 Found 111 hits of Enzyme Inhibition Constant Data
Found 111 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073585
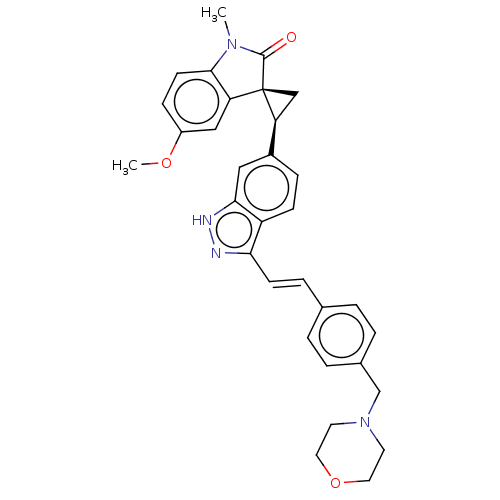
(CHEMBL3408945 | US10358436, Example A102 | US99078...)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073586
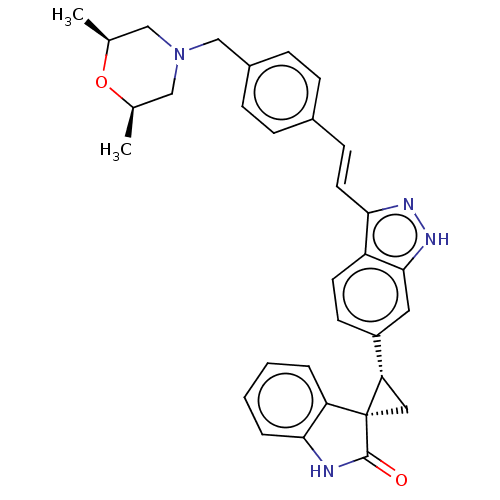
(CHEMBL3408946 | US10358436, Example A198 | US99078...)Show SMILES C[C@H]1CN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C[C@@H](C)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive binding to PLK4 (unknown origin) by double reciprocal plot analysis in presence of ATP |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50044656
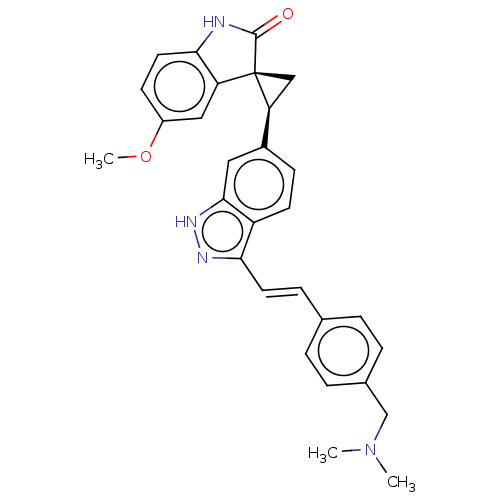
(CHEMBL3353348 | US10358436, Example A35 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN(C)C)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073551
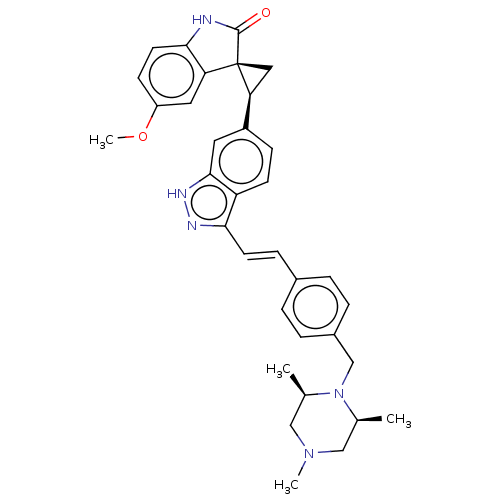
(CHEMBL3408955)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6[C@@H](C)CN(C)C[C@H]6C)cc5)n[nH]c4c3)c2c1 |r| Show InChI InChI=1S/C34H37N5O2/c1-21-18-38(3)19-22(2)39(21)20-24-7-5-23(6-8-24)9-13-30-27-12-10-25(15-32(27)37-36-30)29-17-34(29)28-16-26(41-4)11-14-31(28)35-33(34)40/h5-16,21-22,29H,17-20H2,1-4H3,(H,35,40)(H,36,37)/b13-9+/t21-,22+,29-,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073584
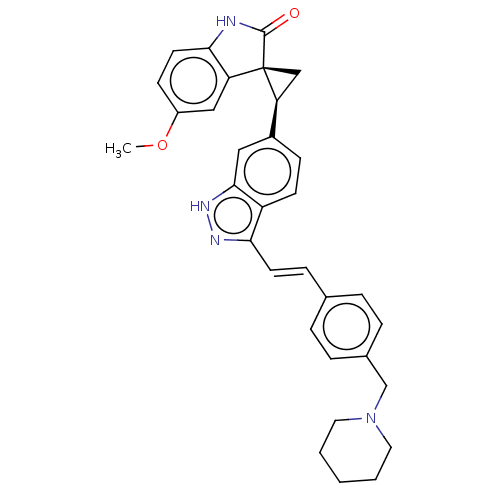
(CHEMBL3408944 | US10358436, Example A59 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCCCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073550
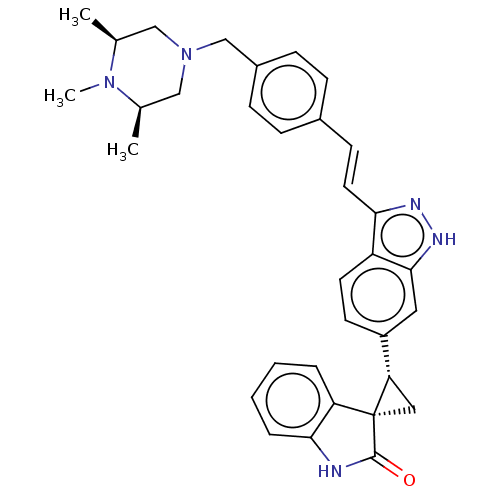
(CHEMBL3408954)Show SMILES C[C@H]1CN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C[C@@H](C)N1C |r| Show InChI InChI=1S/C33H35N5O/c1-21-18-38(19-22(2)37(21)3)20-24-10-8-23(9-11-24)12-15-29-26-14-13-25(16-31(26)36-35-29)28-17-33(28)27-6-4-5-7-30(27)34-32(33)39/h4-16,21-22,28H,17-20H2,1-3H3,(H,34,39)(H,35,36)/b15-12+/t21-,22+,28-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50044665
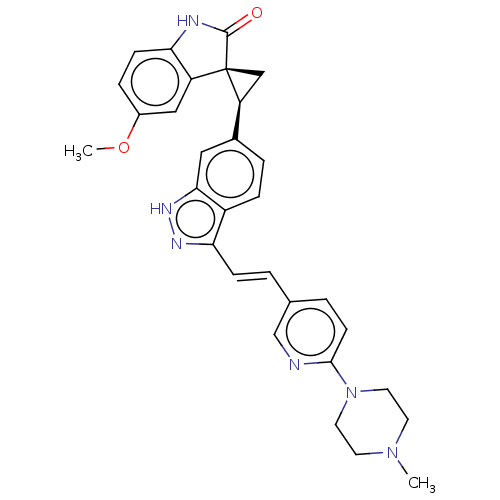
(CHEMBL3353341 | US10358436, Example A33 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(nc5)N5CCN(C)CC5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50044662
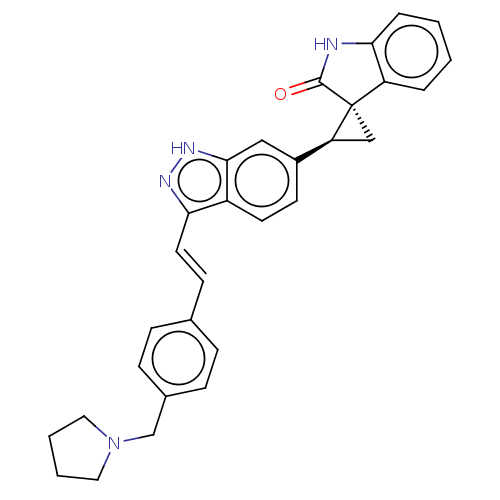
(CHEMBL3353361 | US10358436, Example A61 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(CN4CCCC4)cc3)n[nH]c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50044667

(CHEMBL3353362 | US10358436, Example A58 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(CN4CCCCC4)cc3)n[nH]c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073555
(CHEMBL3408941 | US10358436, Example A92 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(cc3)N3CCNCC3)n[nH]c2c1 |r| Show InChI InChI=1S/C29H27N5O/c35-28-29(23-3-1-2-4-26(23)31-28)18-24(29)20-8-11-22-25(32-33-27(22)17-20)12-7-19-5-9-21(10-6-19)34-15-13-30-14-16-34/h1-12,17,24,30H,13-16,18H2,(H,31,35)(H,32,33)/b12-7+/t24-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073552

(CHEMBL3408956 | US10358436, Example A78 | US990780...)Show SMILES CN1CCC(CC1)Oc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| Show InChI InChI=1S/C31H30N4O2/c1-35-16-14-23(15-17-35)37-22-10-6-20(7-11-22)8-13-27-24-12-9-21(18-29(24)34-33-27)26-19-31(26)25-4-2-3-5-28(25)32-30(31)36/h2-13,18,23,26H,14-17,19H2,1H3,(H,32,36)(H,33,34)/b13-8+/t26-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50044657

(CHEMBL3353347 | US10358436, Example A24 | US990780...)Show SMILES CN(C)Cc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50044657

(CHEMBL3353347 | US10358436, Example A24 | US990780...)Show SMILES CN(C)Cc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073585

(CHEMBL3408945 | US10358436, Example A102 | US99078...)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073554
(CHEMBL3408940 | US10358436, Example A110 | US99078...)Show SMILES CN1C(=O)[C@@]2(C[C@H]2c2ccc3c(\C=C\c4ccc(nc4)N4CCN(C)CC4)n[nH]c3c2)c2ccccc12 |r| Show InChI InChI=1S/C30H30N6O/c1-34-13-15-36(16-14-34)28-12-8-20(19-31-28)7-11-25-22-10-9-21(17-26(22)33-32-25)24-18-30(24)23-5-3-4-6-27(23)35(2)29(30)37/h3-12,17,19,24H,13-16,18H2,1-2H3,(H,32,33)/b11-7+/t24-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50044675
(CHEMBL3353354 | US10358436, Example A56 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(CN4CCOCC4)cc3)n[nH]c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073582
(CHEMBL3408942 | US10358436, Example A109 | US99078...)Show SMILES CN(C)Cc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)N(C)c3ccccc23)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073547

(CHEMBL3408951)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(cc5)[C@H](C)N5CCOCC5)n[nH]c4c3)c2c1 |r| Show InChI InChI=1S/C33H34N4O3/c1-21(37-14-16-40-17-15-37)23-7-4-22(5-8-23)6-12-29-26-11-9-24(18-30(26)35-34-29)28-20-33(28)27-19-25(39-3)10-13-31(27)36(2)32(33)38/h4-13,18-19,21,28H,14-17,20H2,1-3H3,(H,34,35)/b12-6+/t21-,28-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073553
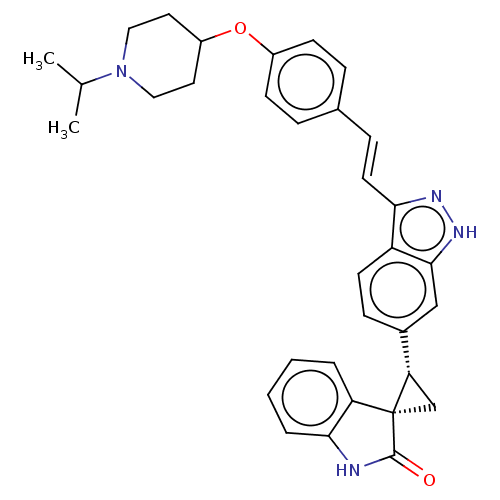
(CHEMBL3408957)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| Show InChI InChI=1S/C33H34N4O2/c1-21(2)37-17-15-25(16-18-37)39-24-11-7-22(8-12-24)9-14-29-26-13-10-23(19-31(26)36-35-29)28-20-33(28)27-5-3-4-6-30(27)34-32(33)38/h3-14,19,21,25,28H,15-18,20H2,1-2H3,(H,34,38)(H,35,36)/b14-9+/t28-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073549

(CHEMBL3408953 | US10358436, Example A72 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(CN4CCCOCC4)cc3)n[nH]c2c1 |r| Show InChI InChI=1S/C31H30N4O2/c36-30-31(25-4-1-2-5-28(25)32-30)19-26(31)23-11-12-24-27(33-34-29(24)18-23)13-10-21-6-8-22(9-7-21)20-35-14-3-16-37-17-15-35/h1-2,4-13,18,26H,3,14-17,19-20H2,(H,32,36)(H,33,34)/b13-10+/t26-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50044674
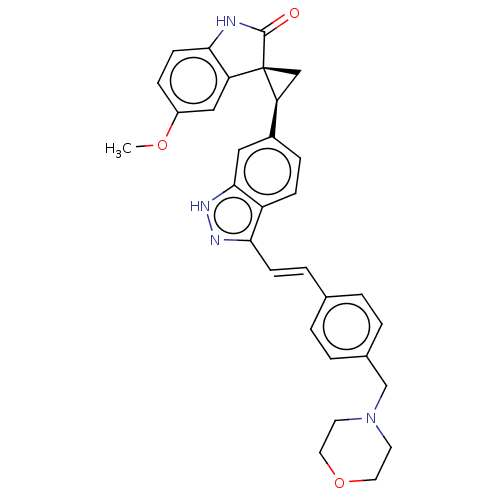
(CHEMBL3353355 | US10358436, Example A42 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50044674

(CHEMBL3353355 | US10358436, Example A42 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50044668
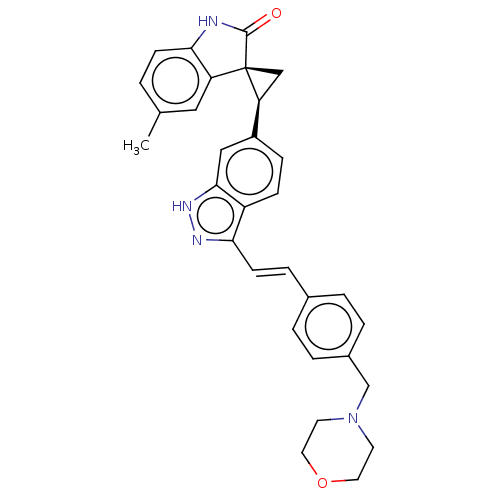
(CHEMBL3353357 | US10358436, Example A195 | US99078...)Show SMILES Cc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50044660
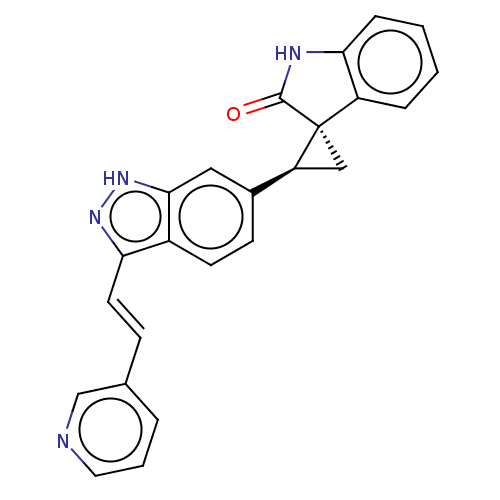
(CHEMBL3353342 | US10358436, Example A29 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3cccnc3)n[nH]c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073583
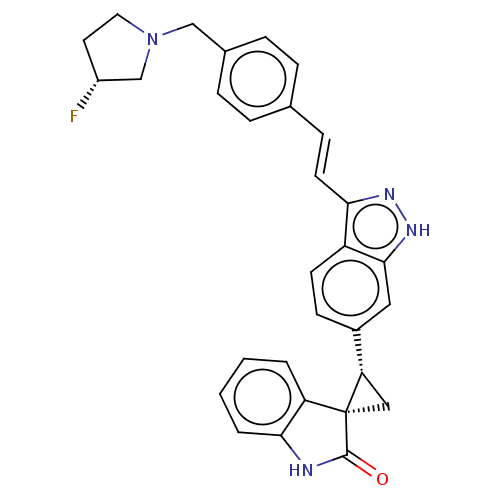
(CHEMBL3408943 | US9907800, Example A106)Show SMILES F[C@@H]1CCN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073548
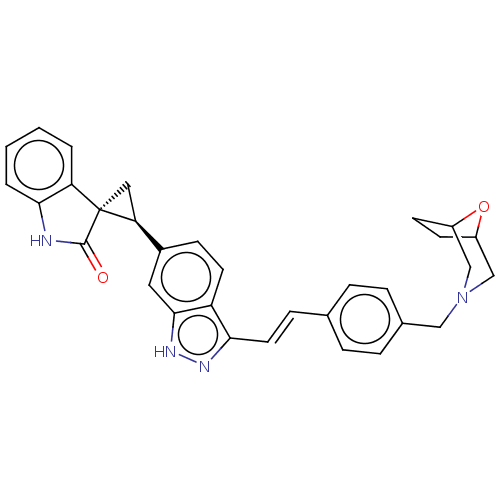
(CHEMBL3408952)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(CN4CC5CCC(C4)O5)cc3)n[nH]c2c1 |r| Show InChI InChI=1S/C32H30N4O2/c37-31-32(26-3-1-2-4-29(26)33-31)16-27(32)22-10-13-25-28(34-35-30(25)15-22)14-9-20-5-7-21(8-6-20)17-36-18-23-11-12-24(19-36)38-23/h1-10,13-15,23-24,27H,11-12,16-19H2,(H,33,37)(H,34,35)/b14-9+/t23?,24?,27-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073544
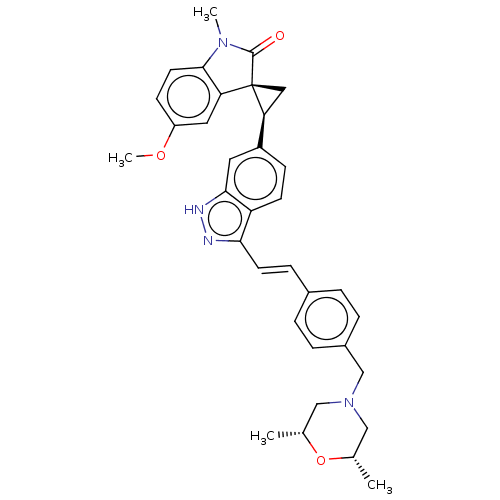
(CHEMBL3408948)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TRKA (unknown origin) by FRET analysis |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073586

(CHEMBL3408946 | US10358436, Example A198 | US99078...)Show SMILES C[C@H]1CN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C[C@@H](C)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073545
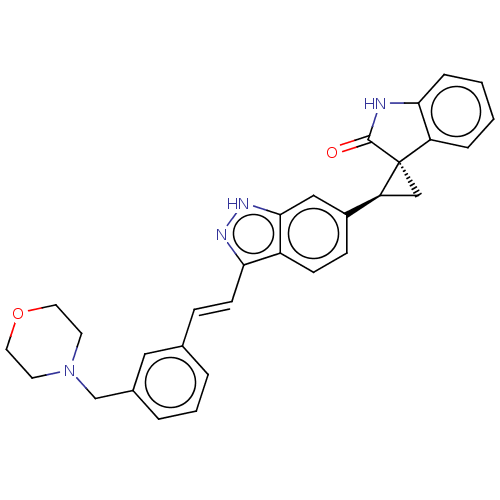
(CHEMBL3408949 | US10358436, Example A151 | US99078...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3cccc(CN4CCOCC4)c3)n[nH]c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TRKB (unknown origin) by FRET analysis |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073546

(CHEMBL3408950)Show SMILES C[C@H]1CN(Cc2cccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)c2)C[C@@H](C)O1 |r| Show InChI InChI=1S/C32H32N4O2/c1-20-17-36(18-21(2)38-20)19-23-7-5-6-22(14-23)10-13-28-25-12-11-24(15-30(25)35-34-28)27-16-32(27)26-8-3-4-9-29(26)33-31(32)37/h3-15,20-21,27H,16-19H2,1-2H3,(H,33,37)(H,34,35)/b13-10+/t20-,21+,27-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by ELISA |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TIE2 (unknown origin) by FRET analysis |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of AURKA (unknown origin) by FRET analysis |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073554

(CHEMBL3408940 | US10358436, Example A110 | US99078...)Show SMILES CN1C(=O)[C@@]2(C[C@H]2c2ccc3c(\C=C\c4ccc(nc4)N4CCN(C)CC4)n[nH]c3c2)c2ccccc12 |r| Show InChI InChI=1S/C30H30N6O/c1-34-13-15-36(16-14-34)28-12-8-20(19-31-28)7-11-25-22-10-9-21(17-26(22)33-32-25)24-18-30(24)23-5-3-4-6-27(23)35(2)29(30)37/h3-12,17,19,24H,13-16,18H2,1-2H3,(H,32,33)/b11-7+/t24-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073544

(CHEMBL3408948)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073547

(CHEMBL3408951)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(cc5)[C@H](C)N5CCOCC5)n[nH]c4c3)c2c1 |r| Show InChI InChI=1S/C33H34N4O3/c1-21(37-14-16-40-17-15-37)23-7-4-22(5-8-23)6-12-29-26-11-9-24(18-30(26)35-34-29)28-20-33(28)27-19-25(39-3)10-13-31(27)36(2)32(33)38/h4-13,18-19,21,28H,14-17,20H2,1-3H3,(H,34,35)/b12-6+/t21-,28-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073547

(CHEMBL3408951)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(cc5)[C@H](C)N5CCOCC5)n[nH]c4c3)c2c1 |r| Show InChI InChI=1S/C33H34N4O3/c1-21(37-14-16-40-17-15-37)23-7-4-22(5-8-23)6-12-29-26-11-9-24(18-30(26)35-34-29)28-20-33(28)27-19-25(39-3)10-13-31(27)36(2)32(33)38/h4-13,18-19,21,28H,14-17,20H2,1-3H3,(H,34,35)/b12-6+/t21-,28-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073551

(CHEMBL3408955)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6[C@@H](C)CN(C)C[C@H]6C)cc5)n[nH]c4c3)c2c1 |r| Show InChI InChI=1S/C34H37N5O2/c1-21-18-38(3)19-22(2)39(21)20-24-7-5-23(6-8-24)9-13-30-27-12-10-25(15-32(27)37-36-30)29-17-34(29)28-16-26(41-4)11-14-31(28)35-33(34)40/h5-16,21-22,29H,17-20H2,1-4H3,(H,35,40)(H,36,37)/b13-9+/t21-,22+,29-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50044675

(CHEMBL3353354 | US10358436, Example A56 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(CN4CCOCC4)cc3)n[nH]c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073544

(CHEMBL3408948)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50044675

(CHEMBL3353354 | US10358436, Example A56 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(CN4CCOCC4)cc3)n[nH]c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50044674

(CHEMBL3353355 | US10358436, Example A42 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073586

(CHEMBL3408946 | US10358436, Example A198 | US99078...)Show SMILES C[C@H]1CN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C[C@@H](C)O1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073551

(CHEMBL3408955)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6[C@@H](C)CN(C)C[C@H]6C)cc5)n[nH]c4c3)c2c1 |r| Show InChI InChI=1S/C34H37N5O2/c1-21-18-38(3)19-22(2)39(21)20-24-7-5-23(6-8-24)9-13-30-27-12-10-25(15-32(27)37-36-30)29-17-34(29)28-16-26(41-4)11-14-31(28)35-33(34)40/h5-16,21-22,29H,17-20H2,1-4H3,(H,35,40)(H,36,37)/b13-9+/t21-,22+,29-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073555

(CHEMBL3408941 | US10358436, Example A92 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(cc3)N3CCNCC3)n[nH]c2c1 |r| Show InChI InChI=1S/C29H27N5O/c35-28-29(23-3-1-2-4-26(23)31-28)18-24(29)20-8-11-22-25(32-33-27(22)17-20)12-7-19-5-9-21(10-6-19)34-15-13-30-14-16-34/h1-12,17,24,30H,13-16,18H2,(H,31,35)(H,32,33)/b12-7+/t24-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073583

(CHEMBL3408943 | US9907800, Example A106)Show SMILES F[C@@H]1CCN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073547

(CHEMBL3408951)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(cc5)[C@H](C)N5CCOCC5)n[nH]c4c3)c2c1 |r| Show InChI InChI=1S/C33H34N4O3/c1-21(37-14-16-40-17-15-37)23-7-4-22(5-8-23)6-12-29-26-11-9-24(18-30(26)35-34-29)28-20-33(28)27-19-25(39-3)10-13-31(27)36(2)32(33)38/h4-13,18-19,21,28H,14-17,20H2,1-3H3,(H,34,35)/b12-6+/t21-,28-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073582

(CHEMBL3408942 | US10358436, Example A109 | US99078...)Show SMILES CN(C)Cc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)N(C)c3ccccc23)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50044657

(CHEMBL3353347 | US10358436, Example A24 | US990780...)Show SMILES CN(C)Cc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50044657

(CHEMBL3353347 | US10358436, Example A24 | US990780...)Show SMILES CN(C)Cc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073584

(CHEMBL3408944 | US10358436, Example A59 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCCCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073585

(CHEMBL3408945 | US10358436, Example A102 | US99078...)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50044668

(CHEMBL3353357 | US10358436, Example A195 | US99078...)Show SMILES Cc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073583

(CHEMBL3408943 | US9907800, Example A106)Show SMILES F[C@@H]1CCN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073585

(CHEMBL3408945 | US10358436, Example A102 | US99078...)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073553

(CHEMBL3408957)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| Show InChI InChI=1S/C33H34N4O2/c1-21(2)37-17-15-25(16-18-37)39-24-11-7-22(8-12-24)9-14-29-26-13-10-23(19-31(26)36-35-29)28-20-33(28)27-5-3-4-6-30(27)34-32(33)38/h3-14,19,21,25,28H,15-18,20H2,1-2H3,(H,34,38)(H,35,36)/b14-9+/t28-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073544

(CHEMBL3408948)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073554

(CHEMBL3408940 | US10358436, Example A110 | US99078...)Show SMILES CN1C(=O)[C@@]2(C[C@H]2c2ccc3c(\C=C\c4ccc(nc4)N4CCN(C)CC4)n[nH]c3c2)c2ccccc12 |r| Show InChI InChI=1S/C30H30N6O/c1-34-13-15-36(16-14-34)28-12-8-20(19-31-28)7-11-25-22-10-9-21(17-26(22)33-32-25)24-18-30(24)23-5-3-4-6-27(23)35(2)29(30)37/h3-12,17,19,24H,13-16,18H2,1-2H3,(H,32,33)/b11-7+/t24-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50044668

(CHEMBL3353357 | US10358436, Example A195 | US99078...)Show SMILES Cc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50044674

(CHEMBL3353355 | US10358436, Example A42 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073584

(CHEMBL3408944 | US10358436, Example A59 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCCCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50044665

(CHEMBL3353341 | US10358436, Example A33 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(nc5)N5CCN(C)CC5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073583

(CHEMBL3408943 | US9907800, Example A106)Show SMILES F[C@@H]1CCN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073553

(CHEMBL3408957)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| Show InChI InChI=1S/C33H34N4O2/c1-21(2)37-17-15-25(16-18-37)39-24-11-7-22(8-12-24)9-14-29-26-13-10-23(19-31(26)36-35-29)28-20-33(28)27-5-3-4-6-30(27)34-32(33)38/h3-14,19,21,25,28H,15-18,20H2,1-2H3,(H,34,38)(H,35,36)/b14-9+/t28-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073551

(CHEMBL3408955)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6[C@@H](C)CN(C)C[C@H]6C)cc5)n[nH]c4c3)c2c1 |r| Show InChI InChI=1S/C34H37N5O2/c1-21-18-38(3)19-22(2)39(21)20-24-7-5-23(6-8-24)9-13-30-27-12-10-25(15-32(27)37-36-30)29-17-34(29)28-16-26(41-4)11-14-31(28)35-33(34)40/h5-16,21-22,29H,17-20H2,1-4H3,(H,35,40)(H,36,37)/b13-9+/t21-,22+,29-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073555

(CHEMBL3408941 | US10358436, Example A92 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(cc3)N3CCNCC3)n[nH]c2c1 |r| Show InChI InChI=1S/C29H27N5O/c35-28-29(23-3-1-2-4-26(23)31-28)18-24(29)20-8-11-22-25(32-33-27(22)17-20)12-7-19-5-9-21(10-6-19)34-15-13-30-14-16-34/h1-12,17,24,30H,13-16,18H2,(H,31,35)(H,32,33)/b12-7+/t24-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073586

(CHEMBL3408946 | US10358436, Example A198 | US99078...)Show SMILES C[C@H]1CN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C[C@@H](C)O1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073553

(CHEMBL3408957)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| Show InChI InChI=1S/C33H34N4O2/c1-21(2)37-17-15-25(16-18-37)39-24-11-7-22(8-12-24)9-14-29-26-13-10-23(19-31(26)36-35-29)28-20-33(28)27-5-3-4-6-30(27)34-32(33)38/h3-14,19,21,25,28H,15-18,20H2,1-2H3,(H,34,38)(H,35,36)/b14-9+/t28-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50073582

(CHEMBL3408942 | US10358436, Example A109 | US99078...)Show SMILES CN(C)Cc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)N(C)c3ccccc23)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50044665

(CHEMBL3353341 | US10358436, Example A33 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(nc5)N5CCN(C)CC5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50044656

(CHEMBL3353348 | US10358436, Example A35 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN(C)C)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073582

(CHEMBL3408942 | US10358436, Example A109 | US99078...)Show SMILES CN(C)Cc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)N(C)c3ccccc23)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073553

(CHEMBL3408957)Show SMILES CC(C)N1CCC(CC1)Oc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| Show InChI InChI=1S/C33H34N4O2/c1-21(2)37-17-15-25(16-18-37)39-24-11-7-22(8-12-24)9-14-29-26-13-10-23(19-31(26)36-35-29)28-20-33(28)27-5-3-4-6-30(27)34-32(33)38/h3-14,19,21,25,28H,15-18,20H2,1-2H3,(H,34,38)(H,35,36)/b14-9+/t28-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044674

(CHEMBL3353355 | US10358436, Example A42 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073582

(CHEMBL3408942 | US10358436, Example A109 | US99078...)Show SMILES CN(C)Cc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)N(C)c3ccccc23)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044675

(CHEMBL3353354 | US10358436, Example A56 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(CN4CCOCC4)cc3)n[nH]c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073555

(CHEMBL3408941 | US10358436, Example A92 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(cc3)N3CCNCC3)n[nH]c2c1 |r| Show InChI InChI=1S/C29H27N5O/c35-28-29(23-3-1-2-4-26(23)31-28)18-24(29)20-8-11-22-25(32-33-27(22)17-20)12-7-19-5-9-21(10-6-19)34-15-13-30-14-16-34/h1-12,17,24,30H,13-16,18H2,(H,31,35)(H,32,33)/b12-7+/t24-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073554

(CHEMBL3408940 | US10358436, Example A110 | US99078...)Show SMILES CN1C(=O)[C@@]2(C[C@H]2c2ccc3c(\C=C\c4ccc(nc4)N4CCN(C)CC4)n[nH]c3c2)c2ccccc12 |r| Show InChI InChI=1S/C30H30N6O/c1-34-13-15-36(16-14-34)28-12-8-20(19-31-28)7-11-25-22-10-9-21(17-26(22)33-32-25)24-18-30(24)23-5-3-4-6-27(23)35(2)29(30)37/h3-12,17,19,24H,13-16,18H2,1-2H3,(H,32,33)/b11-7+/t24-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044668

(CHEMBL3353357 | US10358436, Example A195 | US99078...)Show SMILES Cc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073584

(CHEMBL3408944 | US10358436, Example A59 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCCCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50044656

(CHEMBL3353348 | US10358436, Example A35 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN(C)C)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50073554

(CHEMBL3408940 | US10358436, Example A110 | US99078...)Show SMILES CN1C(=O)[C@@]2(C[C@H]2c2ccc3c(\C=C\c4ccc(nc4)N4CCN(C)CC4)n[nH]c3c2)c2ccccc12 |r| Show InChI InChI=1S/C30H30N6O/c1-34-13-15-36(16-14-34)28-12-8-20(19-31-28)7-11-25-22-10-9-21(17-26(22)33-32-25)24-18-30(24)23-5-3-4-6-27(23)35(2)29(30)37/h3-12,17,19,24H,13-16,18H2,1-2H3,(H,32,33)/b11-7+/t24-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073586

(CHEMBL3408946 | US10358436, Example A198 | US99078...)Show SMILES C[C@H]1CN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C[C@@H](C)O1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044657

(CHEMBL3353347 | US10358436, Example A24 | US990780...)Show SMILES CN(C)Cc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073586

(CHEMBL3408946 | US10358436, Example A198 | US99078...)Show SMILES C[C@H]1CN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C[C@@H](C)O1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073584

(CHEMBL3408944 | US10358436, Example A59 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCCCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044665

(CHEMBL3353341 | US10358436, Example A33 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(nc5)N5CCN(C)CC5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073585

(CHEMBL3408945 | US10358436, Example A102 | US99078...)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044668

(CHEMBL3353357 | US10358436, Example A195 | US99078...)Show SMILES Cc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044675

(CHEMBL3353354 | US10358436, Example A56 | US990780...)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccc(CN4CCOCC4)cc3)n[nH]c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044674

(CHEMBL3353355 | US10358436, Example A42 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044656

(CHEMBL3353348 | US10358436, Example A35 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN(C)C)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073544

(CHEMBL3408948)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073551

(CHEMBL3408955)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6[C@@H](C)CN(C)C[C@H]6C)cc5)n[nH]c4c3)c2c1 |r| Show InChI InChI=1S/C34H37N5O2/c1-21-18-38(3)19-22(2)39(21)20-24-7-5-23(6-8-24)9-13-30-27-12-10-25(15-32(27)37-36-30)29-17-34(29)28-16-26(41-4)11-14-31(28)35-33(34)40/h5-16,21-22,29H,17-20H2,1-4H3,(H,35,40)(H,36,37)/b13-9+/t21-,22+,29-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044657

(CHEMBL3353347 | US10358436, Example A24 | US990780...)Show SMILES CN(C)Cc1ccc(\C=C\c2n[nH]c3cc(ccc23)[C@@H]2C[C@@]22C(=O)Nc3ccccc23)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073583

(CHEMBL3408943 | US9907800, Example A106)Show SMILES F[C@@H]1CCN(Cc2ccc(\C=C\c3n[nH]c4cc(ccc34)[C@@H]3C[C@@]33C(=O)Nc4ccccc34)cc2)C1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50044665

(CHEMBL3353341 | US10358436, Example A33 | US990780...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(nc5)N5CCN(C)CC5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073547

(CHEMBL3408951)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(cc5)[C@H](C)N5CCOCC5)n[nH]c4c3)c2c1 |r| Show InChI InChI=1S/C33H34N4O3/c1-21(37-14-16-40-17-15-37)23-7-4-22(5-8-23)6-12-29-26-11-9-24(18-30(26)35-34-29)28-20-33(28)27-19-25(39-3)10-13-31(27)36(2)32(33)38/h4-13,18-19,21,28H,14-17,20H2,1-3H3,(H,34,35)/b12-6+/t21-,28-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using DBF as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50073585

(CHEMBL3408945 | US10358436, Example A102 | US99078...)Show SMILES COc1ccc2N(C)C(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6CCOCC6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using BFC as substrate by fluorescence assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 88 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TRKB (unknown origin) by cell-based assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 510 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of AURKA (unknown origin) by cell-based assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TIE2 (unknown origin) by cell-based assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TRKA (unknown origin) by cell-based assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50073587

(CHEMBL3408947 | US10358436, Example A185 | US20230...)Show SMILES COc1ccc2NC(=O)[C@@]3(C[C@H]3c3ccc4c(\C=C\c5ccc(CN6C[C@H](C)O[C@H](C)C6)cc5)n[nH]c4c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 (unknown origin) by cell-based assay |
J Med Chem 58: 147-69 (2015)
Article DOI: 10.1021/jm5005336
BindingDB Entry DOI: 10.7270/Q2M0475V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data