 Found 136 hits of Enzyme Inhibition Constant Data
Found 136 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297321

(CHEMBL551613 | Isoindolin-2-yl(4-(spiro[chromene-2...)Show SMILES O=C(N1Cc2ccccc2C1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:20| Show InChI InChI=1S/C28H26N2O2/c31-27(30-18-22-5-1-2-6-23(22)19-30)21-11-9-20(10-12-21)25-17-28(13-15-29-16-14-28)32-26-8-4-3-7-24(25)26/h1-12,17,29H,13-16,18-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297339
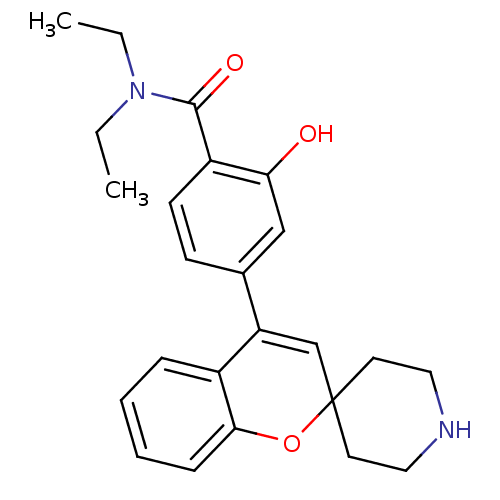
(CHEMBL557458 | N,N-Diethyl-2-hydroxy-4-(spiro[chro...)Show SMILES CCN(CC)C(=O)c1ccc(cc1O)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)19-10-9-17(15-21(19)27)20-16-24(11-13-25-14-12-24)29-22-8-6-5-7-18(20)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297329
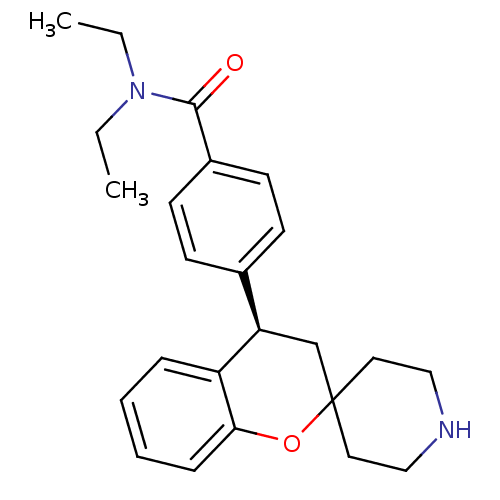
((R)-N,N-diethyl-4-(spiro[chroman-2,4'-piperidine]-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@H]1CC2(CCNCC2)Oc2ccccc12 |r| Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297316
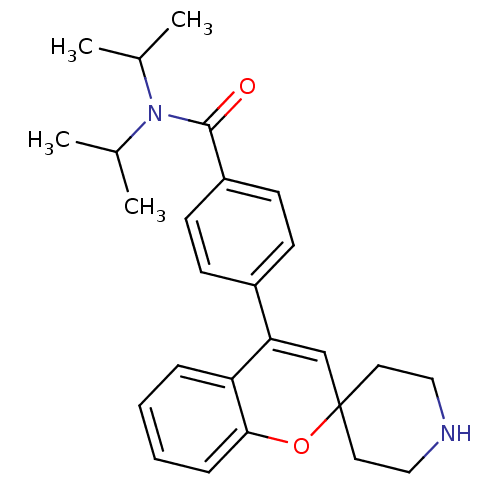
(CHEMBL561882 | N,N-Diisopropyl-4-(spiro[chromene-2...)Show SMILES CC(C)N(C(C)C)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C26H32N2O2/c1-18(2)28(19(3)4)25(29)21-11-9-20(10-12-21)23-17-26(13-15-27-16-14-26)30-24-8-6-5-7-22(23)24/h5-12,17-19,27H,13-16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297338
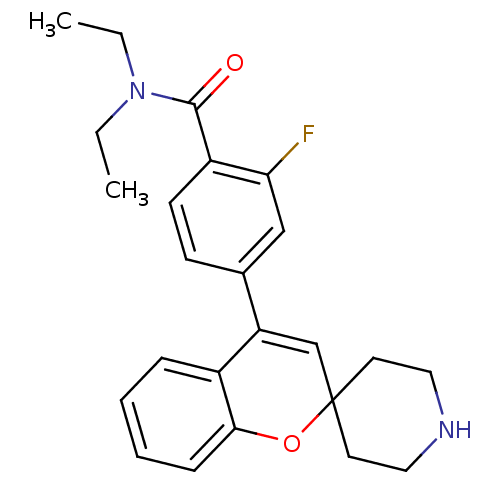
(CHEMBL562873 | N,N-Diethyl-2-fluoro-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(cc1F)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H27FN2O2/c1-3-27(4-2)23(28)19-10-9-17(15-21(19)25)20-16-24(11-13-26-14-12-24)29-22-8-6-5-7-18(20)22/h5-10,15-16,26H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297331

(CHEMBL550472 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(s1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O2S/c1-3-24(4-2)21(25)20-10-9-19(27-20)17-15-22(11-13-23-14-12-22)26-18-8-6-5-7-16(17)18/h5-10,15,23H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297328
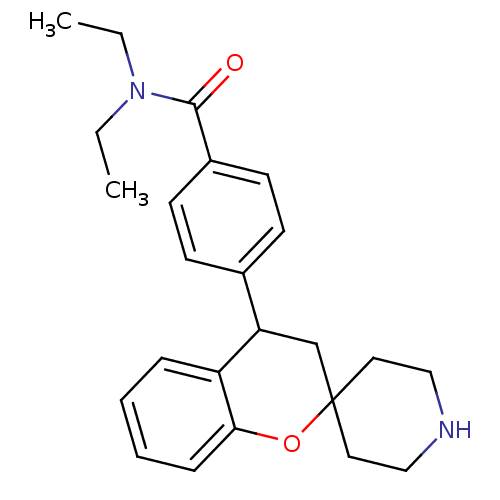
((+/-)-N,N-Diethyl-4-(spiro[chroman-2,4'-piperidine...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1CC2(CCNCC2)Oc2ccccc12 Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297324
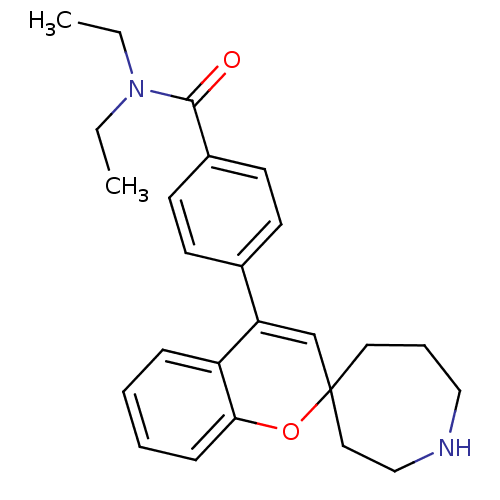
(CHEMBL563893 | N,N-Diethyl-4-(spiro[azepane-4,2'-c...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C25H30N2O2/c1-3-27(4-2)24(28)20-12-10-19(11-13-20)22-18-25(14-7-16-26-17-15-25)29-23-9-6-5-8-21(22)23/h5-6,8-13,18,26H,3-4,7,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50252876
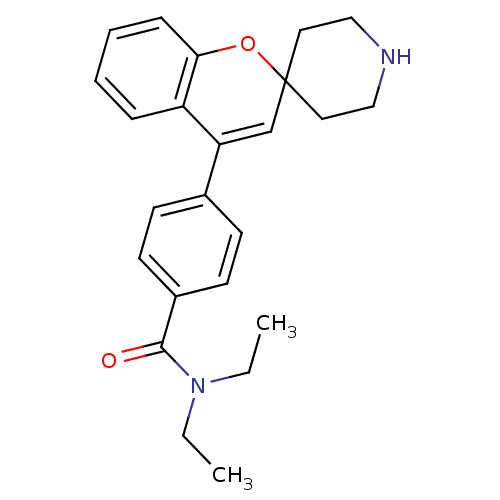
(CHEMBL494462 | N,N-Diethyl-4-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,17,25H,3-4,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297340
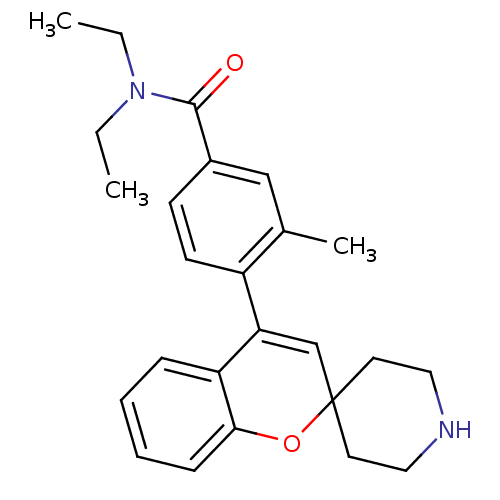
(CHEMBL562478 | N,N-Diethyl-3-methyl-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(C)c1 |t:11| Show InChI InChI=1S/C25H30N2O2/c1-4-27(5-2)24(28)19-10-11-20(18(3)16-19)22-17-25(12-14-26-15-13-25)29-23-9-7-6-8-21(22)23/h6-11,16-17,26H,4-5,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297334
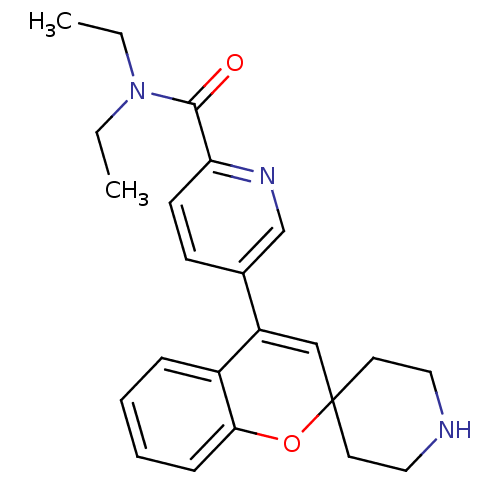
(CHEMBL557054 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(cn1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H27N3O2/c1-3-26(4-2)22(27)20-10-9-17(16-25-20)19-15-23(11-13-24-14-12-23)28-21-8-6-5-7-18(19)21/h5-10,15-16,24H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297342
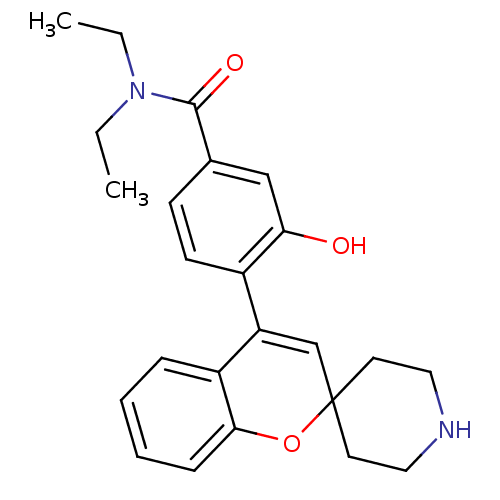
(CHEMBL561339 | N,N-Diethyl-3-hydroxy-4-(spiro[chro...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(O)c1 |t:11| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)17-9-10-18(21(27)15-17)20-16-24(11-13-25-14-12-24)29-22-8-6-5-7-19(20)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297337
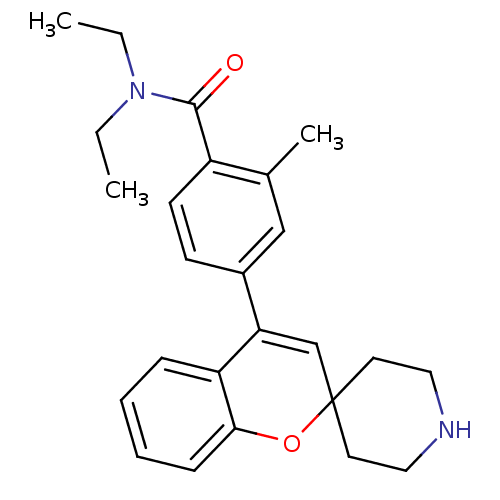
(CHEMBL557257 | N,N-Diethyl-2-methyl-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(cc1C)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C25H30N2O2/c1-4-27(5-2)24(28)20-11-10-19(16-18(20)3)22-17-25(12-14-26-15-13-25)29-23-9-7-6-8-21(22)23/h6-11,16-17,26H,4-5,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297336
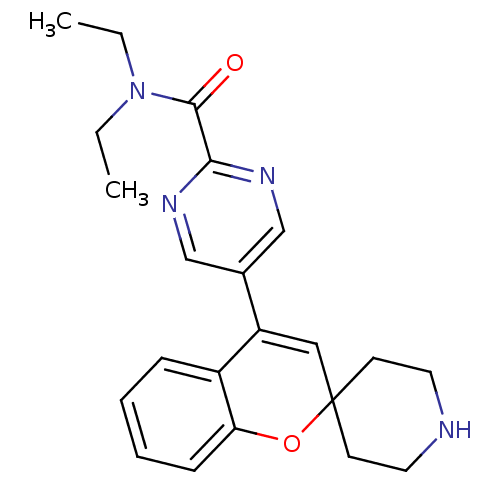
(CHEMBL562280 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ncc(cn1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C22H26N4O2/c1-3-26(4-2)21(27)20-24-14-16(15-25-20)18-13-22(9-11-23-12-10-22)28-19-8-6-5-7-17(18)19/h5-8,13-15,23H,3-4,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297312
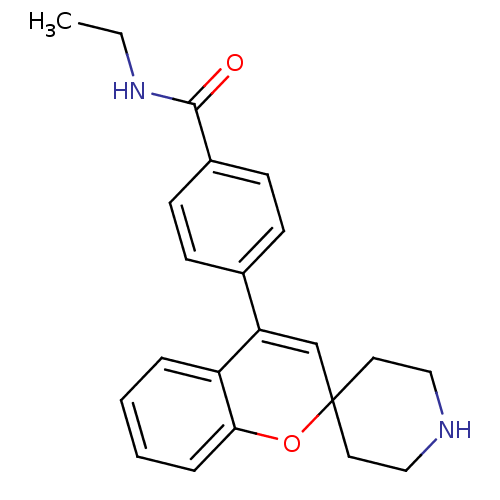
(CHEMBL551412 | N-Ethyl-4-(spiro[chromene-2,4'-pipe...)Show SMILES CCNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:12| Show InChI InChI=1S/C22H24N2O2/c1-2-24-21(25)17-9-7-16(8-10-17)19-15-22(11-13-23-14-12-22)26-20-6-4-3-5-18(19)20/h3-10,15,23H,2,11-14H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297333
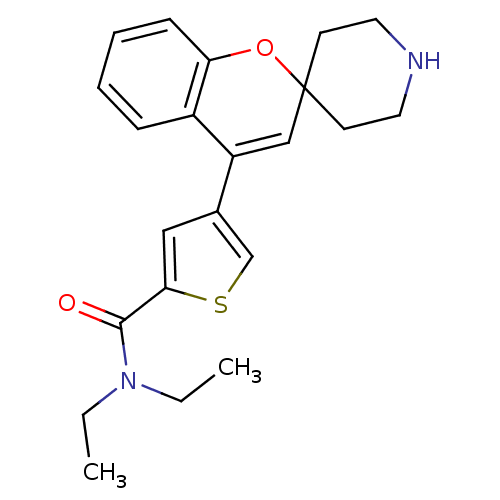
(CHEMBL562898 | N,N-Diethyl-4-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1cc(cs1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O2S/c1-3-24(4-2)21(25)20-13-16(15-27-20)18-14-22(9-11-23-12-10-22)26-19-8-6-5-7-17(18)19/h5-8,13-15,23H,3-4,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297323
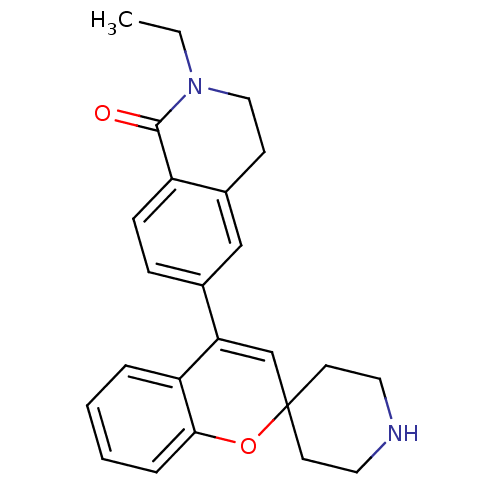
(2-Ethyl-6-(spiro[chromene-2,4'-piperidine]-4-yl)-3...)Show SMILES CCN1CCc2cc(ccc2C1=O)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H26N2O2/c1-2-26-14-9-18-15-17(7-8-19(18)23(26)27)21-16-24(10-12-25-13-11-24)28-22-6-4-3-5-20(21)22/h3-8,15-16,25H,2,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297317
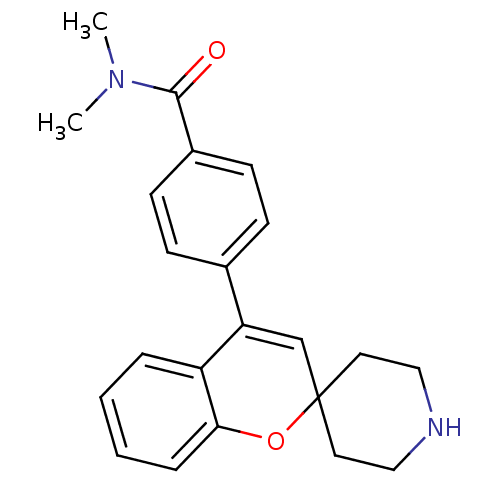
(CHEMBL561805 | N,N-Dimethyl-4-(spiro[chromene-2,4'...)Show SMILES CN(C)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:12| Show InChI InChI=1S/C22H24N2O2/c1-24(2)21(25)17-9-7-16(8-10-17)19-15-22(11-13-23-14-12-22)26-20-6-4-3-5-18(19)20/h3-10,15,23H,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297335
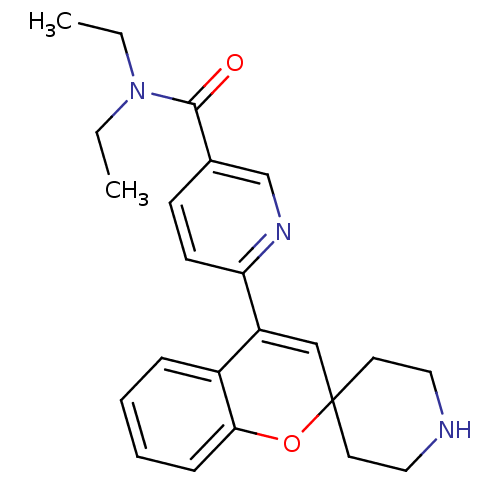
(CHEMBL561138 | N,N-Diethyl-6-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(nc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H27N3O2/c1-3-26(4-2)22(27)17-9-10-20(25-16-17)19-15-23(11-13-24-14-12-23)28-21-8-6-5-7-18(19)21/h5-10,15-16,24H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297341

(CHEMBL559414 | N,N-Diethyl-3-fluoro-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(F)c1 |t:11| Show InChI InChI=1S/C24H27FN2O2/c1-3-27(4-2)23(28)17-9-10-18(21(25)15-17)20-16-24(11-13-26-14-12-24)29-22-8-6-5-7-19(20)22/h5-10,15-16,26H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297315
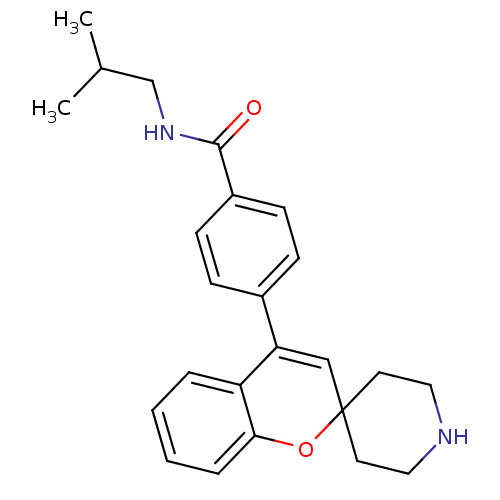
(CHEMBL563137 | N-Isobutyl-4-(spiro[chromene-2,4'-p...)Show SMILES CC(C)CNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-17(2)16-26-23(27)19-9-7-18(8-10-19)21-15-24(11-13-25-14-12-24)28-22-6-4-3-5-20(21)22/h3-10,15,17,25H,11-14,16H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297319
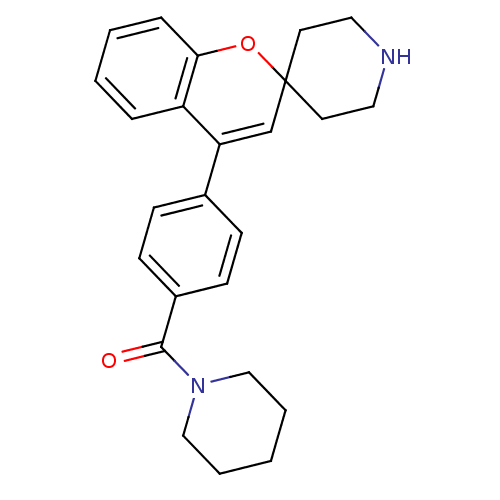
(CHEMBL550261 | Piperidin-1-yl(4-(spiro[chromene-2,...)Show SMILES O=C(N1CCCCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C25H28N2O2/c28-24(27-16-4-1-5-17-27)20-10-8-19(9-11-20)22-18-25(12-14-26-15-13-25)29-23-7-3-2-6-21(22)23/h2-3,6-11,18,26H,1,4-5,12-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297313

(4-(Spiro[chromene-2,4'-piperidine]-4-yl)benzamide ...)Show SMILES NC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:10| Show InChI InChI=1S/C20H20N2O2/c21-19(23)15-7-5-14(6-8-15)17-13-20(9-11-22-12-10-20)24-18-4-2-1-3-16(17)18/h1-8,13,22H,9-12H2,(H2,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297322
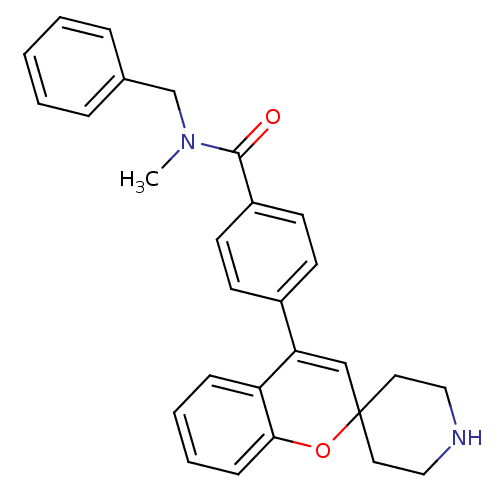
(CHEMBL551536 | N-Benzyl-N-methyl-4-(spiro[chromene...)Show SMILES CN(Cc1ccccc1)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:19| Show InChI InChI=1S/C28H28N2O2/c1-30(20-21-7-3-2-4-8-21)27(31)23-13-11-22(12-14-23)25-19-28(15-17-29-18-16-28)32-26-10-6-5-9-24(25)26/h2-14,19,29H,15-18,20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297314
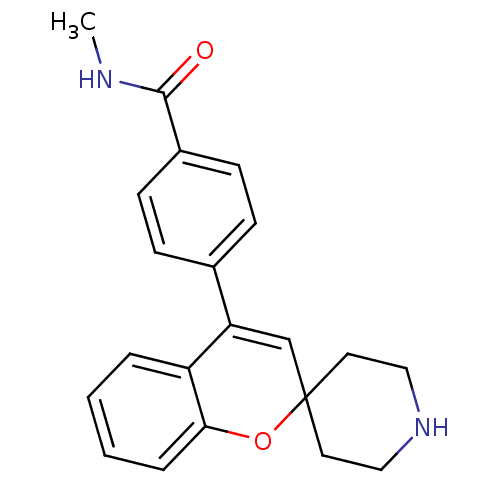
(CHEMBL551413 | N-Methyl-4-(spiro[chromene-2,4'-pip...)Show SMILES CNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:11| Show InChI InChI=1S/C21H22N2O2/c1-22-20(24)16-8-6-15(7-9-16)18-14-21(10-12-23-13-11-21)25-19-5-3-2-4-17(18)19/h2-9,14,23H,10-13H2,1H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297325
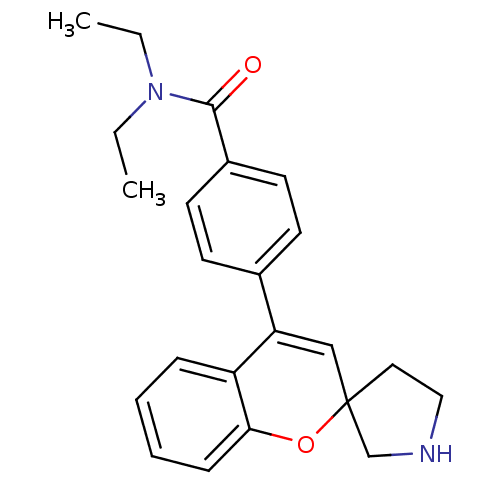
(CHEMBL556439 | N,N-Diethyl-4-(spiro[chromene-2,3'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H26N2O2/c1-3-25(4-2)22(26)18-11-9-17(10-12-18)20-15-23(13-14-24-16-23)27-21-8-6-5-7-19(20)21/h5-12,15,24H,3-4,13-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297332
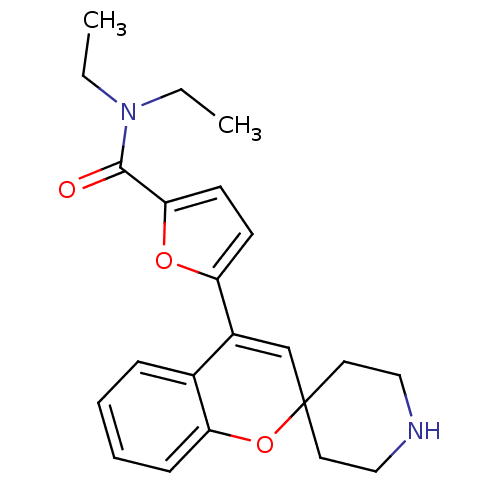
(CHEMBL550669 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(o1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O3/c1-3-24(4-2)21(25)20-10-9-18(26-20)17-15-22(11-13-23-14-12-22)27-19-8-6-5-7-16(17)19/h5-10,15,23H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297318

(CHEMBL564922 | Pyrrolidin-1-yl(4-(spiro[chromene-2...)Show SMILES O=C(N1CCCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H26N2O2/c27-23(26-15-3-4-16-26)19-9-7-18(8-10-19)21-17-24(11-13-25-14-12-24)28-22-6-2-1-5-20(21)22/h1-2,5-10,17,25H,3-4,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297330
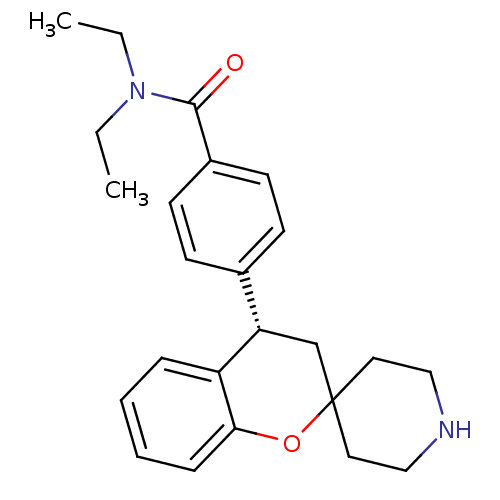
((S)-N,N-diethyl-4-(spiro[chroman-2,4'-piperidine]-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H]1CC2(CCNCC2)Oc2ccccc12 |r| Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297320
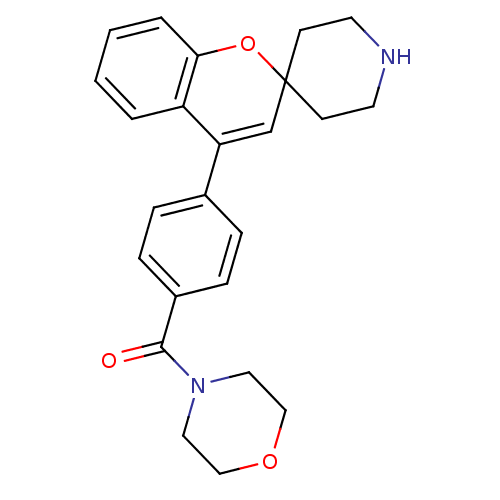
(CHEMBL551414 | Morpholino(4-(spiro[chromene-2,4'-p...)Show SMILES O=C(N1CCOCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C24H26N2O3/c27-23(26-13-15-28-16-14-26)19-7-5-18(6-8-19)21-17-24(9-11-25-12-10-24)29-22-4-2-1-3-20(21)22/h1-8,17,25H,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297326
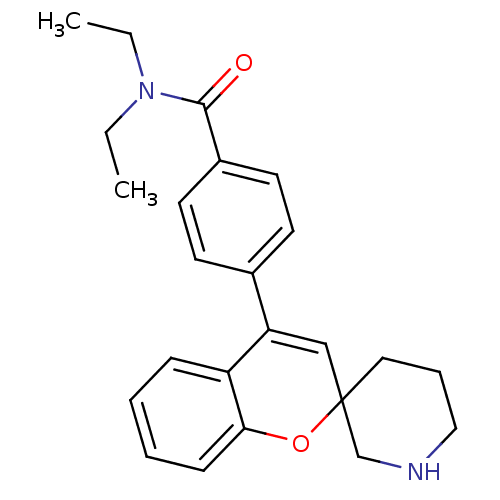
(CHEMBL563700 | N,N-Diethyl-4-(spiro[chromene-2,3'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCCNC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-3-26(4-2)23(27)19-12-10-18(11-13-19)21-16-24(14-7-15-25-17-24)28-22-9-6-5-8-20(21)22/h5-6,8-13,16,25H,3-4,7,14-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50297333

(CHEMBL562898 | N,N-Diethyl-4-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1cc(cs1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O2S/c1-3-24(4-2)21(25)20-13-16(15-27-20)18-14-22(9-11-23-12-10-22)26-19-8-6-5-7-17(18)19/h5-8,13-15,23H,3-4,9-12H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50297340

(CHEMBL562478 | N,N-Diethyl-3-methyl-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(C)c1 |t:11| Show InChI InChI=1S/C25H30N2O2/c1-4-27(5-2)24(28)19-10-11-20(18(3)16-19)22-17-25(12-14-26-15-13-25)29-23-9-7-6-8-21(22)23/h6-11,16-17,26H,4-5,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50297321

(CHEMBL551613 | Isoindolin-2-yl(4-(spiro[chromene-2...)Show SMILES O=C(N1Cc2ccccc2C1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:20| Show InChI InChI=1S/C28H26N2O2/c31-27(30-18-22-5-1-2-6-23(22)19-30)21-11-9-20(10-12-21)25-17-28(13-15-29-16-14-28)32-26-8-4-3-7-24(25)26/h1-12,17,29H,13-16,18-19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50297313

(4-(Spiro[chromene-2,4'-piperidine]-4-yl)benzamide ...)Show SMILES NC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:10| Show InChI InChI=1S/C20H20N2O2/c21-19(23)15-7-5-14(6-8-15)17-13-20(9-11-22-12-10-20)24-18-4-2-1-3-16(17)18/h1-8,13,22H,9-12H2,(H2,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50297312

(CHEMBL551412 | N-Ethyl-4-(spiro[chromene-2,4'-pipe...)Show SMILES CCNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:12| Show InChI InChI=1S/C22H24N2O2/c1-2-24-21(25)17-9-7-16(8-10-17)19-15-22(11-13-23-14-12-22)26-20-6-4-3-5-18(19)20/h3-10,15,23H,2,11-14H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50297321

(CHEMBL551613 | Isoindolin-2-yl(4-(spiro[chromene-2...)Show SMILES O=C(N1Cc2ccccc2C1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:20| Show InChI InChI=1S/C28H26N2O2/c31-27(30-18-22-5-1-2-6-23(22)19-30)21-11-9-20(10-12-21)25-17-28(13-15-29-16-14-28)32-26-8-4-3-7-24(25)26/h1-12,17,29H,13-16,18-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297327
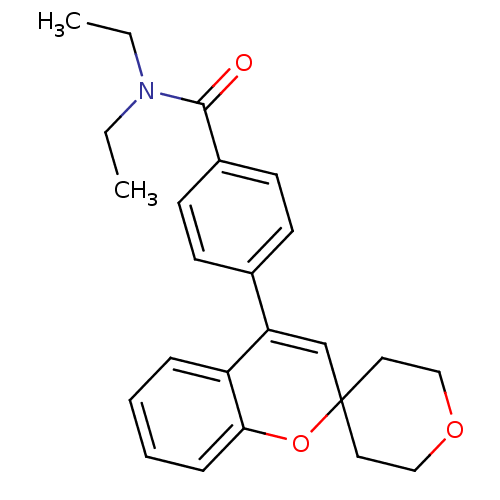
(CHEMBL556923 | N,N-Diethyl-4-(2',3',5',6'-tetrahyd...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCOCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H27NO3/c1-3-25(4-2)23(26)19-11-9-18(10-12-19)21-17-24(13-15-27-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,17H,3-4,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human delta opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50297314

(CHEMBL551413 | N-Methyl-4-(spiro[chromene-2,4'-pip...)Show SMILES CNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:11| Show InChI InChI=1S/C21H22N2O2/c1-22-20(24)16-8-6-15(7-9-16)18-14-21(10-12-23-13-11-21)25-19-5-3-2-4-17(18)19/h2-9,14,23H,10-13H2,1H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50297322

(CHEMBL551536 | N-Benzyl-N-methyl-4-(spiro[chromene...)Show SMILES CN(Cc1ccccc1)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:19| Show InChI InChI=1S/C28H28N2O2/c1-30(20-21-7-3-2-4-8-21)27(31)23-13-11-22(12-14-23)25-19-28(15-17-29-18-16-28)32-26-10-6-5-9-24(25)26/h2-14,19,29H,15-18,20H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50297313

(4-(Spiro[chromene-2,4'-piperidine]-4-yl)benzamide ...)Show SMILES NC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:10| Show InChI InChI=1S/C20H20N2O2/c21-19(23)15-7-5-14(6-8-15)17-13-20(9-11-22-12-10-20)24-18-4-2-1-3-16(17)18/h1-8,13,22H,9-12H2,(H2,21,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50297332

(CHEMBL550669 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(o1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O3/c1-3-24(4-2)21(25)20-10-9-18(26-20)17-15-22(11-13-23-14-12-22)27-19-8-6-5-7-16(17)19/h5-10,15,23H,3-4,11-14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50297340

(CHEMBL562478 | N,N-Diethyl-3-methyl-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(C)c1 |t:11| Show InChI InChI=1S/C25H30N2O2/c1-4-27(5-2)24(28)19-10-11-20(18(3)16-19)22-17-25(12-14-26-15-13-25)29-23-9-7-6-8-21(22)23/h6-11,16-17,26H,4-5,12-15H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297312

(CHEMBL551412 | N-Ethyl-4-(spiro[chromene-2,4'-pipe...)Show SMILES CCNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:12| Show InChI InChI=1S/C22H24N2O2/c1-2-24-21(25)17-9-7-16(8-10-17)19-15-22(11-13-23-14-12-22)26-20-6-4-3-5-18(19)20/h3-10,15,23H,2,11-14H2,1H3,(H,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297341

(CHEMBL559414 | N,N-Diethyl-3-fluoro-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(F)c1 |t:11| Show InChI InChI=1S/C24H27FN2O2/c1-3-27(4-2)23(28)17-9-10-18(21(25)15-17)20-16-24(11-13-26-14-12-24)29-22-8-6-5-7-19(20)22/h5-10,15-16,26H,3-4,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 418 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297313

(4-(Spiro[chromene-2,4'-piperidine]-4-yl)benzamide ...)Show SMILES NC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:10| Show InChI InChI=1S/C20H20N2O2/c21-19(23)15-7-5-14(6-8-15)17-13-20(9-11-22-12-10-20)24-18-4-2-1-3-16(17)18/h1-8,13,22H,9-12H2,(H2,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297332

(CHEMBL550669 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(o1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O3/c1-3-24(4-2)21(25)20-10-9-18(26-20)17-15-22(11-13-23-14-12-22)27-19-8-6-5-7-16(17)19/h5-10,15,23H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297315

(CHEMBL563137 | N-Isobutyl-4-(spiro[chromene-2,4'-p...)Show SMILES CC(C)CNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-17(2)16-26-23(27)19-9-7-18(8-10-19)21-15-24(11-13-25-14-12-24)28-22-6-4-3-5-20(21)22/h3-10,15,17,25H,11-14,16H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297322

(CHEMBL551536 | N-Benzyl-N-methyl-4-(spiro[chromene...)Show SMILES CN(Cc1ccccc1)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:19| Show InChI InChI=1S/C28H28N2O2/c1-30(20-21-7-3-2-4-8-21)27(31)23-13-11-22(12-14-23)25-19-28(15-17-29-18-16-28)32-26-10-6-5-9-24(25)26/h2-14,19,29H,15-18,20H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297314

(CHEMBL551413 | N-Methyl-4-(spiro[chromene-2,4'-pip...)Show SMILES CNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:11| Show InChI InChI=1S/C21H22N2O2/c1-22-20(24)16-8-6-15(7-9-16)18-14-21(10-12-23-13-11-21)25-19-5-3-2-4-17(18)19/h2-9,14,23H,10-13H2,1H3,(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297331

(CHEMBL550472 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(s1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O2S/c1-3-24(4-2)21(25)20-10-9-19(27-20)17-15-22(11-13-23-14-12-22)26-18-8-6-5-7-16(17)18/h5-10,15,23H,3-4,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297322

(CHEMBL551536 | N-Benzyl-N-methyl-4-(spiro[chromene...)Show SMILES CN(Cc1ccccc1)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:19| Show InChI InChI=1S/C28H28N2O2/c1-30(20-21-7-3-2-4-8-21)27(31)23-13-11-22(12-14-23)25-19-28(15-17-29-18-16-28)32-26-10-6-5-9-24(25)26/h2-14,19,29H,15-18,20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297321

(CHEMBL551613 | Isoindolin-2-yl(4-(spiro[chromene-2...)Show SMILES O=C(N1Cc2ccccc2C1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:20| Show InChI InChI=1S/C28H26N2O2/c31-27(30-18-22-5-1-2-6-23(22)19-30)21-11-9-20(10-12-21)25-17-28(13-15-29-16-14-28)32-26-8-4-3-7-24(25)26/h1-12,17,29H,13-16,18-19H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297331

(CHEMBL550472 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(s1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O2S/c1-3-24(4-2)21(25)20-10-9-19(27-20)17-15-22(11-13-23-14-12-22)26-18-8-6-5-7-16(17)18/h5-10,15,23H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297326

(CHEMBL563700 | N,N-Diethyl-4-(spiro[chromene-2,3'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCCNC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-3-26(4-2)23(27)19-12-10-18(11-13-19)21-16-24(14-7-15-25-17-24)28-22-9-6-5-8-20(21)22/h5-6,8-13,16,25H,3-4,7,14-15,17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297325

(CHEMBL556439 | N,N-Diethyl-4-(spiro[chromene-2,3'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H26N2O2/c1-3-25(4-2)22(26)18-11-9-17(10-12-18)20-15-23(13-14-24-16-23)27-21-8-6-5-7-19(20)21/h5-12,15,24H,3-4,13-14,16H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297329

((R)-N,N-diethyl-4-(spiro[chroman-2,4'-piperidine]-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@H]1CC2(CCNCC2)Oc2ccccc12 |r| Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297324

(CHEMBL563893 | N,N-Diethyl-4-(spiro[azepane-4,2'-c...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C25H30N2O2/c1-3-27(4-2)24(28)20-12-10-19(11-13-20)22-18-25(14-7-16-26-17-15-25)29-23-9-6-5-8-21(22)23/h5-6,8-13,18,26H,3-4,7,14-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297323

(2-Ethyl-6-(spiro[chromene-2,4'-piperidine]-4-yl)-3...)Show SMILES CCN1CCc2cc(ccc2C1=O)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H26N2O2/c1-2-26-14-9-18-15-17(7-8-19(18)23(26)27)21-16-24(10-12-25-13-11-24)28-22-6-4-3-5-20(21)22/h3-8,15-16,25H,2,9-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297317

(CHEMBL561805 | N,N-Dimethyl-4-(spiro[chromene-2,4'...)Show SMILES CN(C)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:12| Show InChI InChI=1S/C22H24N2O2/c1-24(2)21(25)17-9-7-16(8-10-17)19-15-22(11-13-23-14-12-22)26-20-6-4-3-5-18(19)20/h3-10,15,23H,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297319

(CHEMBL550261 | Piperidin-1-yl(4-(spiro[chromene-2,...)Show SMILES O=C(N1CCCCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C25H28N2O2/c28-24(27-16-4-1-5-17-27)20-10-8-19(9-11-20)22-18-25(12-14-26-15-13-25)29-23-7-3-2-6-21(22)23/h2-3,6-11,18,26H,1,4-5,12-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297330

((S)-N,N-diethyl-4-(spiro[chroman-2,4'-piperidine]-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H]1CC2(CCNCC2)Oc2ccccc12 |r| Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297315

(CHEMBL563137 | N-Isobutyl-4-(spiro[chromene-2,4'-p...)Show SMILES CC(C)CNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-17(2)16-26-23(27)19-9-7-18(8-10-19)21-15-24(11-13-25-14-12-24)28-22-6-4-3-5-20(21)22/h3-10,15,17,25H,11-14,16H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297333

(CHEMBL562898 | N,N-Diethyl-4-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1cc(cs1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O2S/c1-3-24(4-2)21(25)20-13-16(15-27-20)18-14-22(9-11-23-12-10-22)26-19-8-6-5-7-17(18)19/h5-8,13-15,23H,3-4,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297321

(CHEMBL551613 | Isoindolin-2-yl(4-(spiro[chromene-2...)Show SMILES O=C(N1Cc2ccccc2C1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:20| Show InChI InChI=1S/C28H26N2O2/c31-27(30-18-22-5-1-2-6-23(22)19-30)21-11-9-20(10-12-21)25-17-28(13-15-29-16-14-28)32-26-8-4-3-7-24(25)26/h1-12,17,29H,13-16,18-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297338

(CHEMBL562873 | N,N-Diethyl-2-fluoro-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(cc1F)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H27FN2O2/c1-3-27(4-2)23(28)19-10-9-17(15-21(19)25)20-16-24(11-13-26-14-12-24)29-22-8-6-5-7-18(20)22/h5-10,15-16,26H,3-4,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297316

(CHEMBL561882 | N,N-Diisopropyl-4-(spiro[chromene-2...)Show SMILES CC(C)N(C(C)C)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C26H32N2O2/c1-18(2)28(19(3)4)25(29)21-11-9-20(10-12-21)23-17-26(13-15-27-16-14-26)30-24-8-6-5-7-22(23)24/h5-12,17-19,27H,13-16H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297340

(CHEMBL562478 | N,N-Diethyl-3-methyl-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(C)c1 |t:11| Show InChI InChI=1S/C25H30N2O2/c1-4-27(5-2)24(28)19-10-11-20(18(3)16-19)22-17-25(12-14-26-15-13-25)29-23-9-7-6-8-21(22)23/h6-11,16-17,26H,4-5,12-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50252876

(CHEMBL494462 | N,N-Diethyl-4-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,17,25H,3-4,13-16H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297319

(CHEMBL550261 | Piperidin-1-yl(4-(spiro[chromene-2,...)Show SMILES O=C(N1CCCCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C25H28N2O2/c28-24(27-16-4-1-5-17-27)20-10-8-19(9-11-20)22-18-25(12-14-26-15-13-25)29-23-7-3-2-6-21(22)23/h2-3,6-11,18,26H,1,4-5,12-17H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297337

(CHEMBL557257 | N,N-Diethyl-2-methyl-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(cc1C)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C25H30N2O2/c1-4-27(5-2)24(28)20-11-10-19(16-18(20)3)22-17-25(12-14-26-15-13-25)29-23-9-7-6-8-21(22)23/h6-11,16-17,26H,4-5,12-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297340

(CHEMBL562478 | N,N-Diethyl-3-methyl-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(C)c1 |t:11| Show InChI InChI=1S/C25H30N2O2/c1-4-27(5-2)24(28)19-10-11-20(18(3)16-19)22-17-25(12-14-26-15-13-25)29-23-9-7-6-8-21(22)23/h6-11,16-17,26H,4-5,12-15H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297318

(CHEMBL564922 | Pyrrolidin-1-yl(4-(spiro[chromene-2...)Show SMILES O=C(N1CCCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H26N2O2/c27-23(26-15-3-4-16-26)19-9-7-18(8-10-19)21-17-24(11-13-25-14-12-24)28-22-6-2-1-5-20(21)22/h1-2,5-10,17,25H,3-4,11-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297328

((+/-)-N,N-Diethyl-4-(spiro[chroman-2,4'-piperidine...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1CC2(CCNCC2)Oc2ccccc12 Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297332

(CHEMBL550669 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(o1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O3/c1-3-24(4-2)21(25)20-10-9-18(26-20)17-15-22(11-13-23-14-12-22)27-19-8-6-5-7-16(17)19/h5-10,15,23H,3-4,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297338

(CHEMBL562873 | N,N-Diethyl-2-fluoro-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(cc1F)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H27FN2O2/c1-3-27(4-2)23(28)19-10-9-17(15-21(19)25)20-16-24(11-13-26-14-12-24)29-22-8-6-5-7-18(20)22/h5-10,15-16,26H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297333

(CHEMBL562898 | N,N-Diethyl-4-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1cc(cs1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O2S/c1-3-24(4-2)21(25)20-13-16(15-27-20)18-14-22(9-11-23-12-10-22)26-19-8-6-5-7-17(18)19/h5-8,13-15,23H,3-4,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297328

((+/-)-N,N-Diethyl-4-(spiro[chroman-2,4'-piperidine...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1CC2(CCNCC2)Oc2ccccc12 Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297325

(CHEMBL556439 | N,N-Diethyl-4-(spiro[chromene-2,3'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H26N2O2/c1-3-25(4-2)22(26)18-11-9-17(10-12-18)20-15-23(13-14-24-16-23)27-21-8-6-5-7-19(20)21/h5-12,15,24H,3-4,13-14,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297316

(CHEMBL561882 | N,N-Diisopropyl-4-(spiro[chromene-2...)Show SMILES CC(C)N(C(C)C)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C26H32N2O2/c1-18(2)28(19(3)4)25(29)21-11-9-20(10-12-21)23-17-26(13-15-27-16-14-26)30-24-8-6-5-7-22(23)24/h5-12,17-19,27H,13-16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297329

((R)-N,N-diethyl-4-(spiro[chroman-2,4'-piperidine]-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@H]1CC2(CCNCC2)Oc2ccccc12 |r| Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297341

(CHEMBL559414 | N,N-Diethyl-3-fluoro-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(F)c1 |t:11| Show InChI InChI=1S/C24H27FN2O2/c1-3-27(4-2)23(28)17-9-10-18(21(25)15-17)20-16-24(11-13-26-14-12-24)29-22-8-6-5-7-19(20)22/h5-10,15-16,26H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297320

(CHEMBL551414 | Morpholino(4-(spiro[chromene-2,4'-p...)Show SMILES O=C(N1CCOCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C24H26N2O3/c27-23(26-13-15-28-16-14-26)19-7-5-18(6-8-19)21-17-24(9-11-25-12-10-24)29-22-4-2-1-3-20(21)22/h1-8,17,25H,9-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50252876

(CHEMBL494462 | N,N-Diethyl-4-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,17,25H,3-4,13-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297337

(CHEMBL557257 | N,N-Diethyl-2-methyl-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(cc1C)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C25H30N2O2/c1-4-27(5-2)24(28)20-11-10-19(16-18(20)3)22-17-25(12-14-26-15-13-25)29-23-9-7-6-8-21(22)23/h6-11,16-17,26H,4-5,12-15H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297339

(CHEMBL557458 | N,N-Diethyl-2-hydroxy-4-(spiro[chro...)Show SMILES CCN(CC)C(=O)c1ccc(cc1O)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)19-10-9-17(15-21(19)27)20-16-24(11-13-25-14-12-24)29-22-8-6-5-7-18(20)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297318

(CHEMBL564922 | Pyrrolidin-1-yl(4-(spiro[chromene-2...)Show SMILES O=C(N1CCCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H26N2O2/c27-23(26-15-3-4-16-26)19-9-7-18(8-10-19)21-17-24(11-13-25-14-12-24)28-22-6-2-1-5-20(21)22/h1-2,5-10,17,25H,3-4,11-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297313

(4-(Spiro[chromene-2,4'-piperidine]-4-yl)benzamide ...)Show SMILES NC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:10| Show InChI InChI=1S/C20H20N2O2/c21-19(23)15-7-5-14(6-8-15)17-13-20(9-11-22-12-10-20)24-18-4-2-1-3-16(17)18/h1-8,13,22H,9-12H2,(H2,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297324

(CHEMBL563893 | N,N-Diethyl-4-(spiro[azepane-4,2'-c...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C25H30N2O2/c1-3-27(4-2)24(28)20-12-10-19(11-13-20)22-18-25(14-7-16-26-17-15-25)29-23-9-6-5-8-21(22)23/h5-6,8-13,18,26H,3-4,7,14-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297312

(CHEMBL551412 | N-Ethyl-4-(spiro[chromene-2,4'-pipe...)Show SMILES CCNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:12| Show InChI InChI=1S/C22H24N2O2/c1-2-24-21(25)17-9-7-16(8-10-17)19-15-22(11-13-23-14-12-22)26-20-6-4-3-5-18(19)20/h3-10,15,23H,2,11-14H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297326

(CHEMBL563700 | N,N-Diethyl-4-(spiro[chromene-2,3'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCCNC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-3-26(4-2)23(27)19-12-10-18(11-13-19)21-16-24(14-7-15-25-17-24)28-22-9-6-5-8-20(21)22/h5-6,8-13,16,25H,3-4,7,14-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297323

(2-Ethyl-6-(spiro[chromene-2,4'-piperidine]-4-yl)-3...)Show SMILES CCN1CCc2cc(ccc2C1=O)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H26N2O2/c1-2-26-14-9-18-15-17(7-8-19(18)23(26)27)21-16-24(10-12-25-13-11-24)28-22-6-4-3-5-20(21)22/h3-8,15-16,25H,2,9-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297330

((S)-N,N-diethyl-4-(spiro[chroman-2,4'-piperidine]-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H]1CC2(CCNCC2)Oc2ccccc12 |r| Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297336

(CHEMBL562280 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ncc(cn1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C22H26N4O2/c1-3-26(4-2)21(27)20-24-14-16(15-25-20)18-13-22(9-11-23-12-10-22)28-19-8-6-5-7-17(18)19/h5-8,13-15,23H,3-4,9-12H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297339

(CHEMBL557458 | N,N-Diethyl-2-hydroxy-4-(spiro[chro...)Show SMILES CCN(CC)C(=O)c1ccc(cc1O)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)19-10-9-17(15-21(19)27)20-16-24(11-13-25-14-12-24)29-22-8-6-5-7-18(20)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297317

(CHEMBL561805 | N,N-Dimethyl-4-(spiro[chromene-2,4'...)Show SMILES CN(C)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:12| Show InChI InChI=1S/C22H24N2O2/c1-24(2)21(25)17-9-7-16(8-10-17)19-15-22(11-13-23-14-12-22)26-20-6-4-3-5-18(19)20/h3-10,15,23H,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297334

(CHEMBL557054 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(cn1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H27N3O2/c1-3-26(4-2)22(27)20-10-9-17(16-25-20)19-15-23(11-13-24-14-12-23)28-21-8-6-5-7-18(19)21/h5-10,15-16,24H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297335

(CHEMBL561138 | N,N-Diethyl-6-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(nc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H27N3O2/c1-3-26(4-2)22(27)17-9-10-20(25-16-17)19-15-23(11-13-24-14-12-23)28-21-8-6-5-7-18(19)21/h5-10,15-16,24H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50297342

(CHEMBL561339 | N,N-Diethyl-3-hydroxy-4-(spiro[chro...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(O)c1 |t:11| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)17-9-10-18(21(27)15-17)20-16-24(11-13-25-14-12-24)29-22-8-6-5-7-19(20)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence-based assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297334

(CHEMBL557054 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(cn1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H27N3O2/c1-3-26(4-2)22(27)20-10-9-17(16-25-20)19-15-23(11-13-24-14-12-23)28-21-8-6-5-7-18(19)21/h5-10,15-16,24H,3-4,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297320

(CHEMBL551414 | Morpholino(4-(spiro[chromene-2,4'-p...)Show SMILES O=C(N1CCOCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C24H26N2O3/c27-23(26-13-15-28-16-14-26)19-7-5-18(6-8-19)21-17-24(9-11-25-12-10-24)29-22-4-2-1-3-20(21)22/h1-8,17,25H,9-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297342

(CHEMBL561339 | N,N-Diethyl-3-hydroxy-4-(spiro[chro...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(O)c1 |t:11| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)17-9-10-18(21(27)15-17)20-16-24(11-13-25-14-12-24)29-22-8-6-5-7-19(20)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297335

(CHEMBL561138 | N,N-Diethyl-6-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(nc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H27N3O2/c1-3-26(4-2)22(27)17-9-10-20(25-16-17)19-15-23(11-13-24-14-12-23)28-21-8-6-5-7-18(19)21/h5-10,15-16,24H,3-4,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297314

(CHEMBL551413 | N-Methyl-4-(spiro[chromene-2,4'-pip...)Show SMILES CNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:11| Show InChI InChI=1S/C21H22N2O2/c1-22-20(24)16-8-6-15(7-9-16)18-14-21(10-12-23-13-11-21)25-19-5-3-2-4-17(18)19/h2-9,14,23H,10-13H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50297336

(CHEMBL562280 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ncc(cn1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C22H26N4O2/c1-3-26(4-2)21(27)20-24-14-16(15-25-20)18-13-22(9-11-23-12-10-22)28-19-8-6-5-7-17(18)19/h5-8,13-15,23H,3-4,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG channel expressed in HEK293 cells by voltage clamp assay |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297329

((R)-N,N-diethyl-4-(spiro[chroman-2,4'-piperidine]-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@H]1CC2(CCNCC2)Oc2ccccc12 |r| Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297339

(CHEMBL557458 | N,N-Diethyl-2-hydroxy-4-(spiro[chro...)Show SMILES CCN(CC)C(=O)c1ccc(cc1O)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)19-10-9-17(15-21(19)27)20-16-24(11-13-25-14-12-24)29-22-8-6-5-7-18(20)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297337

(CHEMBL557257 | N,N-Diethyl-2-methyl-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(cc1C)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C25H30N2O2/c1-4-27(5-2)24(28)20-11-10-19(16-18(20)3)22-17-25(12-14-26-15-13-25)29-23-9-7-6-8-21(22)23/h6-11,16-17,26H,4-5,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297323

(2-Ethyl-6-(spiro[chromene-2,4'-piperidine]-4-yl)-3...)Show SMILES CCN1CCc2cc(ccc2C1=O)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H26N2O2/c1-2-26-14-9-18-15-17(7-8-19(18)23(26)27)21-16-24(10-12-25-13-11-24)28-22-6-4-3-5-20(21)22/h3-8,15-16,25H,2,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297328

((+/-)-N,N-Diethyl-4-(spiro[chroman-2,4'-piperidine...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1CC2(CCNCC2)Oc2ccccc12 Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297313

(4-(Spiro[chromene-2,4'-piperidine]-4-yl)benzamide ...)Show SMILES NC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:10| Show InChI InChI=1S/C20H20N2O2/c21-19(23)15-7-5-14(6-8-15)17-13-20(9-11-22-12-10-20)24-18-4-2-1-3-16(17)18/h1-8,13,22H,9-12H2,(H2,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297312

(CHEMBL551412 | N-Ethyl-4-(spiro[chromene-2,4'-pipe...)Show SMILES CCNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:12| Show InChI InChI=1S/C22H24N2O2/c1-2-24-21(25)17-9-7-16(8-10-17)19-15-22(11-13-23-14-12-22)26-20-6-4-3-5-18(19)20/h3-10,15,23H,2,11-14H2,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297340

(CHEMBL562478 | N,N-Diethyl-3-methyl-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(C)c1 |t:11| Show InChI InChI=1S/C25H30N2O2/c1-4-27(5-2)24(28)19-10-11-20(18(3)16-19)22-17-25(12-14-26-15-13-25)29-23-9-7-6-8-21(22)23/h6-11,16-17,26H,4-5,12-15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297317

(CHEMBL561805 | N,N-Dimethyl-4-(spiro[chromene-2,4'...)Show SMILES CN(C)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:12| Show InChI InChI=1S/C22H24N2O2/c1-24(2)21(25)17-9-7-16(8-10-17)19-15-22(11-13-23-14-12-22)26-20-6-4-3-5-18(19)20/h3-10,15,23H,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297322

(CHEMBL551536 | N-Benzyl-N-methyl-4-(spiro[chromene...)Show SMILES CN(Cc1ccccc1)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:19| Show InChI InChI=1S/C28H28N2O2/c1-30(20-21-7-3-2-4-8-21)27(31)23-13-11-22(12-14-23)25-19-28(15-17-29-18-16-28)32-26-10-6-5-9-24(25)26/h2-14,19,29H,15-18,20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297315

(CHEMBL563137 | N-Isobutyl-4-(spiro[chromene-2,4'-p...)Show SMILES CC(C)CNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-17(2)16-26-23(27)19-9-7-18(8-10-19)21-15-24(11-13-25-14-12-24)28-22-6-4-3-5-20(21)22/h3-10,15,17,25H,11-14,16H2,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297335

(CHEMBL561138 | N,N-Diethyl-6-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(nc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H27N3O2/c1-3-26(4-2)22(27)17-9-10-20(25-16-17)19-15-23(11-13-24-14-12-23)28-21-8-6-5-7-18(19)21/h5-10,15-16,24H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297331

(CHEMBL550472 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(s1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O2S/c1-3-24(4-2)21(25)20-10-9-19(27-20)17-15-22(11-13-23-14-12-22)26-18-8-6-5-7-16(17)18/h5-10,15,23H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 69 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297338

(CHEMBL562873 | N,N-Diethyl-2-fluoro-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(cc1F)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H27FN2O2/c1-3-27(4-2)23(28)19-10-9-17(15-21(19)25)20-16-24(11-13-26-14-12-24)29-22-8-6-5-7-18(20)22/h5-10,15-16,26H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297321

(CHEMBL551613 | Isoindolin-2-yl(4-(spiro[chromene-2...)Show SMILES O=C(N1Cc2ccccc2C1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:20| Show InChI InChI=1S/C28H26N2O2/c31-27(30-18-22-5-1-2-6-23(22)19-30)21-11-9-20(10-12-21)25-17-28(13-15-29-16-14-28)32-26-8-4-3-7-24(25)26/h1-12,17,29H,13-16,18-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297330

((S)-N,N-diethyl-4-(spiro[chroman-2,4'-piperidine]-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H]1CC2(CCNCC2)Oc2ccccc12 |r| Show InChI InChI=1S/C24H30N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,21,25H,3-4,13-17H2,1-2H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297326

(CHEMBL563700 | N,N-Diethyl-4-(spiro[chromene-2,3'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCCNC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-3-26(4-2)23(27)19-12-10-18(11-13-19)21-16-24(14-7-15-25-17-24)28-22-9-6-5-8-20(21)22/h5-6,8-13,16,25H,3-4,7,14-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297333

(CHEMBL562898 | N,N-Diethyl-4-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1cc(cs1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O2S/c1-3-24(4-2)21(25)20-13-16(15-27-20)18-14-22(9-11-23-12-10-22)26-19-8-6-5-7-17(18)19/h5-8,13-15,23H,3-4,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297324

(CHEMBL563893 | N,N-Diethyl-4-(spiro[azepane-4,2'-c...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C25H30N2O2/c1-3-27(4-2)24(28)20-12-10-19(11-13-20)22-18-25(14-7-16-26-17-15-25)29-23-9-6-5-8-21(22)23/h5-6,8-13,18,26H,3-4,7,14-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297325

(CHEMBL556439 | N,N-Diethyl-4-(spiro[chromene-2,3'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H26N2O2/c1-3-25(4-2)22(26)18-11-9-17(10-12-18)20-15-23(13-14-24-16-23)27-21-8-6-5-7-19(20)21/h5-12,15,24H,3-4,13-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297332

(CHEMBL550669 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(o1)C1=CC2(CCNCC2)Oc2ccccc12 |t:13| Show InChI InChI=1S/C22H26N2O3/c1-3-24(4-2)21(25)20-10-9-18(26-20)17-15-22(11-13-23-14-12-22)27-19-8-6-5-7-16(17)19/h5-10,15,23H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 980 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297336

(CHEMBL562280 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ncc(cn1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C22H26N4O2/c1-3-26(4-2)21(27)20-24-14-16(15-25-20)18-13-22(9-11-23-12-10-22)28-19-8-6-5-7-17(18)19/h5-8,13-15,23H,3-4,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 92 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297320

(CHEMBL551414 | Morpholino(4-(spiro[chromene-2,4'-p...)Show SMILES O=C(N1CCOCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C24H26N2O3/c27-23(26-13-15-28-16-14-26)19-7-5-18(6-8-19)21-17-24(9-11-25-12-10-24)29-22-4-2-1-3-20(21)22/h1-8,17,25H,9-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297342

(CHEMBL561339 | N,N-Diethyl-3-hydroxy-4-(spiro[chro...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(O)c1 |t:11| Show InChI InChI=1S/C24H28N2O3/c1-3-26(4-2)23(28)17-9-10-18(21(27)15-17)20-16-24(11-13-25-14-12-24)29-22-8-6-5-7-19(20)22/h5-10,15-16,25,27H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 94 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50252876

(CHEMBL494462 | N,N-Diethyl-4-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C24H28N2O2/c1-3-26(4-2)23(27)19-11-9-18(10-12-19)21-17-24(13-15-25-16-14-24)28-22-8-6-5-7-20(21)22/h5-12,17,25H,3-4,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297334

(CHEMBL557054 | N,N-Diethyl-5-(spiro[chromene-2,4'-...)Show SMILES CCN(CC)C(=O)c1ccc(cn1)C1=CC2(CCNCC2)Oc2ccccc12 |t:14| Show InChI InChI=1S/C23H27N3O2/c1-3-26(4-2)22(27)20-10-9-17(16-25-20)19-15-23(11-13-24-14-12-23)28-21-8-6-5-7-18(19)21/h5-10,15-16,24H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297341

(CHEMBL559414 | N,N-Diethyl-3-fluoro-4-(spiro[chrom...)Show SMILES CCN(CC)C(=O)c1ccc(C2=CC3(CCNCC3)Oc3ccccc23)c(F)c1 |t:11| Show InChI InChI=1S/C24H27FN2O2/c1-3-27(4-2)23(28)17-9-10-18(21(25)15-17)20-16-24(11-13-26-14-12-24)29-22-8-6-5-7-19(20)22/h5-10,15-16,26H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 980 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297314

(CHEMBL551413 | N-Methyl-4-(spiro[chromene-2,4'-pip...)Show SMILES CNC(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:11| Show InChI InChI=1S/C21H22N2O2/c1-22-20(24)16-8-6-15(7-9-16)18-14-21(10-12-23-13-11-21)25-19-5-3-2-4-17(18)19/h2-9,14,23H,10-13H2,1H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297318

(CHEMBL564922 | Pyrrolidin-1-yl(4-(spiro[chromene-2...)Show SMILES O=C(N1CCCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:15| Show InChI InChI=1S/C24H26N2O2/c27-23(26-15-3-4-16-26)19-9-7-18(8-10-19)21-17-24(11-13-25-14-12-24)28-22-6-2-1-5-20(21)22/h1-2,5-10,17,25H,3-4,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297316

(CHEMBL561882 | N,N-Diisopropyl-4-(spiro[chromene-2...)Show SMILES CC(C)N(C(C)C)C(=O)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C26H32N2O2/c1-18(2)28(19(3)4)25(29)21-11-9-20(10-12-21)23-17-26(13-15-27-16-14-26)30-24-8-6-5-7-22(23)24/h5-12,17-19,27H,13-16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 47 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50297319

(CHEMBL550261 | Piperidin-1-yl(4-(spiro[chromene-2,...)Show SMILES O=C(N1CCCCC1)c1ccc(cc1)C1=CC2(CCNCC2)Oc2ccccc12 |t:16| Show InChI InChI=1S/C25H28N2O2/c28-24(27-16-4-1-5-17-27)20-10-8-19(9-11-20)22-18-25(12-14-26-15-13-25)29-23-7-3-2-6-21(22)23/h2-3,6-11,18,26H,1,4-5,12-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Agonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding |
J Med Chem 52: 5685-702 (2009)
Article DOI: 10.1021/jm900773n
BindingDB Entry DOI: 10.7270/Q2VD6ZH3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data