 Found 64 hits of Enzyme Inhibition Constant Data
Found 64 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428292

(CHEMBL2338356)Show SMILES CCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C9H18N2O7P2/c1-2-3-4-10-5-6-11(8-10)7-9(12,19(13,14)15)20(16,17)18/h5-6,8,12H,2-4,7H2,1H3,(H3-,13,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428292

(CHEMBL2338356)Show SMILES CCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C9H18N2O7P2/c1-2-3-4-10-5-6-11(8-10)7-9(12,19(13,14)15)20(16,17)18/h5-6,8,12H,2-4,7H2,1H3,(H3-,13,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428291
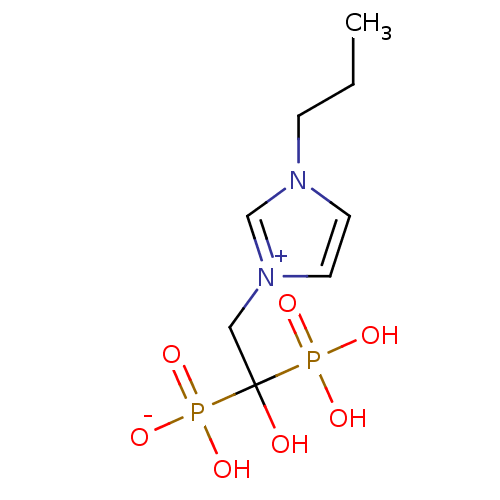
(CHEMBL2338355)Show SMILES CCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C8H16N2O7P2/c1-2-3-9-4-5-10(7-9)6-8(11,18(12,13)14)19(15,16)17/h4-5,7,11H,2-3,6H2,1H3,(H3-,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428296

(CHEMBL2338360)Show SMILES CCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C13H26N2O7P2/c1-2-3-4-5-6-7-8-14-9-10-15(12-14)11-13(16,23(17,18)19)24(20,21)22/h9-10,12,16H,2-8,11H2,1H3,(H3-,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428295

(CHEMBL2338359)Show SMILES CCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C12H24N2O7P2/c1-2-3-4-5-6-7-13-8-9-14(11-13)10-12(15,22(16,17)18)23(19,20)21/h8-9,11,15H,2-7,10H2,1H3,(H3-,16,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428312
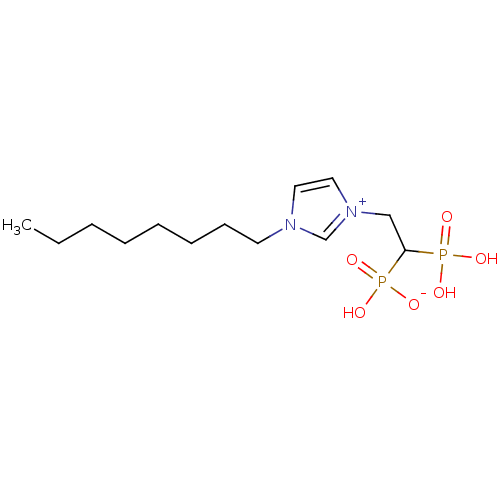
(CHEMBL2338376)Show SMILES CCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C13H26N2O6P2/c1-2-3-4-5-6-7-8-14-9-10-15(12-14)11-13(22(16,17)18)23(19,20)21/h9-10,12-13H,2-8,11H2,1H3,(H3-,16,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428313

(CHEMBL2338377)Show SMILES CCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C14H28N2O6P2/c1-2-3-4-5-6-7-8-9-15-10-11-16(13-15)12-14(23(17,18)19)24(20,21)22/h10-11,13-14H,2-9,12H2,1H3,(H3-,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428311

(CHEMBL2338375)Show SMILES CCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C12H24N2O6P2/c1-2-3-4-5-6-7-13-8-9-14(11-13)10-12(21(15,16)17)22(18,19)20/h8-9,11-12H,2-7,10H2,1H3,(H3-,15,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428294

(CHEMBL2338358)Show SMILES CCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C11H22N2O7P2/c1-2-3-4-5-6-12-7-8-13(10-12)9-11(14,21(15,16)17)22(18,19)20/h7-8,10,14H,2-6,9H2,1H3,(H3-,15,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428293
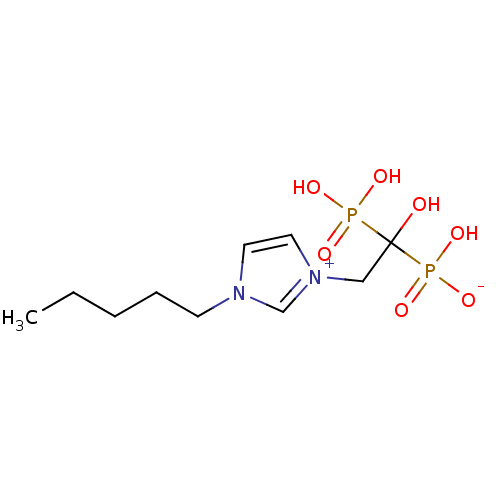
(CHEMBL2338357)Show SMILES CCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C10H20N2O7P2/c1-2-3-4-5-11-6-7-12(9-11)8-10(13,20(14,15)16)21(17,18)19/h6-7,9,13H,2-5,8H2,1H3,(H3-,14,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428290

(CHEMBL2338354)Show InChI InChI=1S/C7H14N2O7P2/c1-2-8-3-4-9(6-8)5-7(10,17(11,12)13)18(14,15)16/h3-4,6,10H,2,5H2,1H3,(H3-,11,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428310
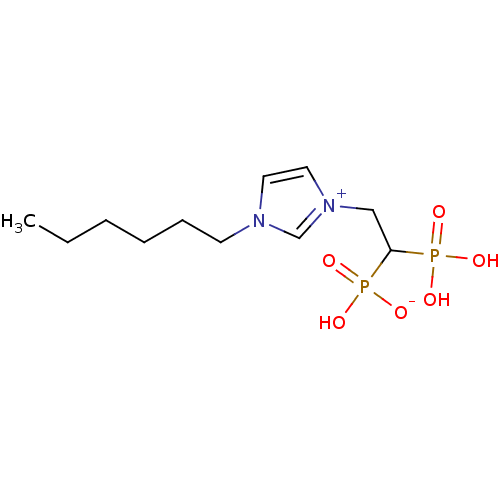
(CHEMBL2338374)Show SMILES CCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C11H22N2O6P2/c1-2-3-4-5-6-12-7-8-13(10-12)9-11(20(14,15)16)21(17,18)19/h7-8,10-11H,2-6,9H2,1H3,(H3-,14,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428307
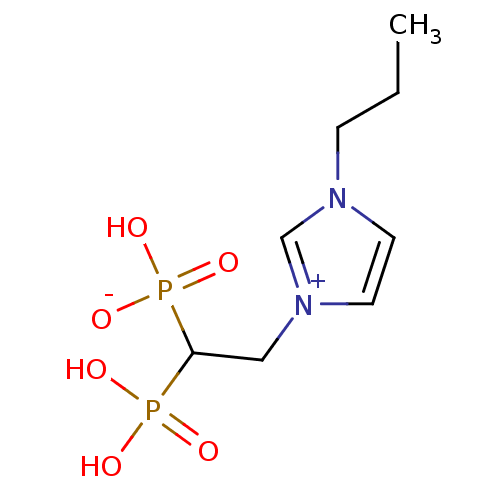
(CHEMBL2338371)Show InChI InChI=1S/C8H16N2O6P2/c1-2-3-9-4-5-10(7-9)6-8(17(11,12)13)18(14,15)16/h4-5,7-8H,2-3,6H2,1H3,(H3-,11,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428308
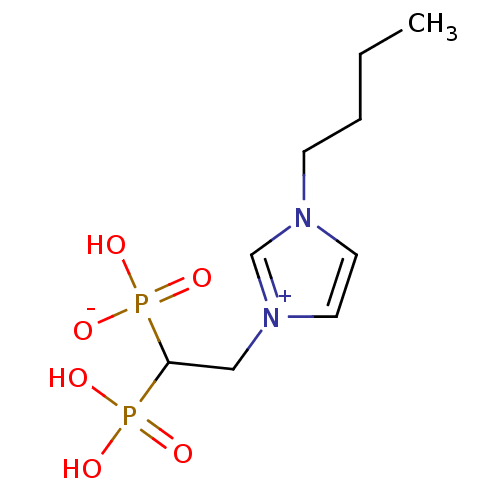
(CHEMBL2338372)Show InChI InChI=1S/C9H18N2O6P2/c1-2-3-4-10-5-6-11(8-10)7-9(18(12,13)14)19(15,16)17/h5-6,8-9H,2-4,7H2,1H3,(H3-,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428297
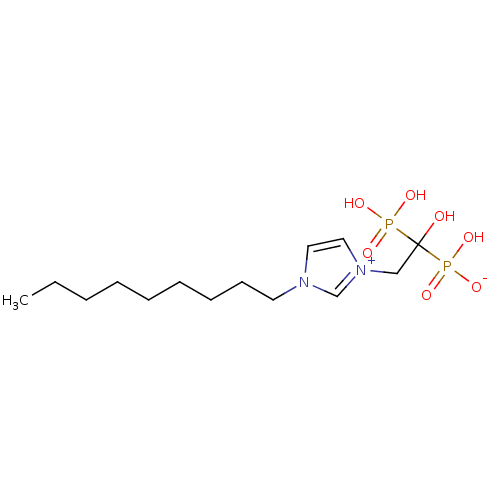
(CHEMBL2338361)Show SMILES CCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C14H28N2O7P2/c1-2-3-4-5-6-7-8-9-15-10-11-16(13-15)12-14(17,24(18,19)20)25(21,22)23/h10-11,13,17H,2-9,12H2,1H3,(H3-,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428289
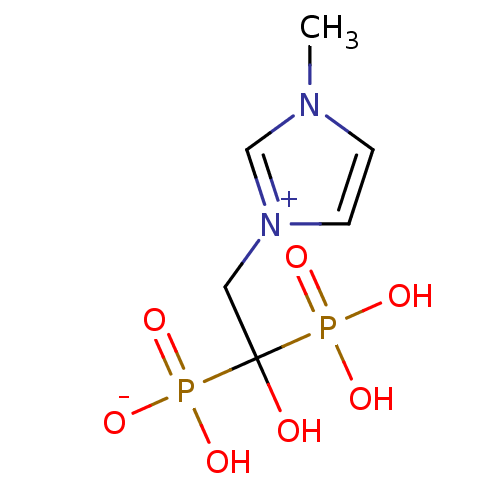
(CHEMBL2338353)Show InChI InChI=1S/C6H12N2O7P2/c1-7-2-3-8(5-7)4-6(9,16(10,11)12)17(13,14)15/h2-3,5,9H,4H2,1H3,(H3-,10,11,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428306

(CHEMBL2338370)Show InChI InChI=1S/C7H14N2O6P2/c1-2-8-3-4-9(6-8)5-7(16(10,11)12)17(13,14)15/h3-4,6-7H,2,5H2,1H3,(H3-,10,11,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428296

(CHEMBL2338360)Show SMILES CCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C13H26N2O7P2/c1-2-3-4-5-6-7-8-14-9-10-15(12-14)11-13(16,23(17,18)19)24(20,21)22/h9-10,12,16H,2-8,11H2,1H3,(H3-,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428313

(CHEMBL2338377)Show SMILES CCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C14H28N2O6P2/c1-2-3-4-5-6-7-8-9-15-10-11-16(13-15)12-14(23(17,18)19)24(20,21)22/h10-11,13-14H,2-9,12H2,1H3,(H3-,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428314
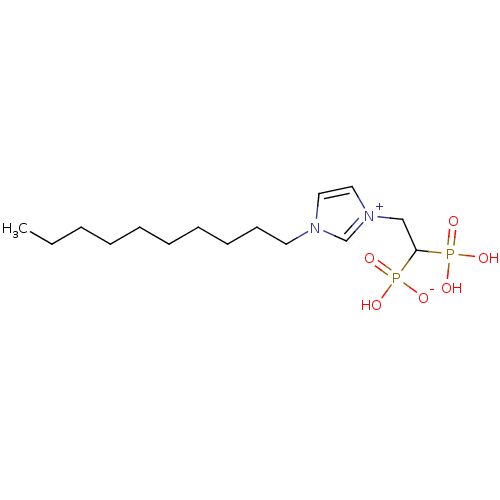
(CHEMBL2338378)Show SMILES CCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C15H30N2O6P2/c1-2-3-4-5-6-7-8-9-10-16-11-12-17(14-16)13-15(24(18,19)20)25(21,22)23/h11-12,14-15H,2-10,13H2,1H3,(H3-,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428314

(CHEMBL2338378)Show SMILES CCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C15H30N2O6P2/c1-2-3-4-5-6-7-8-9-10-16-11-12-17(14-16)13-15(24(18,19)20)25(21,22)23/h11-12,14-15H,2-10,13H2,1H3,(H3-,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428295

(CHEMBL2338359)Show SMILES CCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C12H24N2O7P2/c1-2-3-4-5-6-7-13-8-9-14(11-13)10-12(15,22(16,17)18)23(19,20)21/h8-9,11,15H,2-7,10H2,1H3,(H3-,16,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428309
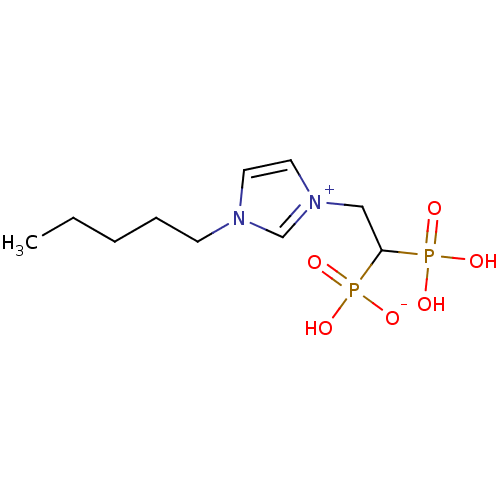
(CHEMBL2338373)Show InChI InChI=1S/C10H20N2O6P2/c1-2-3-4-5-11-6-7-12(9-11)8-10(19(13,14)15)20(16,17)18/h6-7,9-10H,2-5,8H2,1H3,(H3-,13,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428294

(CHEMBL2338358)Show SMILES CCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C11H22N2O7P2/c1-2-3-4-5-6-12-7-8-13(10-12)9-11(14,21(15,16)17)22(18,19)20/h7-8,10,14H,2-6,9H2,1H3,(H3-,15,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428293

(CHEMBL2338357)Show SMILES CCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C10H20N2O7P2/c1-2-3-4-5-11-6-7-12(9-11)8-10(13,20(14,15)16)21(17,18)19/h6-7,9,13H,2-5,8H2,1H3,(H3-,14,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428298
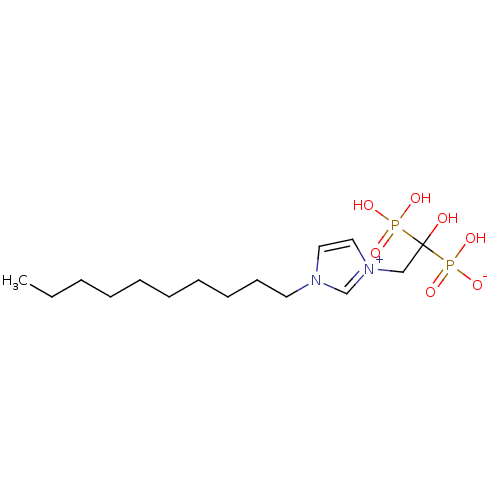
(CHEMBL2338362)Show SMILES CCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C15H30N2O7P2/c1-2-3-4-5-6-7-8-9-10-16-11-12-17(14-16)13-15(18,25(19,20)21)26(22,23)24/h11-12,14,18H,2-10,13H2,1H3,(H3-,19,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428297

(CHEMBL2338361)Show SMILES CCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C14H28N2O7P2/c1-2-3-4-5-6-7-8-9-15-10-11-16(13-15)12-14(17,24(18,19)20)25(21,22)23/h10-11,13,17H,2-9,12H2,1H3,(H3-,18,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428290

(CHEMBL2338354)Show InChI InChI=1S/C7H14N2O7P2/c1-2-8-3-4-9(6-8)5-7(10,17(11,12)13)18(14,15)16/h3-4,6,10H,2,5H2,1H3,(H3-,11,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428308

(CHEMBL2338372)Show InChI InChI=1S/C9H18N2O6P2/c1-2-3-4-10-5-6-11(8-10)7-9(18(12,13)14)19(15,16)17/h5-6,8-9H,2-4,7H2,1H3,(H3-,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428315

(CHEMBL2338379)Show SMILES CCCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C16H32N2O6P2/c1-2-3-4-5-6-7-8-9-10-11-17-12-13-18(15-17)14-16(25(19,20)21)26(22,23)24/h12-13,15-16H,2-11,14H2,1H3,(H3-,19,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428304

(CHEMBL2338368)Show InChI InChI=1S/C5H10N2O6P2/c8-14(9,10)5(15(11,12)13)3-7-2-1-6-4-7/h1-2,4-5H,3H2,(H4,8,9,10,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428307

(CHEMBL2338371)Show InChI InChI=1S/C8H16N2O6P2/c1-2-3-9-4-5-10(7-9)6-8(17(11,12)13)18(14,15)16/h4-5,7-8H,2-3,6H2,1H3,(H3-,11,12,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428289

(CHEMBL2338353)Show InChI InChI=1S/C6H12N2O7P2/c1-7-2-3-8(5-7)4-6(9,16(10,11)12)17(13,14)15/h2-3,5,9H,4H2,1H3,(H3-,10,11,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428305

(CHEMBL2338369)Show InChI InChI=1S/C6H12N2O6P2/c1-7-2-3-8(5-7)4-6(15(9,10)11)16(12,13)14/h2-3,5-6H,4H2,1H3,(H3-,9,10,11,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428304

(CHEMBL2338368)Show InChI InChI=1S/C5H10N2O6P2/c8-14(9,10)5(15(11,12)13)3-7-2-1-6-4-7/h1-2,4-5H,3H2,(H4,8,9,10,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428291

(CHEMBL2338355)Show SMILES CCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C8H16N2O7P2/c1-2-3-9-4-5-10(7-9)6-8(11,18(12,13)14)19(15,16)17/h4-5,7,11H,2-3,6H2,1H3,(H3-,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428312

(CHEMBL2338376)Show SMILES CCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C13H26N2O6P2/c1-2-3-4-5-6-7-8-14-9-10-15(12-14)11-13(22(16,17)18)23(19,20)21/h9-10,12-13H,2-8,11H2,1H3,(H3-,16,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428306

(CHEMBL2338370)Show InChI InChI=1S/C7H14N2O6P2/c1-2-8-3-4-9(6-8)5-7(16(10,11)12)17(13,14)15/h3-4,6-7H,2,5H2,1H3,(H3-,10,11,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428311

(CHEMBL2338375)Show SMILES CCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C12H24N2O6P2/c1-2-3-4-5-6-7-13-8-9-14(11-13)10-12(21(15,16)17)22(18,19)20/h8-9,11-12H,2-7,10H2,1H3,(H3-,15,16,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428305

(CHEMBL2338369)Show InChI InChI=1S/C6H12N2O6P2/c1-7-2-3-8(5-7)4-6(15(9,10)11)16(12,13)14/h2-3,5-6H,4H2,1H3,(H3-,9,10,11,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428309

(CHEMBL2338373)Show InChI InChI=1S/C10H20N2O6P2/c1-2-3-4-5-11-6-7-12(9-11)8-10(19(13,14)15)20(16,17)18/h6-7,9-10H,2-5,8H2,1H3,(H3-,13,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428310

(CHEMBL2338374)Show SMILES CCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C11H22N2O6P2/c1-2-3-4-5-6-12-7-8-13(10-12)9-11(20(14,15)16)21(17,18)19/h7-8,10-11H,2-6,9H2,1H3,(H3-,14,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428299
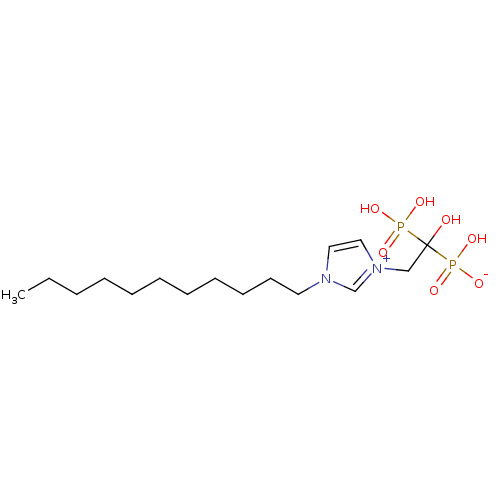
(CHEMBL2338363)Show SMILES CCCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C16H32N2O7P2/c1-2-3-4-5-6-7-8-9-10-11-17-12-13-18(15-17)14-16(19,26(20,21)22)27(23,24)25/h12-13,15,19H,2-11,14H2,1H3,(H3-,20,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428298

(CHEMBL2338362)Show SMILES CCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C15H30N2O7P2/c1-2-3-4-5-6-7-8-9-10-16-11-12-17(14-16)13-15(18,25(19,20)21)26(22,23)24/h11-12,14,18H,2-10,13H2,1H3,(H3-,19,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428315

(CHEMBL2338379)Show SMILES CCCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C16H32N2O6P2/c1-2-3-4-5-6-7-8-9-10-11-17-12-13-18(15-17)14-16(25(19,20)21)26(22,23)24/h12-13,15-16H,2-11,14H2,1H3,(H3-,19,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428316

(CHEMBL2338380)Show SMILES CCCCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C17H34N2O6P2/c1-2-3-4-5-6-7-8-9-10-11-12-18-13-14-19(16-18)15-17(26(20,21)22)27(23,24)25/h13-14,16-17H,2-12,15H2,1H3,(H3-,20,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428299

(CHEMBL2338363)Show SMILES CCCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C16H32N2O7P2/c1-2-3-4-5-6-7-8-9-10-11-17-12-13-18(15-17)14-16(19,26(20,21)22)27(23,24)25/h12-13,15,19H,2-11,14H2,1H3,(H3-,20,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428317
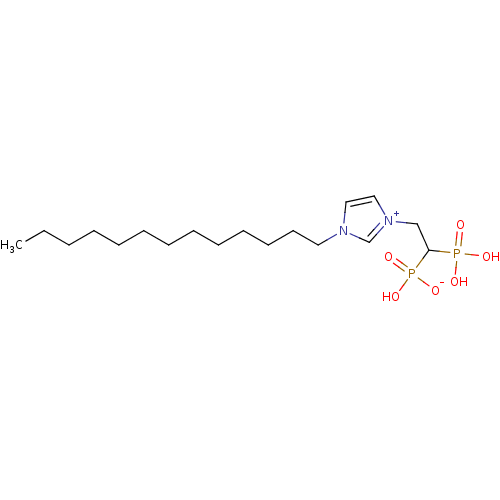
(CHEMBL2338381)Show SMILES CCCCCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C18H36N2O6P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-19-14-15-20(17-19)16-18(27(21,22)23)28(24,25)26/h14-15,17-18H,2-13,16H2,1H3,(H3-,21,22,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428316

(CHEMBL2338380)Show SMILES CCCCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C17H34N2O6P2/c1-2-3-4-5-6-7-8-9-10-11-12-18-13-14-19(16-18)15-17(26(20,21)22)27(23,24)25/h13-14,16-17H,2-12,15H2,1H3,(H3-,20,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428317

(CHEMBL2338381)Show SMILES CCCCCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C18H36N2O6P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-19-14-15-20(17-19)16-18(27(21,22)23)28(24,25)26/h14-15,17-18H,2-13,16H2,1H3,(H3-,21,22,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428300
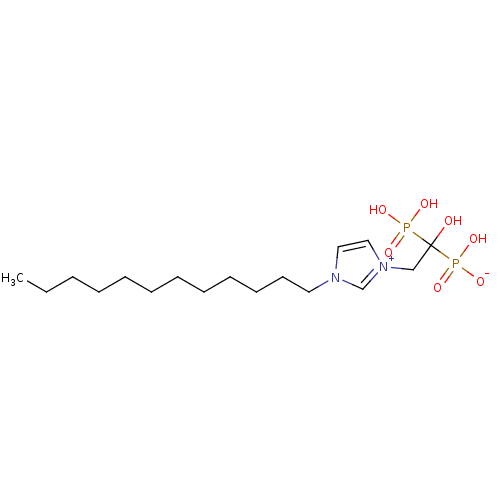
(CHEMBL2338364)Show SMILES CCCCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C17H34N2O7P2/c1-2-3-4-5-6-7-8-9-10-11-12-18-13-14-19(16-18)15-17(20,27(21,22)23)28(24,25)26/h13-14,16,20H,2-12,15H2,1H3,(H3-,21,22,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428300

(CHEMBL2338364)Show SMILES CCCCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C17H34N2O7P2/c1-2-3-4-5-6-7-8-9-10-11-12-18-13-14-19(16-18)15-17(20,27(21,22)23)28(24,25)26/h13-14,16,20H,2-12,15H2,1H3,(H3-,21,22,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428319

(CHEMBL2338382)Show SMILES CCCCCCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C19H38N2O6P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-20-15-16-21(18-20)17-19(28(22,23)24)29(25,26)27/h15-16,18-19H,2-14,17H2,1H3,(H3-,22,23,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428301

(CHEMBL2338365)Show SMILES CCCCCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C18H36N2O7P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-19-14-15-20(17-19)16-18(21,28(22,23)24)29(25,26)27/h14-15,17,21H,2-13,16H2,1H3,(H3-,22,23,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428319

(CHEMBL2338382)Show SMILES CCCCCCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C19H38N2O6P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-20-15-16-21(18-20)17-19(28(22,23)24)29(25,26)27/h15-16,18-19H,2-14,17H2,1H3,(H3-,22,23,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428302

(CHEMBL2338366)Show SMILES CCCCCCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C19H38N2O7P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-20-15-16-21(18-20)17-19(22,29(23,24)25)30(26,27)28/h15-16,18,22H,2-14,17H2,1H3,(H3-,23,24,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428301

(CHEMBL2338365)Show SMILES CCCCCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C18H36N2O7P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-19-14-15-20(17-19)16-18(21,28(22,23)24)29(25,26)27/h14-15,17,21H,2-13,16H2,1H3,(H3-,22,23,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428318

(CHEMBL2338383)Show SMILES CCCCCCCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C20H40N2O6P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-21-16-17-22(19-21)18-20(29(23,24)25)30(26,27)28/h16-17,19-20H,2-15,18H2,1H3,(H3-,23,24,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428303

(CHEMBL2338367)Show SMILES CCCCCCCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C20H40N2O7P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-21-16-17-22(19-21)18-20(23,30(24,25)26)31(27,28)29/h16-17,19,23H,2-15,18H2,1H3,(H3-,24,25,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human FPPS at 1 mM by X-ray crystallographic analysis |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428318

(CHEMBL2338383)Show SMILES CCCCCCCCCCCCCCCn1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C20H40N2O6P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-21-16-17-22(19-21)18-20(29(23,24)25)30(26,27)28/h16-17,19-20H,2-15,18H2,1H3,(H3-,23,24,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428302

(CHEMBL2338366)Show SMILES CCCCCCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C19H38N2O7P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-20-15-16-21(18-20)17-19(22,29(23,24)25)30(26,27)28/h15-16,18,22H,2-14,17H2,1H3,(H3-,23,24,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50428303

(CHEMBL2338367)Show SMILES CCCCCCCCCCCCCCCn1cc[n+](CC(O)(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C20H40N2O7P2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-21-16-17-22(19-21)18-20(23,30(24,25)26)31(27,28)29/h16-17,19,23H,2-15,18H2,1H3,(H3-,24,25,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PrenylX Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged human truncated FPPS (6-353) expressed in Escherichia coli BL21(DE3) cells using geranyl diphosphate and isopentenyl diphos... |
ACS Med Chem Lett 4: 423-427 (2013)
Article DOI: 10.1021/ml4000436
BindingDB Entry DOI: 10.7270/Q2KP83GB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data