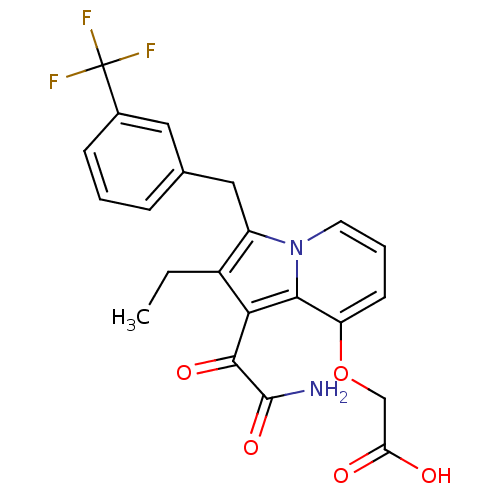
(CHEMBL120112 | [1-Aminooxalyl-2-ethyl-3-(3-trifluo...)Show SMILES CCc1c(Cc2cccc(c2)C(F)(F)F)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C22H19F3N2O5/c1-2-14-15(10-12-5-3-6-13(9-12)22(23,24)25)27-8-4-7-16(32-11-17(28)29)19(27)18(14)20(30)21(26)31/h3-9H,2,10-11H2,1H3,(H2,26,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053108
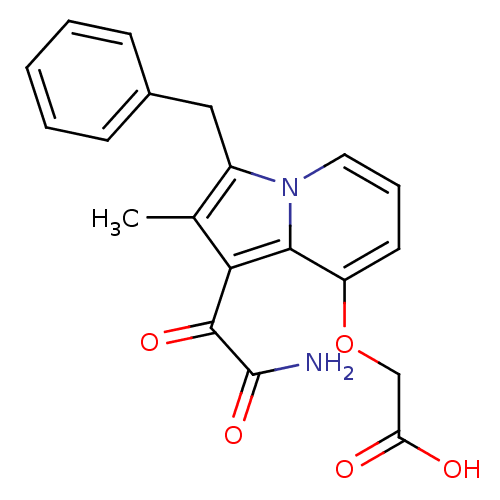
((1-Aminooxalyl-3-benzyl-2-methyl-indolizin-8-yloxy...)Show SMILES Cc1c(Cc2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C20H18N2O5/c1-12-14(10-13-6-3-2-4-7-13)22-9-5-8-15(27-11-16(23)24)18(22)17(12)19(25)20(21)26/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053137

((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-ethyl-indol...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C27H24N2O5/c1-2-19-21(15-18-11-6-7-12-20(18)17-9-4-3-5-10-17)29-14-8-13-22(34-16-23(30)31)25(29)24(19)26(32)27(28)33/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053102
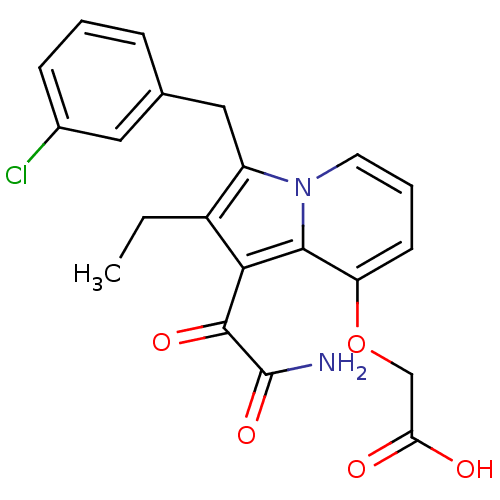
(CHEMBL121245 | [1-Aminooxalyl-3-(3-chloro-benzyl)-...)Show SMILES CCc1c(Cc2cccc(Cl)c2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C21H19ClN2O5/c1-2-14-15(10-12-5-3-6-13(22)9-12)24-8-4-7-16(29-11-17(25)26)19(24)18(14)20(27)21(23)28/h3-9H,2,10-11H2,1H3,(H2,23,28)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50085986

(CHEMBL267258 | N-{4-(Biphenyl-2-ylmethyl-isobutyl-...)Show SMILES CC(C)CN(Cc1ccccc1-c1ccccc1)[C@@H]1C[C@@H](CNC(=O)C=Cc2ccc(C=C3SC(O)=NC3=O)cc2)N(C1)C(=O)c1ccccc1C(=O)c1ccc(F)cc1 |w:31.32,26.28,c:37| Show InChI InChI=1S/C49H45FN4O5S/c1-32(2)29-53(30-37-12-6-7-13-41(37)35-10-4-3-5-11-35)40-27-39(28-51-45(55)25-20-33-16-18-34(19-17-33)26-44-47(57)52-49(59)60-44)54(31-40)48(58)43-15-9-8-14-42(43)46(56)36-21-23-38(50)24-22-36/h3-26,32,39-40H,27-31H2,1-2H3,(H,51,55)(H,52,57,59)/t39-,40+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) |
J Med Chem 43: 1041-4 (2000)
BindingDB Entry DOI: 10.7270/Q27P8XMZ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161305
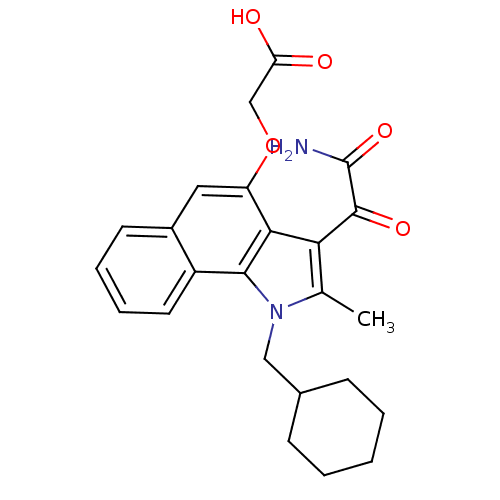
((3-Aminooxalyl-1-cyclohexylmethyl-2-methyl-2,3-dih...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1CC1CCCCC1 Show InChI InChI=1S/C24H26N2O5/c1-14-20(23(29)24(25)30)21-18(31-13-19(27)28)11-16-9-5-6-10-17(16)22(21)26(14)12-15-7-3-2-4-8-15/h5-6,9-11,15H,2-4,7-8,12-13H2,1H3,(H2,25,30)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2 beta
(Homo sapiens (Human)) | BDBM50110881
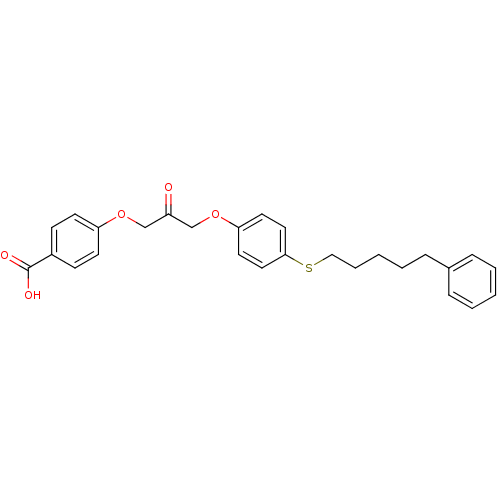
(4-(2-oxo-3-(4-(5-phenylpentylthio)phenoxy)propoxy)...)Show SMILES OC(=O)c1ccc(OCC(=O)COc2ccc(SCCCCCc3ccccc3)cc2)cc1 Show InChI InChI=1S/C27H28O5S/c28-23(19-31-24-12-10-22(11-13-24)27(29)30)20-32-25-14-16-26(17-15-25)33-18-6-2-5-9-21-7-3-1-4-8-21/h1,3-4,7-8,10-17H,2,5-6,9,18-20H2,(H,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood
Curated by ChEMBL
| Assay Description
Inhibitory activity against cytosolic Phospholipase A2 (PLA2) by bilayer assay |
J Med Chem 45: 1348-62 (2002)
BindingDB Entry DOI: 10.7270/Q2F76BVP |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055374
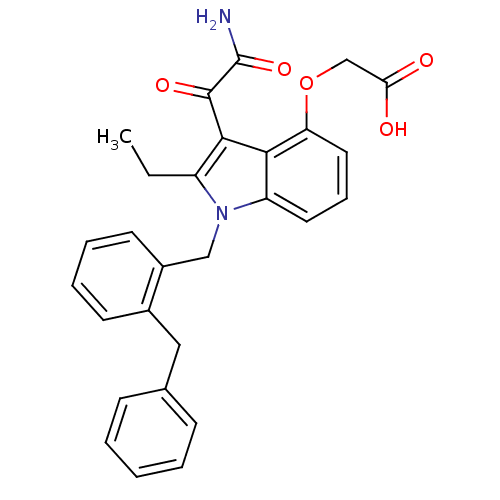
(CHEMBL436456 | [3-Aminooxalyl-1-(2-benzyl-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1Cc1ccccc1 Show InChI InChI=1S/C28H26N2O5/c1-2-21-26(27(33)28(29)34)25-22(13-8-14-23(25)35-17-24(31)32)30(21)16-20-12-7-6-11-19(20)15-18-9-4-3-5-10-18/h3-14H,2,15-17H2,1H3,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055374

(CHEMBL436456 | [3-Aminooxalyl-1-(2-benzyl-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1Cc1ccccc1 Show InChI InChI=1S/C28H26N2O5/c1-2-21-26(27(33)28(29)34)25-22(13-8-14-23(25)35-17-24(31)32)30(21)16-20-12-7-6-11-19(20)15-18-9-4-3-5-10-18/h3-14H,2,15-17H2,1H3,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053114

((1-Aminooxalyl-3-biphenyl-3-ylmethyl-2-ethyl-indol...)Show SMILES CCc1c(Cc2cccc(c2)-c2ccccc2)n2cccc(OCC(=O)OC)c2c1C(=O)C(N)=O Show InChI InChI=1S/C28H26N2O5/c1-3-21-22(16-18-9-7-12-20(15-18)19-10-5-4-6-11-19)30-14-8-13-23(35-17-24(31)34-2)26(30)25(21)27(32)28(29)33/h4-15H,3,16-17H2,1-2H3,(H2,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2 gamma
(Homo sapiens (Human)) | BDBM50097334
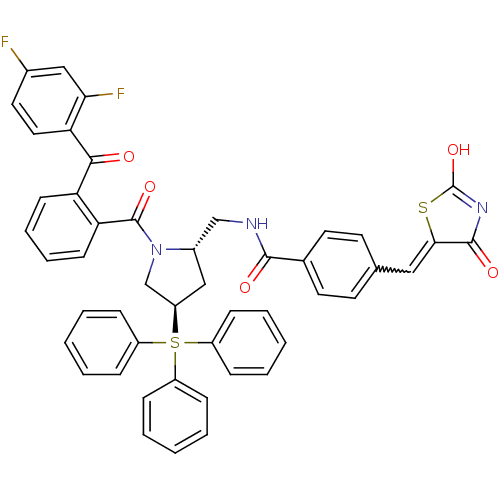
(CHEMBL385730 | N-[(2S,4R)-1-[2-(2,4-Difluoro-benzo...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(cc1)C(=O)NC[C@@H]1C[C@H](CN1C(=O)c1ccccc1C(=O)c1ccc(F)cc1F)S(c1ccccc1)(c1ccccc1)c1ccccc1 |w:7.8,t:1| Show InChI InChI=1S/C48H37F2N3O5S2/c49-33-24-25-41(42(50)27-33)44(54)39-18-10-11-19-40(39)47(57)53-30-38(60(35-12-4-1-5-13-35,36-14-6-2-7-15-36)37-16-8-3-9-17-37)28-34(53)29-51-45(55)32-22-20-31(21-23-32)26-43-46(56)52-48(58)59-43/h1-27,34,38H,28-30H2,(H,51,55)(H,52,56,58)/t34-,38+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit human Cytosolic phospholipase A2 |
Bioorg Med Chem Lett 11: 587-90 (2001)
BindingDB Entry DOI: 10.7270/Q2ST7P4R |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50185354

(3-methylhydrogen 1-[3-(4-decyloxyphenoxy)-2-oxopro...)Show SMILES CCCCCCCCCCOc1ccc(OCC(=O)Cn2cc(C(=O)OC)c3cc(ccc23)C(O)=O)cc1 Show InChI InChI=1S/C30H37NO7/c1-3-4-5-6-7-8-9-10-17-37-24-12-14-25(15-13-24)38-21-23(32)19-31-20-27(30(35)36-2)26-18-22(29(33)34)11-16-28(26)31/h11-16,18,20H,3-10,17,19,21H2,1-2H3,(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Münster
Curated by ChEMBL
| Assay Description
Inhibition of human isolated cPLA2alpha by vescicle assay |
J Med Chem 49: 2611-20 (2006)
Article DOI: 10.1021/jm051243a
BindingDB Entry DOI: 10.7270/Q2668CSF |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50185348

(3-(methoxycarbonyl)-1-(3-(4-octylphenoxy)-2-oxopro...)Show SMILES CCCCCCCCc1ccc(OCC(=O)Cn2cc(C(=O)OC)c3cc(ccc23)C(O)=O)cc1 Show InChI InChI=1S/C28H33NO6/c1-3-4-5-6-7-8-9-20-10-13-23(14-11-20)35-19-22(30)17-29-18-25(28(33)34-2)24-16-21(27(31)32)12-15-26(24)29/h10-16,18H,3-9,17,19H2,1-2H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Münster
Curated by ChEMBL
| Assay Description
Inhibition of human isolated cPLA2alpha by vescicle assay |
J Med Chem 49: 2611-20 (2006)
Article DOI: 10.1021/jm051243a
BindingDB Entry DOI: 10.7270/Q2668CSF |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50277800

(1-(3-(4-octylphenoxy)-2-oxopropyl)-1H-indazole-5-c...)Show SMILES CCCCCCCCc1ccc(OCC(=O)Cn2ncc3cc(ccc23)C(O)=O)cc1 Show InChI InChI=1S/C25H30N2O4/c1-2-3-4-5-6-7-8-19-9-12-23(13-10-19)31-18-22(28)17-27-24-14-11-20(25(29)30)15-21(24)16-26-27/h9-16H,2-8,17-18H2,1H3,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of M£nster
Curated by ChEMBL
| Assay Description
Inhibition of cPLA2 in human platelets assessed as arachidonic acid release |
Bioorg Med Chem Lett 19: 2107-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.019
BindingDB Entry DOI: 10.7270/Q26H4JB8 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50601472
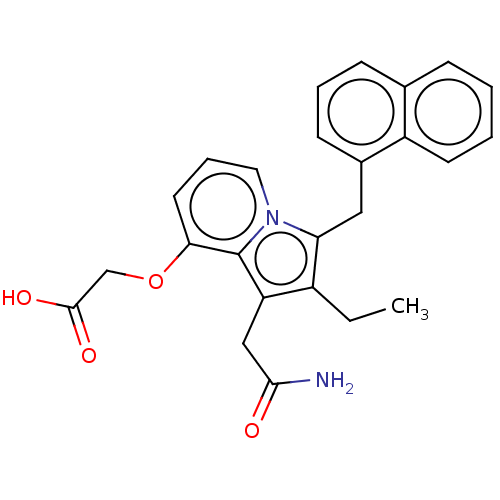
(CHEMBL5208632)Show SMILES CCc1c(CC(N)=O)c2c(OCC(O)=O)cccn2c1Cc1cccc2ccccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01808
BindingDB Entry DOI: 10.7270/Q2SF316G |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50185348

(3-(methoxycarbonyl)-1-(3-(4-octylphenoxy)-2-oxopro...)Show SMILES CCCCCCCCc1ccc(OCC(=O)Cn2cc(C(=O)OC)c3cc(ccc23)C(O)=O)cc1 Show InChI InChI=1S/C28H33NO6/c1-3-4-5-6-7-8-9-20-10-13-23(14-11-20)35-19-22(30)17-29-18-25(28(33)34-2)24-16-21(27(31)32)12-15-26(24)29/h10-16,18H,3-9,17,19H2,1-2H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of M£nster
Curated by ChEMBL
| Assay Description
Inhibition of cPLA2 from human platelets by RP-HPLC |
Bioorg Med Chem 18: 945-52 (2010)
Article DOI: 10.1016/j.bmc.2009.11.028
BindingDB Entry DOI: 10.7270/Q2N016MF |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50277800

(1-(3-(4-octylphenoxy)-2-oxopropyl)-1H-indazole-5-c...)Show SMILES CCCCCCCCc1ccc(OCC(=O)Cn2ncc3cc(ccc23)C(O)=O)cc1 Show InChI InChI=1S/C25H30N2O4/c1-2-3-4-5-6-7-8-19-9-12-23(13-10-19)31-18-22(28)17-27-24-14-11-20(25(29)30)15-21(24)16-26-27/h9-16H,2-8,17-18H2,1H3,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of M£nster
Curated by ChEMBL
| Assay Description
Inhibition of cPLA2 from human platelets by RP-HPLC |
Bioorg Med Chem 18: 945-52 (2010)
Article DOI: 10.1016/j.bmc.2009.11.028
BindingDB Entry DOI: 10.7270/Q2N016MF |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053133
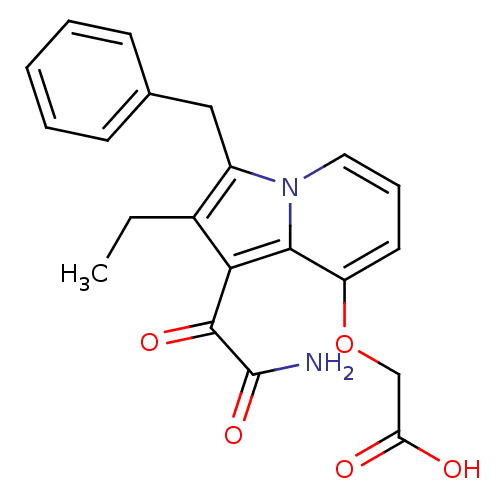
((1-Aminooxalyl-3-benzyl-2-ethyl-indolizin-8-yloxy)...)Show SMILES CCc1c(Cc2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C21H20N2O5/c1-2-14-15(11-13-7-4-3-5-8-13)23-10-6-9-16(28-12-17(24)25)19(23)18(14)20(26)21(22)27/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50185348

(3-(methoxycarbonyl)-1-(3-(4-octylphenoxy)-2-oxopro...)Show SMILES CCCCCCCCc1ccc(OCC(=O)Cn2cc(C(=O)OC)c3cc(ccc23)C(O)=O)cc1 Show InChI InChI=1S/C28H33NO6/c1-3-4-5-6-7-8-9-20-10-13-23(14-11-20)35-19-22(30)17-29-18-25(28(33)34-2)24-16-21(27(31)32)12-15-26(24)29/h10-16,18H,3-9,17,19H2,1-2H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Münster
Curated by ChEMBL
| Assay Description
Inhibition of human platelet cPLA2-alpha assessed as arachidonic acid release by vesicle assay |
Bioorg Med Chem 15: 2883-91 (2007)
Article DOI: 10.1016/j.bmc.2007.02.016
BindingDB Entry DOI: 10.7270/Q24749JP |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053096

(CHEMBL123774 | [1-Aminooxalyl-3-(4-butyl-benzyl)-2...)Show SMILES CCCCc1ccc(Cc2c(CC)c(C(=O)C(N)=O)c3c(OCC(O)=O)cccn23)cc1 Show InChI InChI=1S/C25H28N2O5/c1-3-5-7-16-9-11-17(12-10-16)14-19-18(4-2)22(24(30)25(26)31)23-20(32-15-21(28)29)8-6-13-27(19)23/h6,8-13H,3-5,7,14-15H2,1-2H3,(H2,26,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053123
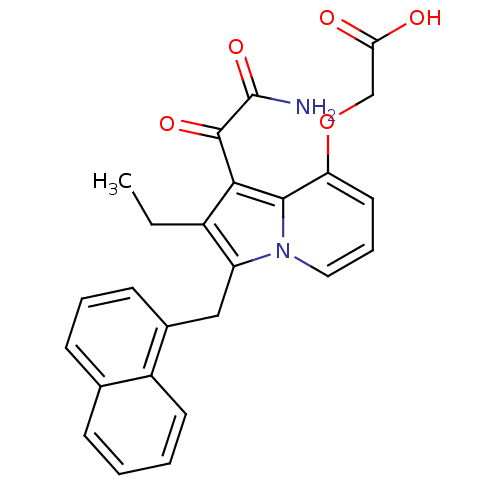
((1-Aminooxalyl-2-ethyl-3-naphthalen-1-ylmethyl-ind...)Show SMILES CCc1c(Cc2cccc3ccccc23)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C25H22N2O5/c1-2-17-19(13-16-9-5-8-15-7-3-4-10-18(15)16)27-12-6-11-20(32-14-21(28)29)23(27)22(17)24(30)25(26)31/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human secretory phospholipase A2 (sPLA2), chromogenic screening assay. |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50085987

(4-(2,4-Dioxo-thiazolidin-5-ylidenemethyl)-N-[1-[2-...)Show SMILES CC(C)Cc1cccc(c1O[C@@H]1C[C@@H](CNC(=O)c2ccc(C=C3SC(O)=NC3=O)cc2)N(C1)C(=O)c1ccccc1C(=O)c1ccc(F)cc1)-c1ccccc1C(C)C |w:22.22,c:27| Show InChI InChI=1S/C49H46FN3O6S/c1-29(2)24-34-10-9-15-41(39-12-6-5-11-38(39)30(3)4)45(34)59-37-26-36(27-51-46(55)33-18-16-31(17-19-33)25-43-47(56)52-49(58)60-43)53(28-37)48(57)42-14-8-7-13-40(42)44(54)32-20-22-35(50)23-21-32/h5-23,25,29-30,36-37H,24,26-28H2,1-4H3,(H,51,55)(H,52,56,58)/t36-,37+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) |
J Med Chem 43: 1041-4 (2000)
BindingDB Entry DOI: 10.7270/Q27P8XMZ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055371

((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01808
BindingDB Entry DOI: 10.7270/Q2SF316G |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053102

(CHEMBL121245 | [1-Aminooxalyl-3-(3-chloro-benzyl)-...)Show SMILES CCc1c(Cc2cccc(Cl)c2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C21H19ClN2O5/c1-2-14-15(10-12-5-3-6-13(22)9-12)24-8-4-7-16(29-11-17(25)26)19(24)18(14)20(27)21(23)28/h3-9H,2,10-11H2,1H3,(H2,23,28)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human secretory phospholipase A2 (sPLA2), chromogenic screening assay. |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50161293
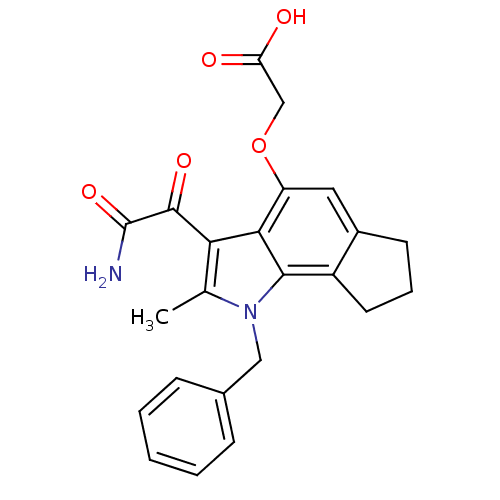
((3-Aminooxalyl-1-benzyl-2-methyl-1,6,7,8-tetrahydr...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3CCCc3c2n1Cc1ccccc1 Show InChI InChI=1S/C23H22N2O5/c1-13-19(22(28)23(24)29)20-17(30-12-18(26)27)10-15-8-5-9-16(15)21(20)25(13)11-14-6-3-2-4-7-14/h2-4,6-7,10H,5,8-9,11-12H2,1H3,(H2,24,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human nonpancreatic secretory phospholipase A2 |
J Med Chem 48: 893-6 (2005)
Article DOI: 10.1021/jm0401309
BindingDB Entry DOI: 10.7270/Q20001M3 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50053137

((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-ethyl-indol...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C27H24N2O5/c1-2-19-21(15-18-11-6-7-12-20(18)17-9-4-3-5-10-17)29-14-8-13-22(34-16-23(30)31)25(29)24(19)26(32)27(28)33/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01808
BindingDB Entry DOI: 10.7270/Q2SF316G |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053137

((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-ethyl-indol...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C27H24N2O5/c1-2-19-21(15-18-11-6-7-12-20(18)17-9-4-3-5-10-17)29-14-8-13-22(34-16-23(30)31)25(29)24(19)26(32)27(28)33/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human secretory phospholipase A2 (sPLA2), chromogenic screening assay. |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50292791

(4-{3-[2-{2-[({2-[(4-Acetylpiperazin-1-yl)methyl]be...)Show SMILES CC(=O)N1CCN(Cc2ccccc2CS(=O)(=O)NCCc2c(CCCc3ccc(cc3)C(O)=O)c3cc(Cl)ccc3n2C(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C47H49ClN4O5S/c1-34(53)51-29-27-50(28-30-51)32-39-16-8-9-17-40(39)33-58(56,57)49-26-25-45-42(18-10-11-35-19-21-38(22-20-35)47(54)55)43-31-41(48)23-24-44(43)52(45)46(36-12-4-2-5-13-36)37-14-6-3-7-15-37/h2-9,12-17,19-24,31,46,49H,10-11,18,25-30,32-33H2,1H3,(H,54,55) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human cytosolic PLA2alpha by GLU micelle assay |
J Med Chem 52: 1156-71 (2009)
Article DOI: 10.1021/jm8009876
BindingDB Entry DOI: 10.7270/Q20C4VSK |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055401

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-ethyl-1H-in...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H24N2O5/c1-2-20-25(26(32)27(28)33)24-21(13-8-14-22(24)34-16-23(30)31)29(20)15-18-11-6-7-12-19(18)17-9-4-3-5-10-17/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055387

((R)-2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-...)Show SMILES C[C@@H](Oc1cccc2n(Cc3ccccc3)c(C)c(C(=O)C(N)=O)c12)C(O)=O Show InChI InChI=1S/C21H20N2O5/c1-12-17(19(24)20(22)25)18-15(23(12)11-14-7-4-3-5-8-14)9-6-10-16(18)28-13(2)21(26)27/h3-10,13H,11H2,1-2H3,(H2,22,25)(H,26,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055371

((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055379

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-cyclopropyl...)Show SMILES NC(=O)C(=O)c1c(C2CC2)n(Cc2ccccc2-c2ccccc2)c2cccc(OCC(O)=O)c12 Show InChI InChI=1S/C28H24N2O5/c29-28(34)27(33)25-24-21(11-6-12-22(24)35-16-23(31)32)30(26(25)18-13-14-18)15-19-9-4-5-10-20(19)17-7-2-1-3-8-17/h1-12,18H,13-16H2,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055401

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-ethyl-1H-in...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H24N2O5/c1-2-20-25(26(32)27(28)33)24-21(13-8-14-22(24)34-16-23(30)31)29(20)15-18-11-6-7-12-19(18)17-9-4-3-5-10-17/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055378

(CHEMBL345488 | [3-Aminooxalyl-1-(2,6-dichloro-benz...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1c(Cl)cccc1Cl Show InChI InChI=1S/C20H16Cl2N2O5/c1-10-17(19(27)20(23)28)18-14(6-3-7-15(18)29-9-16(25)26)24(10)8-11-12(21)4-2-5-13(11)22/h2-7H,8-9H2,1H3,(H2,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053109

(CHEMBL331755 | [1-Aminooxalyl-3-(2-benzyl-benzyl)-...)Show SMILES CCc1c(Cc2ccccc2Cc2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C28H26N2O5/c1-2-21-22(16-20-12-7-6-11-19(20)15-18-9-4-3-5-10-18)30-14-8-13-23(35-17-24(31)32)26(30)25(21)27(33)28(29)34/h3-14H,2,15-17H2,1H3,(H2,29,34)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human secretory phospholipase A2 (sPLA2), chromogenic screening assay. |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50053147
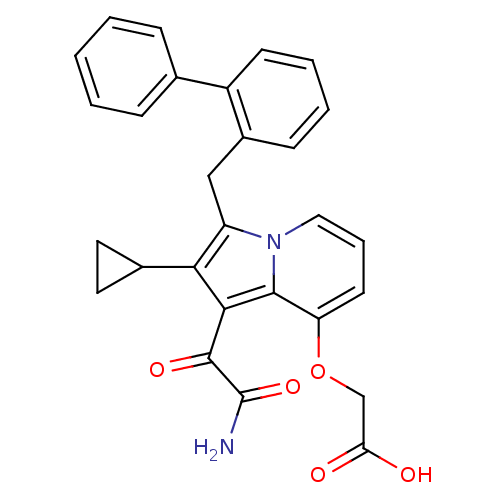
((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-cyclopropyl...)Show SMILES NC(=O)C(=O)c1c(C2CC2)c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c12 Show InChI InChI=1S/C28H24N2O5/c29-28(34)27(33)25-24(18-12-13-18)21(30-14-6-11-22(26(25)30)35-16-23(31)32)15-19-9-4-5-10-20(19)17-7-2-1-3-8-17/h1-11,14,18H,12-13,15-16H2,(H2,29,34)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human secretory phospholipase A2 (s-PLA2) by phosphatidylcholine/deoxycholate assay (PC/DOC). |
J Med Chem 39: 3636-58 (1996)
Article DOI: 10.1021/jm960395q
BindingDB Entry DOI: 10.7270/Q2NC609M |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50085994
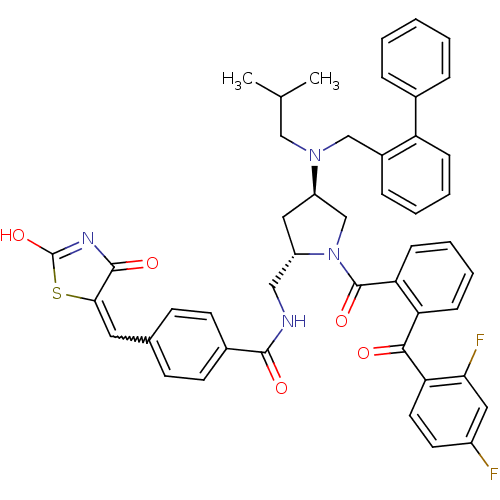
(CHEMBL8970 | N-{4-(Biphenyl-2-ylmethyl-isobutyl-am...)Show SMILES CC(C)CN(Cc1ccccc1-c1ccccc1)[C@@H]1C[C@@H](CNC(=O)c2ccc(C=C3SC(O)=NC3=O)cc2)N(C1)C(=O)c1ccccc1C(=O)c1ccc(F)cc1F |w:29.30,c:35| Show InChI InChI=1S/C47H42F2N4O5S/c1-29(2)26-52(27-33-12-6-7-13-37(33)31-10-4-3-5-11-31)36-24-35(25-50-44(55)32-18-16-30(17-19-32)22-42-45(56)51-47(58)59-42)53(28-36)46(57)39-15-9-8-14-38(39)43(54)40-21-20-34(48)23-41(40)49/h3-23,29,35-36H,24-28H2,1-2H3,(H,50,55)(H,51,56,58)/t35-,36+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Human cPLA2 alpha using Enzyme assay(PC/DOG assay) |
J Med Chem 43: 1041-4 (2000)
BindingDB Entry DOI: 10.7270/Q27P8XMZ |
More data for this
Ligand-Target Pair | |
Cytosolic phospholipase A2
(Homo sapiens (Human)) | BDBM50185351
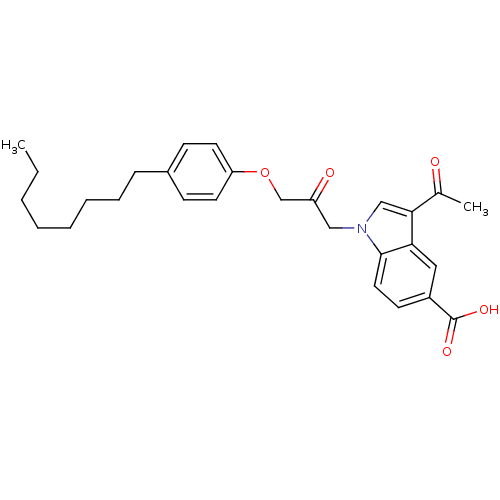
(3-acetyl-1-(3-(4-octylphenoxy)-2-oxopropyl)-1H-ind...)Show SMILES CCCCCCCCc1ccc(OCC(=O)Cn2cc(C(C)=O)c3cc(ccc23)C(O)=O)cc1 Show InChI InChI=1S/C28H33NO5/c1-3-4-5-6-7-8-9-21-10-13-24(14-11-21)34-19-23(31)17-29-18-26(20(2)30)25-16-22(28(32)33)12-15-27(25)29/h10-16,18H,3-9,17,19H2,1-2H3,(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Münster
Curated by ChEMBL
| Assay Description
Inhibition of cPLA2-alpha activity assessed by TPA-induced arachidonic acid release in human platelet |
J Med Chem 49: 2611-20 (2006)
Article DOI: 10.1021/jm051243a
BindingDB Entry DOI: 10.7270/Q2668CSF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data