

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11006 (1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11016 ((-)-(S)4 | (5S)-5-(4-chlorophenyl)-3-(4-fluorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11005 (1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11007 (1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 6 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15586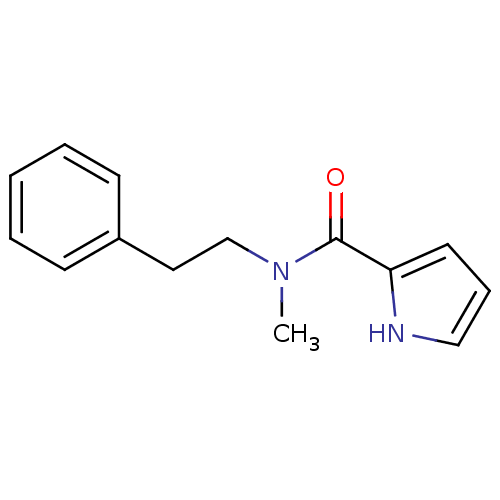 (N-(2-Phenylethyl),N-methyl-1H-pyrrole-2-carboxamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 7 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11008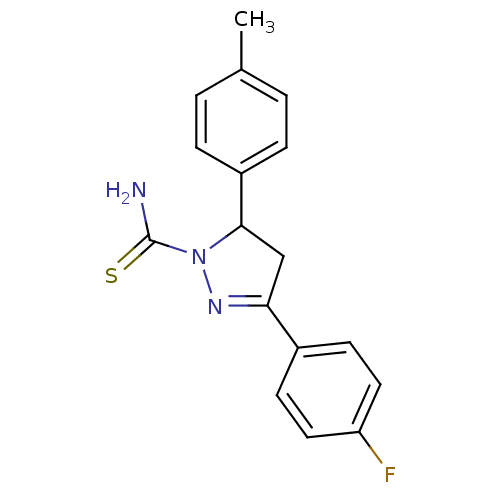 (1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 7 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11009 (1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11017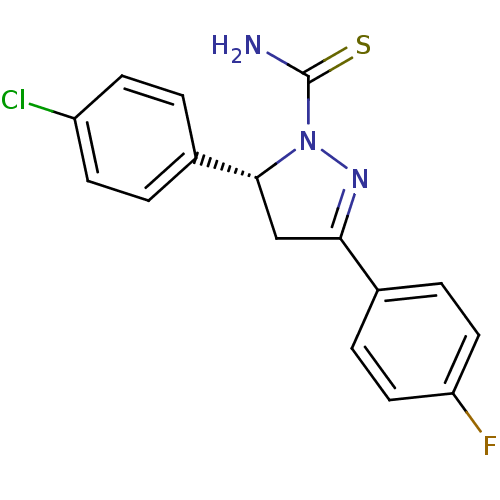 ((+)-(R)4 | (5R)-5-(4-chlorophenyl)-3-(4-fluorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 12 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11015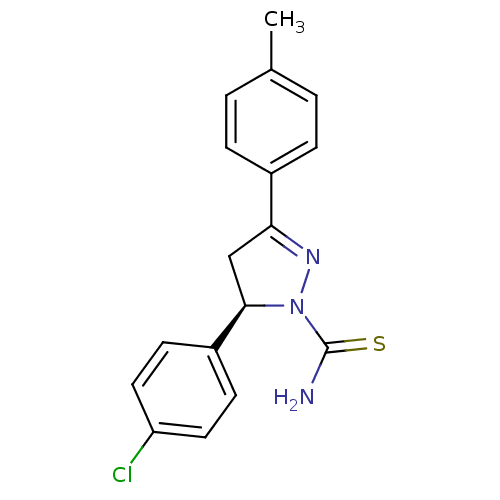 ((+)-(R)1 | (5R)-5-(4-chlorophenyl)-3-(4-methylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 13 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15595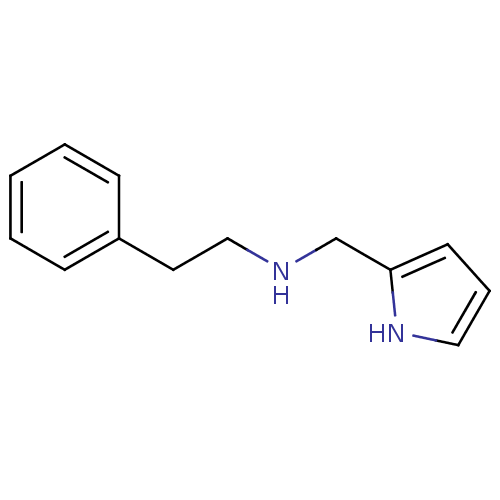 ((2-phenylethyl)(1H-pyrrol-2-ylmethyl)amine | N-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15593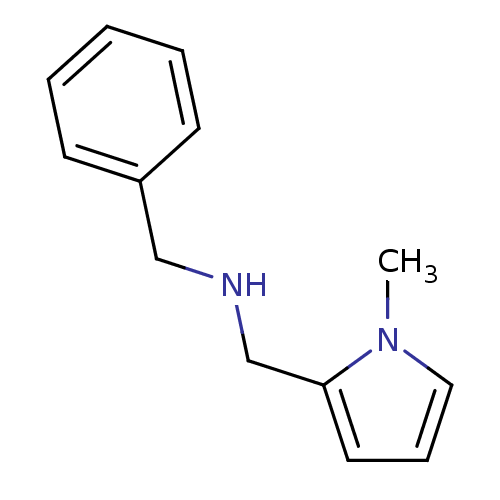 (CHEMBL223300 | N-(2-Benzyl),N-(1-methylpyrrol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11013 (1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 27 | -45.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11004 (1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 31 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15588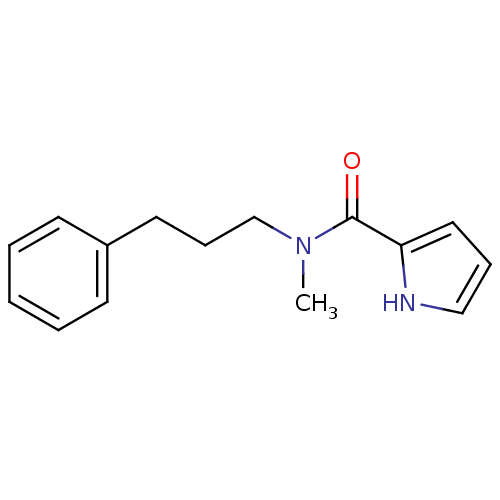 (N-(3-Phenylpropyl),N-methyl-1H-pyrrole-2-carboxami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 40 | -44.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11010 (1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 41 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11014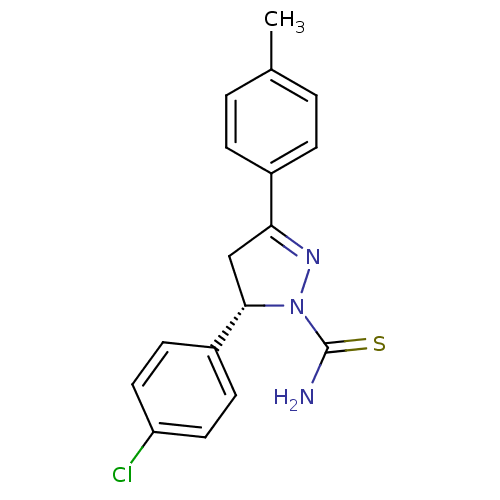 ((-)-(S)1 | (5S)-5-(4-chlorophenyl)-3-(4-methylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 50 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11012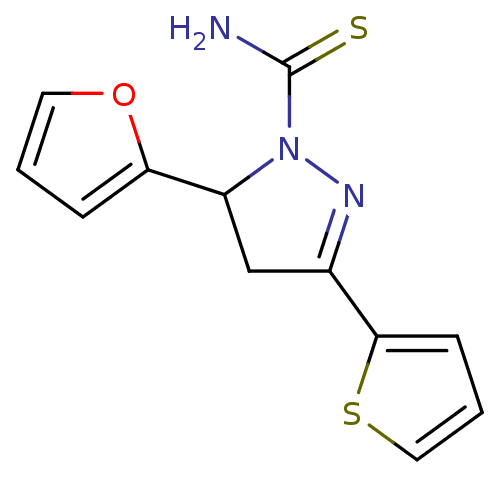 (1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 50 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM11011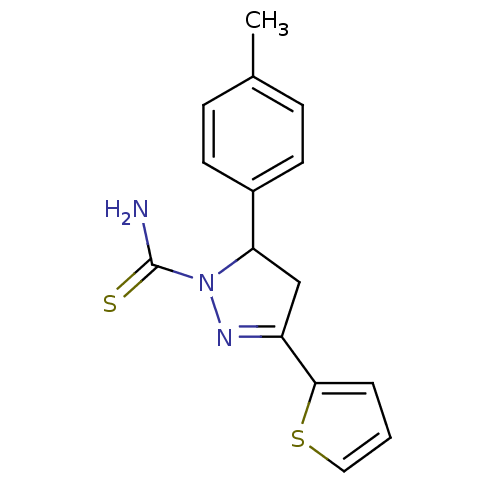 (1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-(1H)-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 50 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita degli Studi di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kynuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 48: 7113-22 (2005) Article DOI: 10.1021/jm040903t BindingDB Entry DOI: 10.7270/Q2JH3JDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15597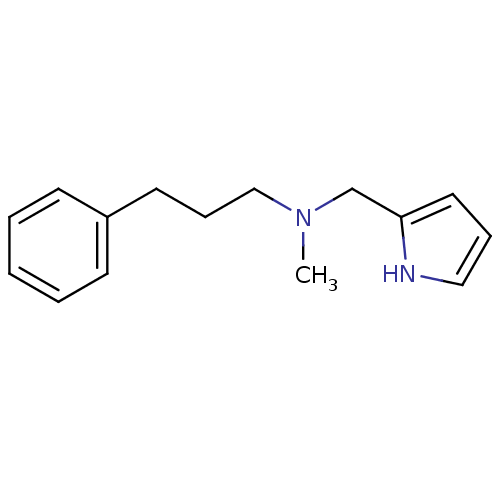 (N-Methyl,N-(3-phenylpropyl),N-(pyrrol-2-ylmethyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 50 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15596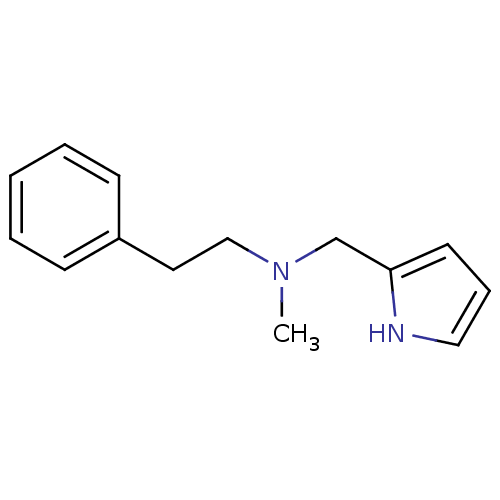 (N-Methyl,N-(2-phenylethylyl),N-(pyrrol-2-ylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 50 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 54 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15589 (N-(4-Phenylbutyl)-1H-pyrrole-2-carboxamide | pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 55 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15590 (N-(4-Phenylbutyl),N-methyl 1H-pyrrole-2-carboxamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 90 | -42.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15598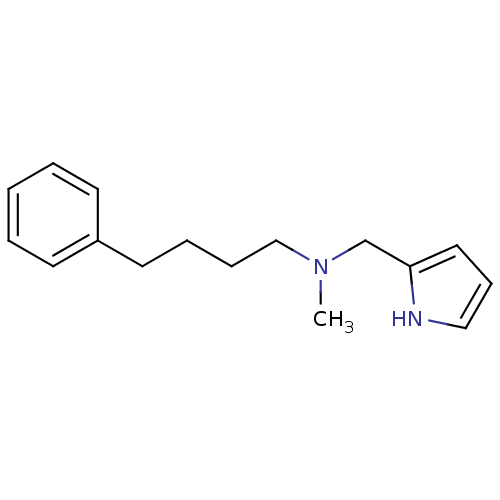 (N-Methyl,N-(3-phenylbutyl),N-(pyrrol-2-ylmethyl)am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 100 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15594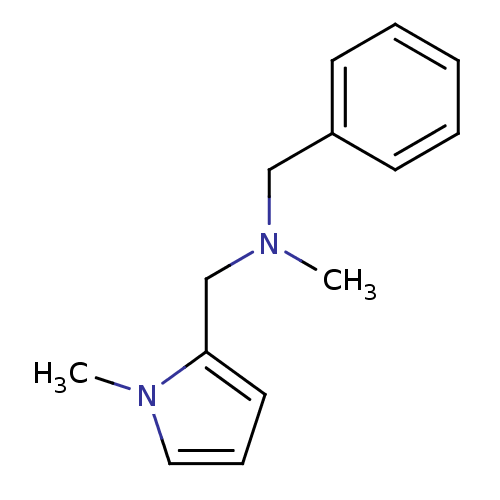 (N-Methyl,N-(benzy),N-(1-methylpyrrol-2-ylmethyl)am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 150 | -40.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15583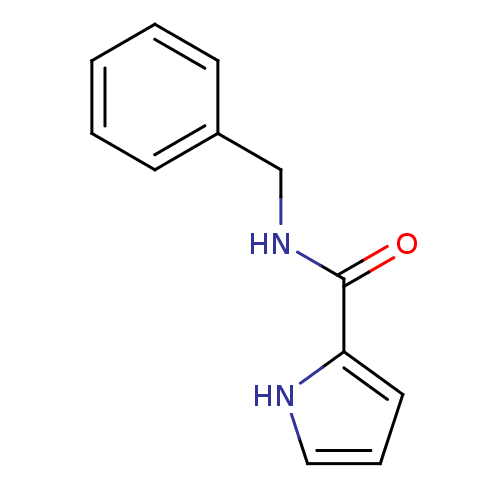 (CHEMBL174040 | N-Benzyl-1H-pyrrole-2-carboxamide |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15591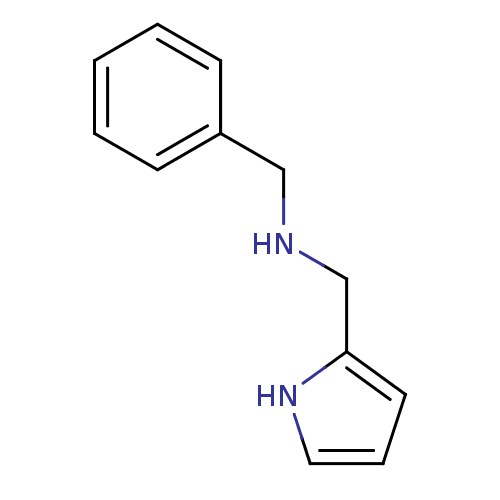 (N-(Benzyl),N-(pyrrol-2-ylmethyl)amine | benzyl(1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 350 | -38.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15582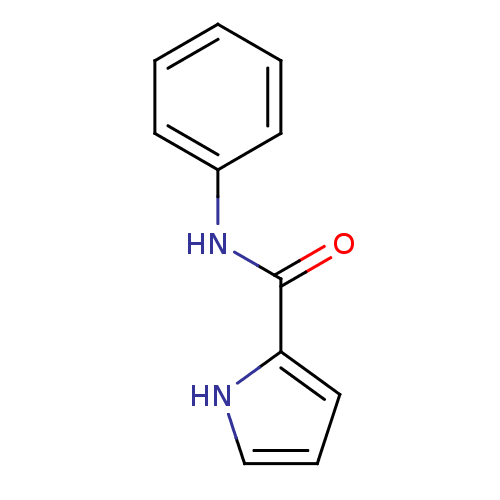 (N-Phenyl-1H-pyrrole-2-carboxamide | pyrrole inhibi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 400 | -38.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15585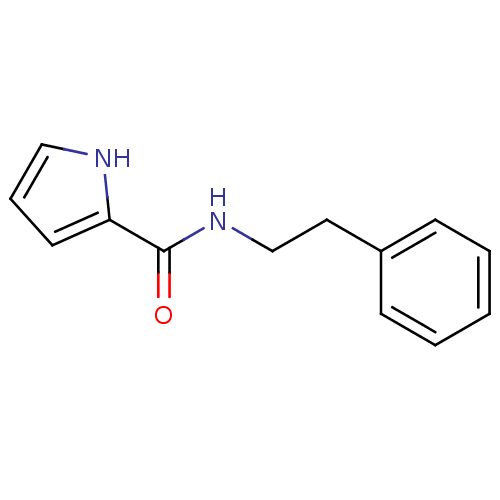 (N-(2-phenylethyl)-1H-pyrrole-2-carboxamide | N-2-P...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 420 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15584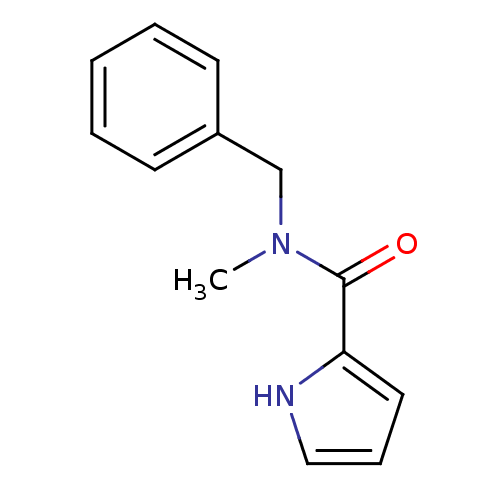 (CHEMBL352538 | N-Benzyl,N-methyl-1H-pyrrole-2-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15587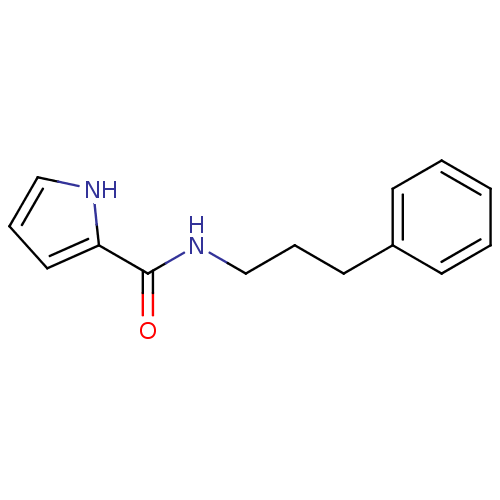 (N-(3-Phenylpropyl)-1H-pyrrole-2-carboxamide | pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 700 | -36.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15592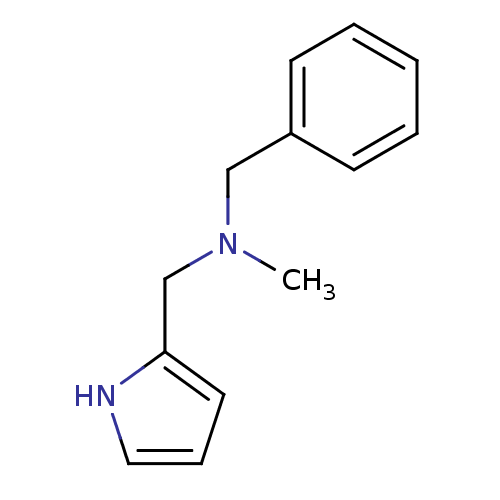 (CHEMBL374335 | N-Methyl,N-(benzyl),N-(pyrrol-2-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Bos taurus) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 38 |
Universita di Roma La Sapienza | Assay Description MAO A and MAO B activities were determined spectrophotometrically using kinuramine as substrates. Fluorimetric measurements were recorded with a Perk... | J Med Chem 50: 922-31 (2007) Article DOI: 10.1021/jm060882y BindingDB Entry DOI: 10.7270/Q2GH9G61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM153654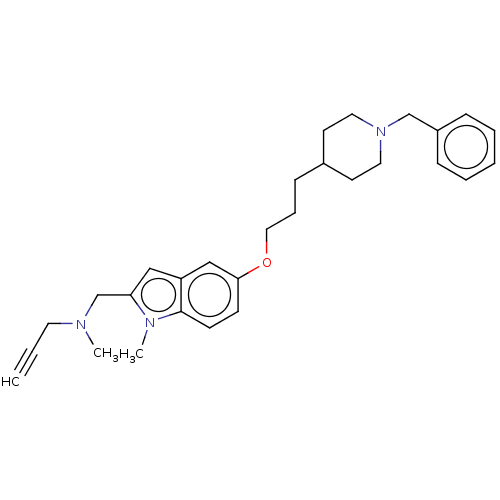 (US8999994, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Consejo Superior de Investigaciones Cientificas; Universitat Autonoma de Barcelona; Universidad de Barcelona US Patent | Assay Description The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi... | US Patent US8999994 (2015) BindingDB Entry DOI: 10.7270/Q2NZ86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM153655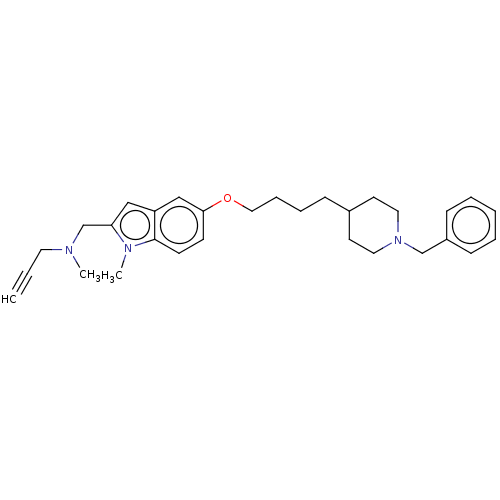 (US8999994, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Consejo Superior de Investigaciones Cientificas; Universitat Autonoma de Barcelona; Universidad de Barcelona US Patent | Assay Description The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi... | US Patent US8999994 (2015) BindingDB Entry DOI: 10.7270/Q2NZ86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 8.55E+8 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Consejo Superior de Investigaciones Cientificas; Universitat Autonoma de Barcelona; Universidad de Barcelona US Patent | Assay Description The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi... | US Patent US8999994 (2015) BindingDB Entry DOI: 10.7270/Q2NZ86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 4.03E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Consejo Superior de Investigaciones Cientificas; Universitat Autonoma de Barcelona; Universidad de Barcelona US Patent | Assay Description The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi... | US Patent US8999994 (2015) BindingDB Entry DOI: 10.7270/Q2NZ86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203828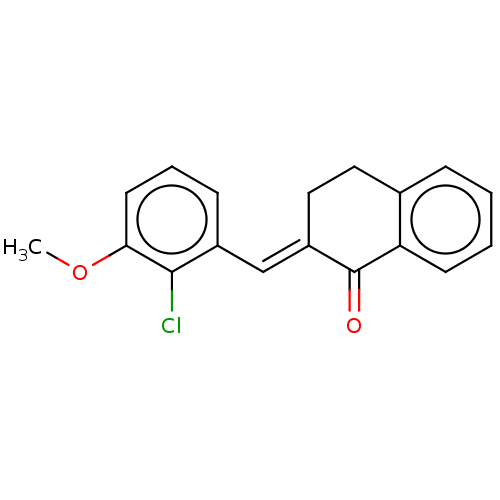 ((2E)-2-[(3-methoxy-2-methylphenyl)methylidene]-1,2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.04E+4 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203829 ((2E)-2-[(3,4-dimethoxy-2-methylphenyl)methylidene]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.69E+4 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203830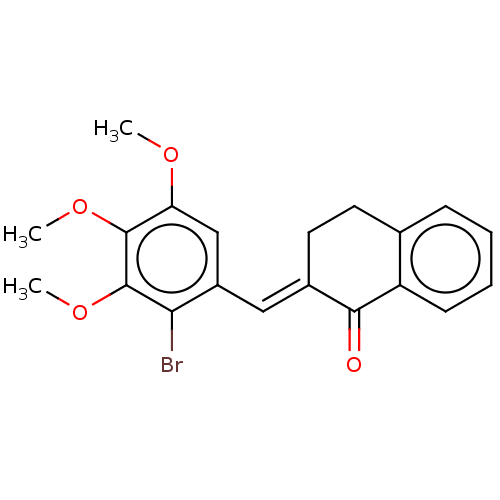 ((2E)-2-[(2-bromo-3,4,5-trimethoxyphenyl)methyliden...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.73E+4 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203831 ((2E)-2-[(2-chloro-3-methoxyphenyl)methylidene]-4-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203832 ((2E)-2-[(2-chloro-3,4-dimethoxyphenyl)methylidene]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203833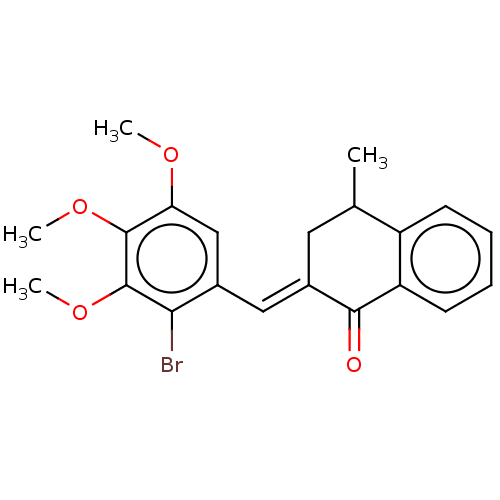 (2-(2-Bromo-3,4,5-trimethoxy-benzylidene)-4-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203834 ((2E)-2-[(2-chloro-3-methoxyphenyl)methylidene]-5-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203835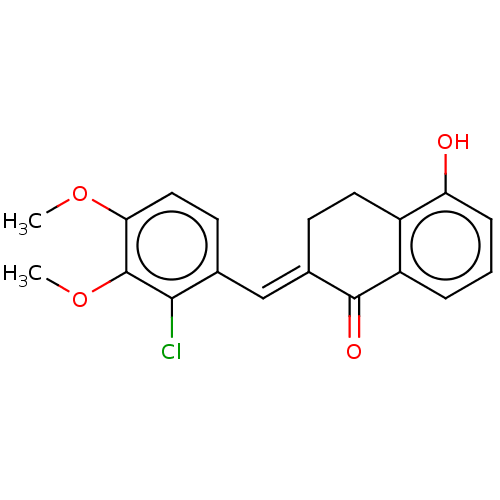 ((2E)-2-[(2-chloro-3,4-dimethoxyphenyl)methylidene]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203836 ((2E)-2-[(2-bromo-3,4,5-trimethoxyphenyl)methyliden...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203837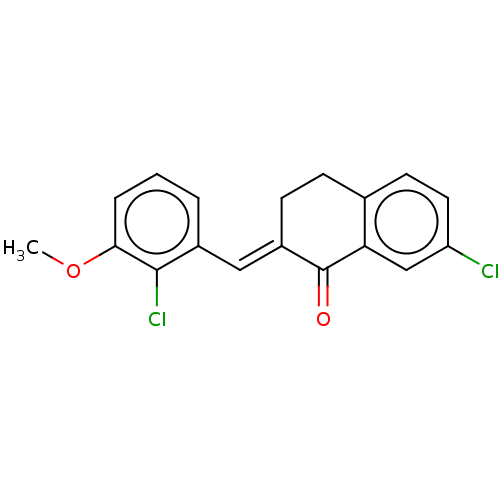 ((2E)-7-chloro-2-[(2-chloro-3-methoxyphenyl)methyli...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203838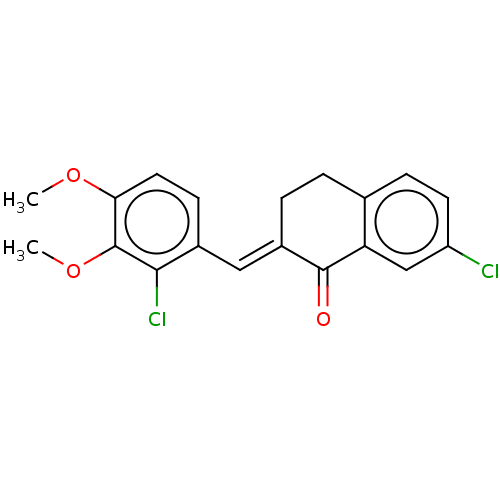 ((2E)-7-chloro-2-[(2-chloro-3,4-dimethoxyphenyl)met...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203839 ((2E)-2-[(2-bromo-3,4,5-trimethoxyphenyl)methyliden...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM203840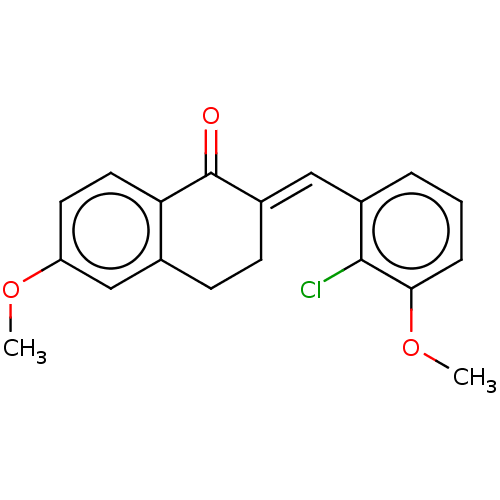 ((2E)-2-[(2-chloro-3-methoxyphenyl)methylidene]-6-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 38 |
Wuhan University of Technology | Assay Description A fluorimetric method reported by Matsumoto et al.[T. Matsumoto, O. Suzuki, T. Furuta, M. Asai, Y. Kurokawa, Y. Nimura, Y. Katsumata, I. Takahashi. C... | Chem Biol Drug Des 88: 889-898 (2016) Article DOI: 10.1111/cbdd.12822 BindingDB Entry DOI: 10.7270/Q2TX3D65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1748 total ) | Next | Last >> |