 Found 443 hits for UniProtKB: O75582
Found 443 hits for UniProtKB: O75582 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50463479

(CHEMBL4249925)Show SMILES CS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C19H17N5O3S2/c1-29(26,27)24-13-5-2-4-12(8-13)9-17(25)23-19-22-16(11-28-19)15-10-21-18-14(15)6-3-7-20-18/h2-8,10-11,24H,9H2,1H3,(H,20,21)(H,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of MSK1 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1/alpha-2/alpha-3/alpha-4/alpha-5/alpha-6/beta-1/beta-2
(Homo sapiens (Human)) | BDBM50463479

(CHEMBL4249925)Show SMILES CS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C19H17N5O3S2/c1-29(26,27)24-13-5-2-4-12(8-13)9-17(25)23-19-22-16(11-28-19)15-10-21-18-14(15)6-3-7-20-18/h2-8,10-11,24H,9H2,1H3,(H,20,21)(H,22,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50463483
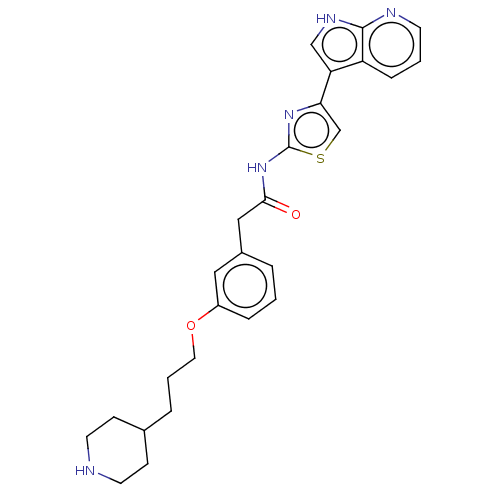
(CHEMBL4245242)Show SMILES O=C(Cc1cccc(OCCCC2CCNCC2)c1)Nc1nc(cs1)-c1c[nH]c2ncccc12 Show InChI InChI=1S/C26H29N5O2S/c32-24(31-26-30-23(17-34-26)22-16-29-25-21(22)7-2-10-28-25)15-19-4-1-6-20(14-19)33-13-3-5-18-8-11-27-12-9-18/h1-2,4,6-7,10,14,16-18,27H,3,5,8-9,11-13,15H2,(H,28,29)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of MSK1 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50463484
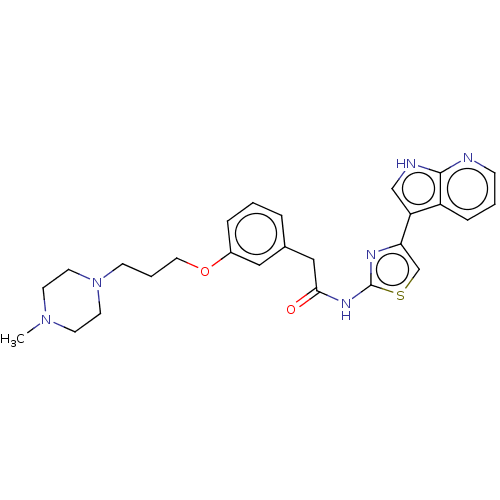
(CHEMBL4248525)Show SMILES CN1CCN(CCCOc2cccc(CC(=O)Nc3nc(cs3)-c3c[nH]c4ncccc34)c2)CC1 Show InChI InChI=1S/C26H30N6O2S/c1-31-10-12-32(13-11-31)9-4-14-34-20-6-2-5-19(15-20)16-24(33)30-26-29-23(18-35-26)22-17-28-25-21(22)7-3-8-27-25/h2-3,5-8,15,17-18H,4,9-14,16H2,1H3,(H,27,28)(H,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of MSK1 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50341519
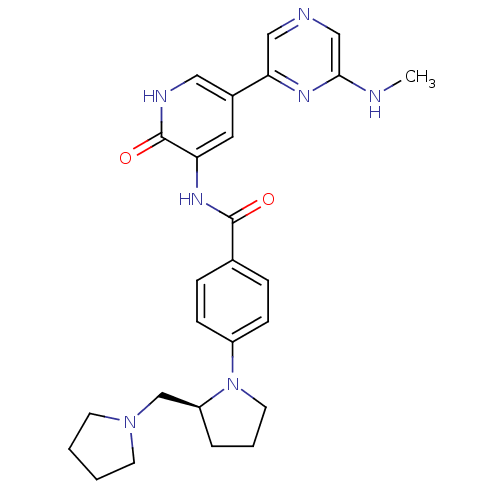
((S)-3-(4-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl...)Show SMILES CNc1cncc(n1)-c1c[nH]c(=O)c(NC(=O)c2ccc(cc2)N2CCC[C@H]2CN2CCCC2)c1 |r| Show InChI InChI=1S/C26H31N7O2/c1-27-24-16-28-15-23(30-24)19-13-22(26(35)29-14-19)31-25(34)18-6-8-20(9-7-18)33-12-4-5-21(33)17-32-10-2-3-11-32/h6-9,13-16,21H,2-5,10-12,17H2,1H3,(H,27,30)(H,29,35)(H,31,34)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of MSK1 |
J Med Chem 54: 2341-50 (2011)
Article DOI: 10.1021/jm101499u
BindingDB Entry DOI: 10.7270/Q2KH0NPW |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50224883
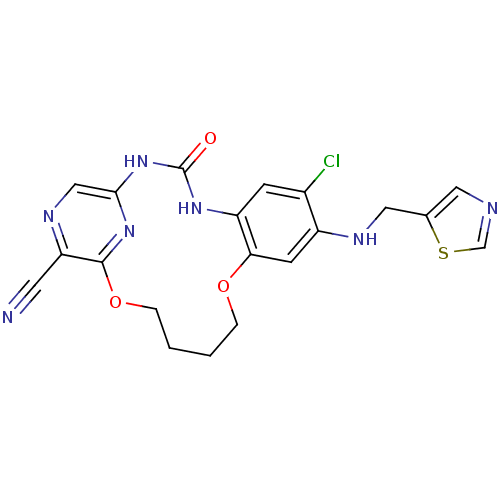
(7-chloro-3-oxo-8-[(thiazol-5-ylmethyl)-amino]-11,1...)Show SMILES Clc1cc2NC(=O)Nc3cnc(C#N)c(OCCCCOc2cc1NCc1cncs1)n3 Show InChI InChI=1S/C20H18ClN7O3S/c21-13-5-15-17(6-14(13)24-9-12-8-23-11-32-12)30-3-1-2-4-31-19-16(7-22)25-10-18(27-19)28-20(29)26-15/h5-6,8,10-11,24H,1-4,9H2,(H2,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MSK1 |
Bioorg Med Chem Lett 17: 6593-601 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.063
BindingDB Entry DOI: 10.7270/Q2X067WT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM31096

(CHEMBL290084 | Staurosporine | cid_451705)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| 8.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VM49NB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2VM49NB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50601136

(CHEMBL5177437)Show SMILES NC(=O)C(=C\c1cccc(c1)-c1ncnc2[nH]ccc12)\C#N | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00134
BindingDB Entry DOI: 10.7270/Q2J38XNB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.426 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MSK1 using GRPRTSSFAEG as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24996
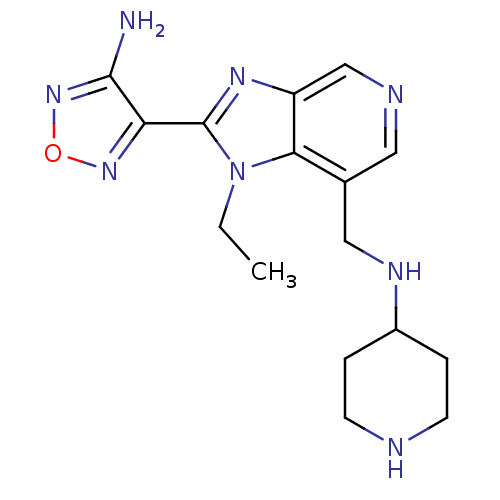
(4-{1-ethyl-7-[(piperidin-4-ylamino)methyl]-1H-imid...)Show InChI InChI=1S/C16H22N8O/c1-2-24-14-10(8-20-11-3-5-18-6-4-11)7-19-9-12(14)21-16(24)13-15(17)23-25-22-13/h7,9,11,18,20H,2-6,8H2,1H3,(H2,17,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24996

(4-{1-ethyl-7-[(piperidin-4-ylamino)methyl]-1H-imid...)Show InChI InChI=1S/C16H22N8O/c1-2-24-14-10(8-20-11-3-5-18-6-4-11)7-19-9-12(14)21-16(24)13-15(17)23-25-22-13/h7,9,11,18,20H,2-6,8H2,1H3,(H2,17,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM259876
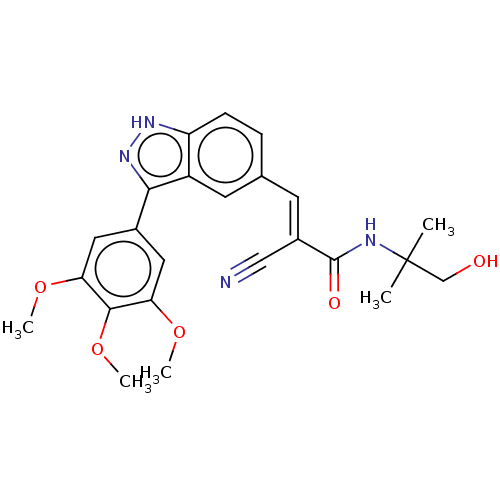
(US9505766, 81)Show SMILES COc1cc(cc(OC)c1OC)-c1n[nH]c2ccc(\C=C(/C#N)C(=O)NC(C)(C)CO)cc12 Show InChI InChI=1S/C24H26N4O5/c1-24(2,13-29)26-23(30)16(12-25)8-14-6-7-18-17(9-14)21(28-27-18)15-10-19(31-3)22(33-5)20(11-15)32-4/h6-11,29H,13H2,1-5H3,(H,26,30)(H,27,28)/b16-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00134
BindingDB Entry DOI: 10.7270/Q2J38XNB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24994
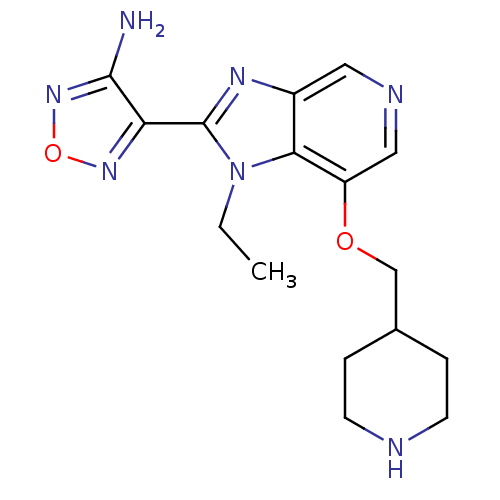
(4-[1-ethyl-7-(piperidin-4-ylmethoxy)-1H-imidazo[4,...)Show InChI InChI=1S/C16H21N7O2/c1-2-23-14-11(20-16(23)13-15(17)22-25-21-13)7-19-8-12(14)24-9-10-3-5-18-6-4-10/h7-8,10,18H,2-6,9H2,1H3,(H2,17,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168854
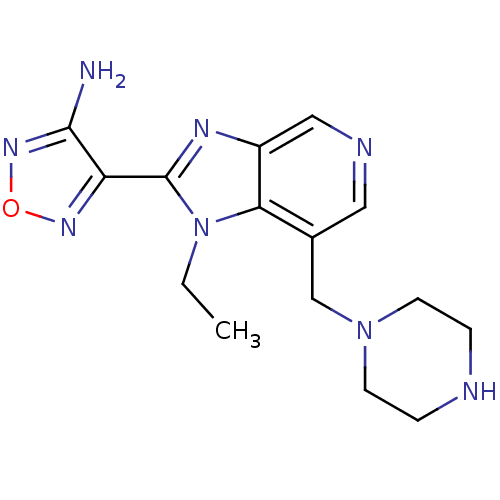
(4-(1-Ethyl-7-piperazin-1-ylmethyl-1H-imidazo[4,5-c...)Show InChI InChI=1S/C15H20N8O/c1-2-23-13-10(9-22-5-3-17-4-6-22)7-18-8-11(13)19-15(23)12-14(16)21-24-20-12/h7-8,17H,2-6,9H2,1H3,(H2,16,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of MSK1 (unknown origin) by mobility shift assay |
Eur J Med Chem 117: 47-58 (2016)
Article DOI: 10.1016/j.ejmech.2016.03.074
BindingDB Entry DOI: 10.7270/Q2QJ7K5K |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human MSK1 using GRPRTSSFAEG as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168577
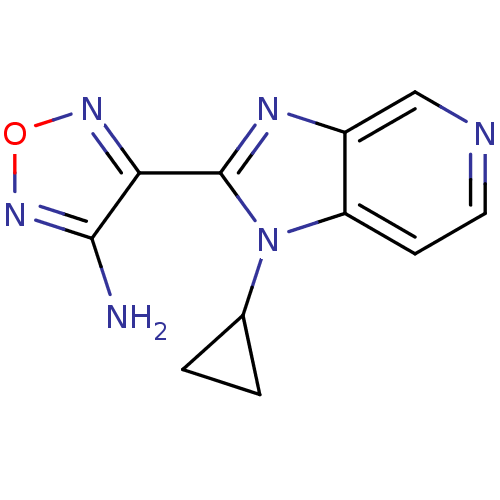
(4-(1-Cyclopropyl-1H-imidazo[4,5-c]pyridin-2-yl)-fu...)Show InChI InChI=1S/C11H10N6O/c12-10-9(15-18-16-10)11-14-7-5-13-4-3-8(7)17(11)6-1-2-6/h3-6H,1-2H2,(H2,12,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168577

(4-(1-Cyclopropyl-1H-imidazo[4,5-c]pyridin-2-yl)-fu...)Show InChI InChI=1S/C11H10N6O/c12-10-9(15-18-16-10)11-14-7-5-13-4-3-8(7)17(11)6-1-2-6/h3-6H,1-2H2,(H2,12,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration exhibited towards MSK-1 |
Bioorg Med Chem Lett 15: 3402-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.021
BindingDB Entry DOI: 10.7270/Q2Z037P9 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168858
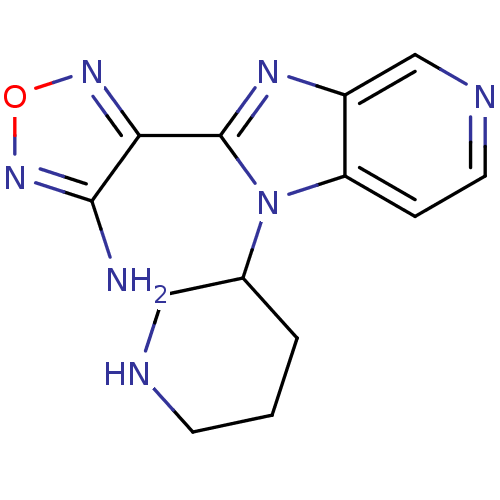
((R,S)-4-(1-Piperidin-3-yl-1H-imidazo[4,5-c]pyridin...)Show InChI InChI=1S/C13H15N7O/c14-12-11(18-21-19-12)13-17-9-7-16-5-3-10(9)20(13)8-2-1-4-15-6-8/h3,5,7-8,15H,1-2,4,6H2,(H2,14,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168856
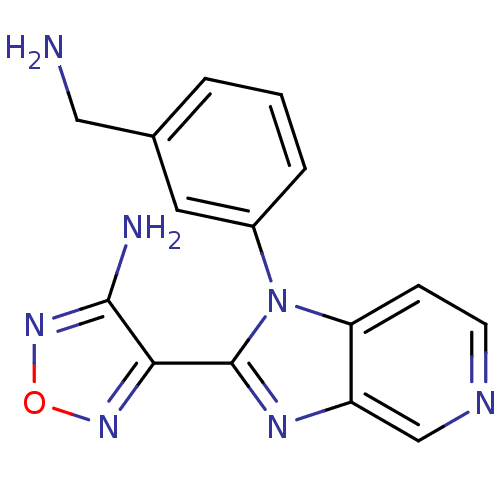
(4-[1-(3-Aminomethyl-phenyl)-1H-imidazo[4,5-c]pyrid...)Show InChI InChI=1S/C15H13N7O/c16-7-9-2-1-3-10(6-9)22-12-4-5-18-8-11(12)19-15(22)13-14(17)21-23-20-13/h1-6,8H,7,16H2,(H2,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM14032
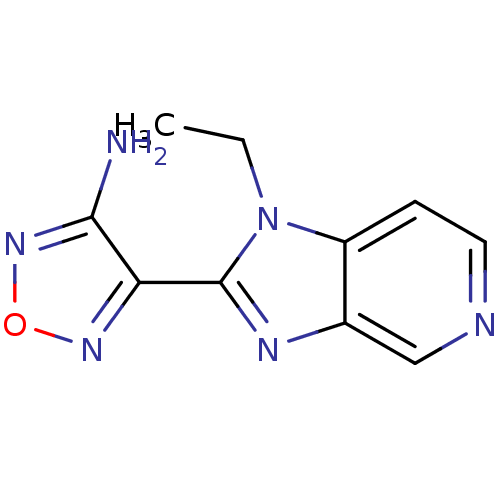
(4-(1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl)-1,2,5-ox...)Show InChI InChI=1S/C10H10N6O/c1-2-16-7-3-4-12-5-6(7)13-10(16)8-9(11)15-17-14-8/h3-5H,2H2,1H3,(H2,11,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24991
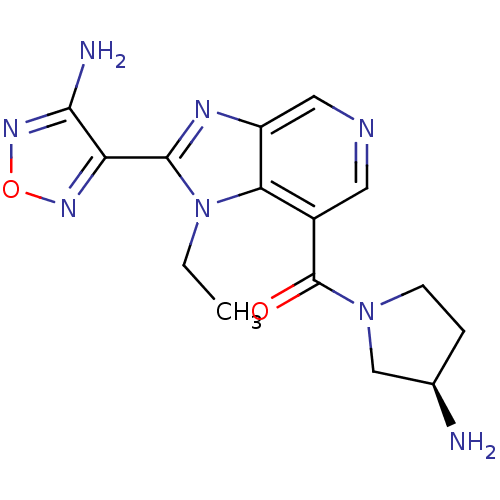
(4-(7-{[(3R)-3-aminopyrrolidin-1-yl]carbonyl}-1-eth...)Show SMILES CCn1c(nc2cncc(C(=O)N3CC[C@@H](N)C3)c12)-c1nonc1N |r| Show InChI InChI=1S/C15H18N8O2/c1-2-23-12-9(15(24)22-4-3-8(16)7-22)5-18-6-10(12)19-14(23)11-13(17)21-25-20-11/h5-6,8H,2-4,7,16H2,1H3,(H2,17,21)/t8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168587

(4-(1-Cyclohexyl-1H-imidazo[4,5-c]pyridin-2-yl)-fur...)Show InChI InChI=1S/C14H16N6O/c15-13-12(18-21-19-13)14-17-10-8-16-7-6-11(10)20(14)9-4-2-1-3-5-9/h6-9H,1-5H2,(H2,15,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM14032

(4-(1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl)-1,2,5-ox...)Show InChI InChI=1S/C10H10N6O/c1-2-16-7-3-4-12-5-6(7)13-10(16)8-9(11)15-17-14-8/h3-5H,2H2,1H3,(H2,11,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration exhibited towards MSK-1 |
Bioorg Med Chem Lett 15: 3402-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.021
BindingDB Entry DOI: 10.7270/Q2Z037P9 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168869
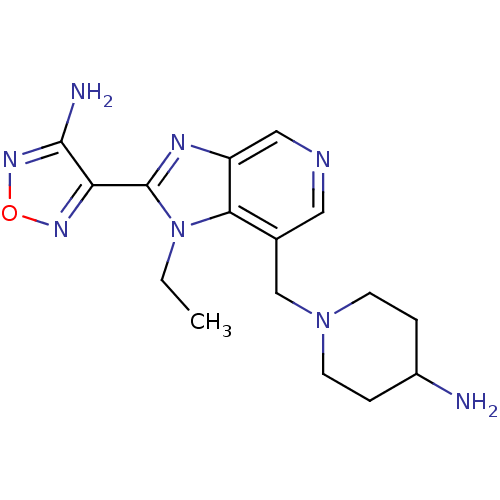
(1-[2-(4-Amino-furazan-3-yl)-1-ethyl-1H-imidazo[4,5...)Show InChI InChI=1S/C16H22N8O/c1-2-24-14-10(9-23-5-3-11(17)4-6-23)7-19-8-12(14)20-16(24)13-15(18)22-25-21-13/h7-8,11H,2-6,9,17H2,1H3,(H2,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168587

(4-(1-Cyclohexyl-1H-imidazo[4,5-c]pyridin-2-yl)-fur...)Show InChI InChI=1S/C14H16N6O/c15-13-12(18-21-19-13)14-17-10-8-16-7-6-11(10)20(14)9-4-2-1-3-5-9/h6-9H,1-5H2,(H2,15,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration exhibited towards MSK-1 |
Bioorg Med Chem Lett 15: 3402-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.021
BindingDB Entry DOI: 10.7270/Q2Z037P9 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM14032

(4-(1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl)-1,2,5-ox...)Show InChI InChI=1S/C10H10N6O/c1-2-16-7-3-4-12-5-6(7)13-10(16)8-9(11)15-17-14-8/h3-5H,2H2,1H3,(H2,11,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50431812
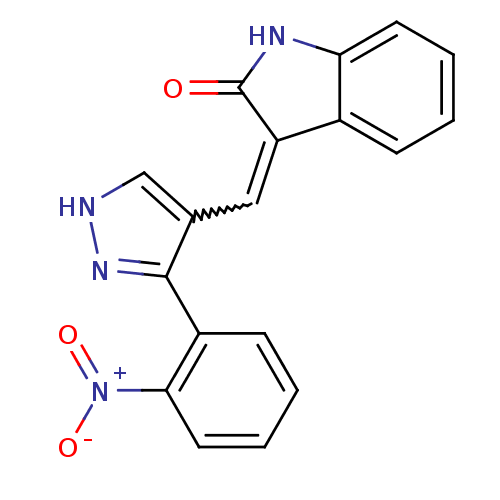
(CHEMBL2347053)Show SMILES [O-][N+](=O)c1ccccc1-c1n[nH]cc1C=C1C(=O)Nc2ccccc12 |w:14.15| Show InChI InChI=1S/C18H12N4O3/c23-18-14(12-5-1-3-7-15(12)20-18)9-11-10-19-21-17(11)13-6-2-4-8-16(13)22(24)25/h1-10H,(H,19,21)(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MSK1 (unknown origin) after 10 mins by mobility shift assay |
Bioorg Med Chem 21: 1724-34 (2013)
Article DOI: 10.1016/j.bmc.2013.01.047
BindingDB Entry DOI: 10.7270/Q2GQ704N |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168861

(4-(4-Chloro-1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl)...)Show InChI InChI=1S/C10H9ClN6O/c1-2-17-5-3-4-13-8(11)6(5)14-10(17)7-9(12)16-18-15-7/h3-4H,2H2,1H3,(H2,12,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168862
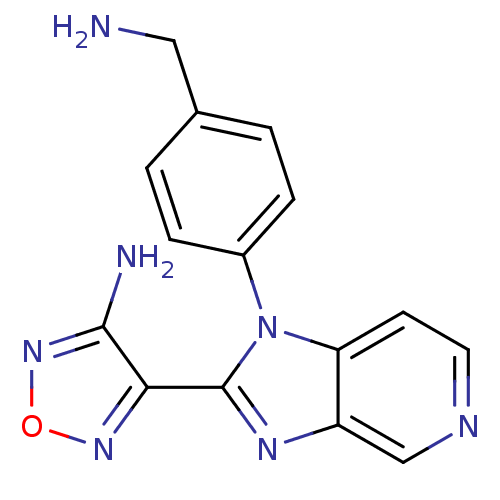
(4-[1-(4-Aminomethyl-phenyl)-1H-imidazo[4,5-c]pyrid...)Show InChI InChI=1S/C15H13N7O/c16-7-9-1-3-10(4-2-9)22-12-5-6-18-8-11(12)19-15(22)13-14(17)21-23-20-13/h1-6,8H,7,16H2,(H2,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM259876

(US9505766, 81)Show SMILES COc1cc(cc(OC)c1OC)-c1n[nH]c2ccc(\C=C(/C#N)C(=O)NC(C)(C)CO)cc12 Show InChI InChI=1S/C24H26N4O5/c1-24(2,13-29)26-23(30)16(12-25)8-14-6-7-18-17(9-14)21(28-27-18)15-10-19(31-3)22(33-5)20(11-15)32-4/h6-11,29H,13H2,1-5H3,(H,26,30)(H,27,28)/b16-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00134
BindingDB Entry DOI: 10.7270/Q2J38XNB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168581
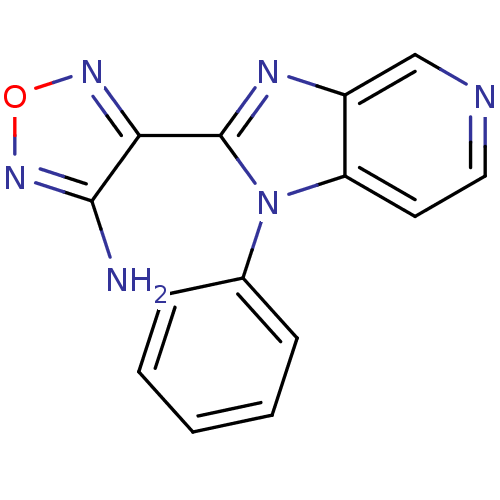
(4-(1-Phenyl-1H-imidazo[4,5-c]pyridin-2-yl)-furazan...)Show InChI InChI=1S/C14H10N6O/c15-13-12(18-21-19-13)14-17-10-8-16-7-6-11(10)20(14)9-4-2-1-3-5-9/h1-8H,(H2,15,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50560259
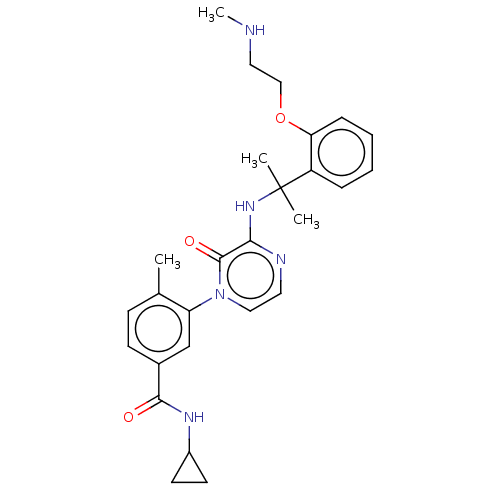
(CHEMBL4792347)Show SMILES CNCCOc1ccccc1C(C)(C)Nc1nccn(-c2cc(ccc2C)C(=O)NC2CC2)c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MSK1 |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127412
BindingDB Entry DOI: 10.7270/Q2V40ZW0 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168853
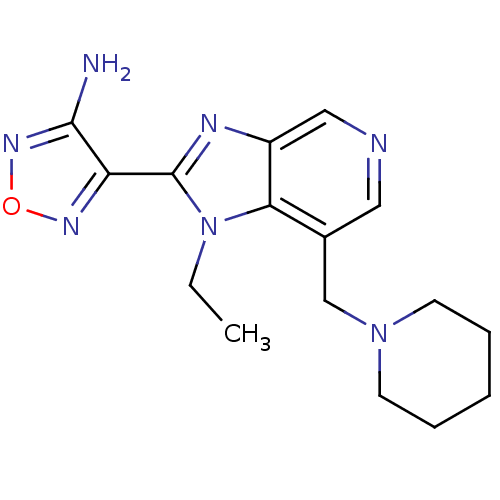
(4-(1-Ethyl-7-piperidin-1-ylmethyl-1H-imidazo[4,5-c...)Show InChI InChI=1S/C16H21N7O/c1-2-23-14-11(10-22-6-4-3-5-7-22)8-18-9-12(14)19-16(23)13-15(17)21-24-20-13/h8-9H,2-7,10H2,1H3,(H2,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24996

(4-{1-ethyl-7-[(piperidin-4-ylamino)methyl]-1H-imid...)Show InChI InChI=1S/C16H22N8O/c1-2-24-14-10(8-20-11-3-5-18-6-4-11)7-19-9-12(14)21-16(24)13-15(17)23-25-22-13/h7,9,11,18,20H,2-6,8H2,1H3,(H2,17,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM3175

(3-[1-[3-(Amidinothio)propyl]-3-indolyl]-4-(1-methy...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2cn(CCCSC(N)=N)c3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C25H23N5O2S/c1-29-13-17(15-7-2-4-9-19(15)29)21-22(24(32)28-23(21)31)18-14-30(11-6-12-33-25(26)27)20-10-5-3-8-16(18)20/h2-5,7-10,13-14H,6,11-12H2,1H3,(H3,26,27)(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human MSK1 expressed in Sf9 cells |
Biochem J 351: 95-105 (2001)
BindingDB Entry DOI: 10.7270/Q24T6JKN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168866
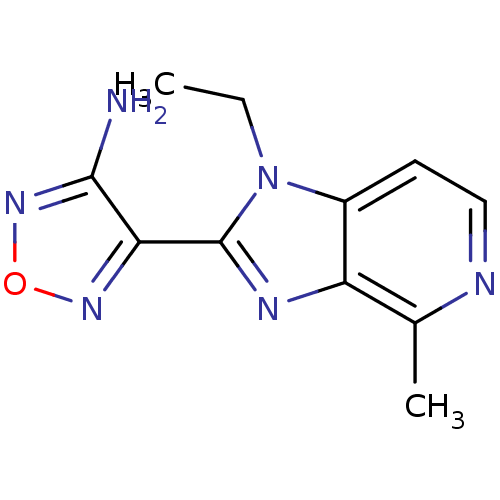
(4-(1-Ethyl-4-methyl-1H-imidazo[4,5-c]pyridin-2-yl)...)Show InChI InChI=1S/C11H12N6O/c1-3-17-7-4-5-13-6(2)8(7)14-11(17)9-10(12)16-18-15-9/h4-5H,3H2,1-2H3,(H2,12,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168864
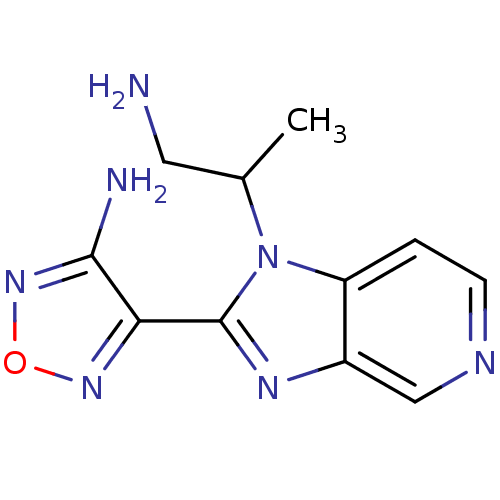
((R,S)-4-[1-(2-Amino-1-methyl-ethyl)-1H-imidazo[4,5...)Show InChI InChI=1S/C11H13N7O/c1-6(4-12)18-8-2-3-14-5-7(8)15-11(18)9-10(13)17-19-16-9/h2-3,5-6H,4,12H2,1H3,(H2,13,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168867
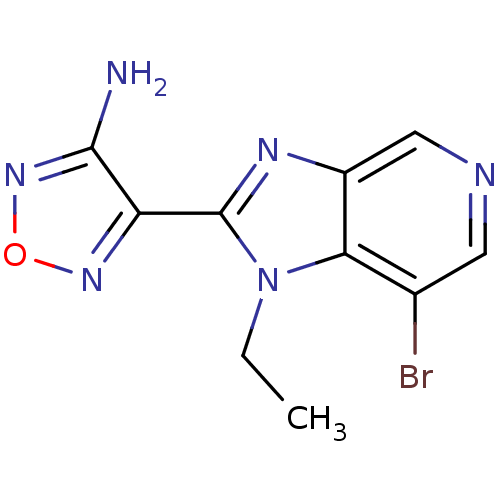
(4-(7-Bromo-1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl)-...)Show InChI InChI=1S/C10H9BrN6O/c1-2-17-8-5(11)3-13-4-6(8)14-10(17)7-9(12)16-18-15-7/h3-4H,2H2,1H3,(H2,12,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50601139
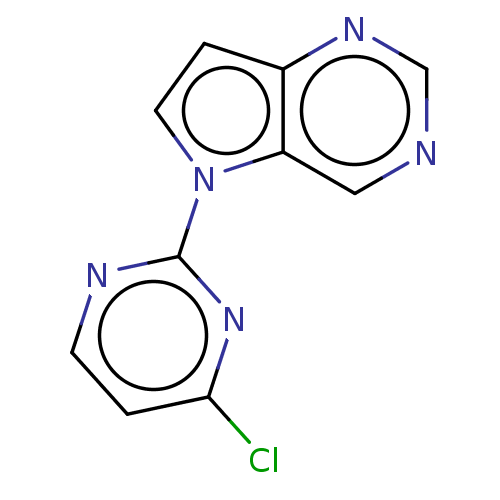
(CHEMBL5190298) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00134
BindingDB Entry DOI: 10.7270/Q2J38XNB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24993
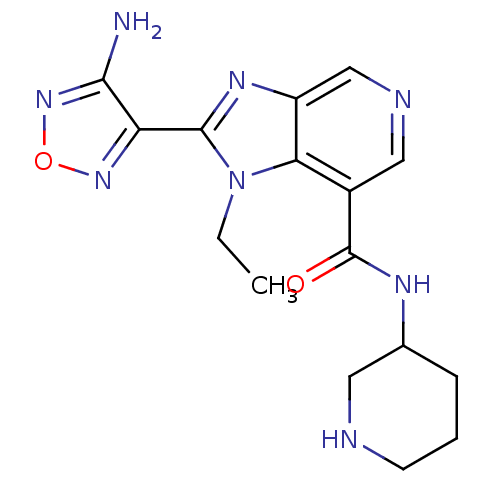
(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-N-(piperi...)Show InChI InChI=1S/C16H20N8O2/c1-2-24-13-10(16(25)20-9-4-3-5-18-6-9)7-19-8-11(13)21-15(24)12-14(17)23-26-22-12/h7-9,18H,2-6H2,1H3,(H2,17,23)(H,20,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168581

(4-(1-Phenyl-1H-imidazo[4,5-c]pyridin-2-yl)-furazan...)Show InChI InChI=1S/C14H10N6O/c15-13-12(18-21-19-13)14-17-10-8-16-7-6-11(10)20(14)9-4-2-1-3-5-9/h1-8H,(H2,15,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration exhibited towards MSK-1 |
Bioorg Med Chem Lett 15: 3402-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.021
BindingDB Entry DOI: 10.7270/Q2Z037P9 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168868
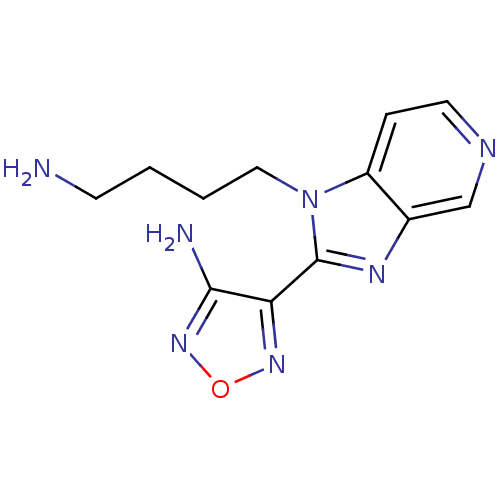
(4-[1-(4-Amino-butyl)-1H-imidazo[4,5-c]pyridin-2-yl...)Show InChI InChI=1S/C12H15N7O/c13-4-1-2-6-19-9-3-5-15-7-8(9)16-12(19)10-11(14)18-20-17-10/h3,5,7H,1-2,4,6,13H2,(H2,14,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50601138
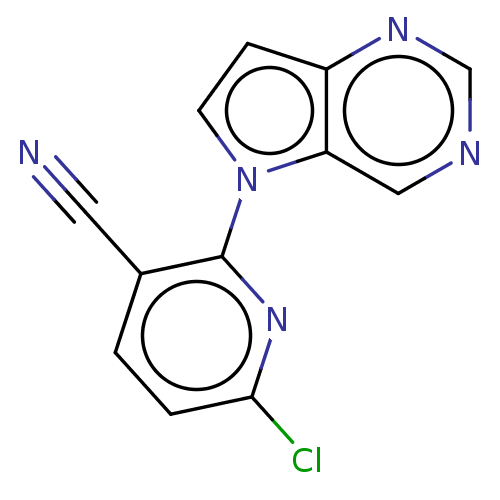
(CHEMBL5184056) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00134
BindingDB Entry DOI: 10.7270/Q2J38XNB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24995
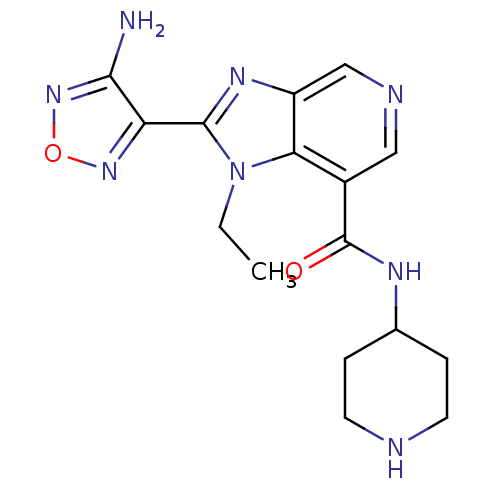
(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-N-(piperi...)Show InChI InChI=1S/C16H20N8O2/c1-2-24-13-10(16(25)20-9-3-5-18-6-4-9)7-19-8-11(13)21-15(24)12-14(17)23-26-22-12/h7-9,18H,2-6H2,1H3,(H2,17,23)(H,20,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168578

(4-[1-(1-Amino-butyl)-1H-imidazo[4,5-c]pyridin-2-yl...)Show InChI InChI=1S/C12H15N7O/c1-2-3-9(13)19-8-4-5-15-6-7(8)16-12(19)10-11(14)18-20-17-10/h4-6,9H,2-3,13H2,1H3,(H2,14,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration exhibited towards MSK-1 |
Bioorg Med Chem Lett 15: 3402-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.021
BindingDB Entry DOI: 10.7270/Q2Z037P9 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50365218
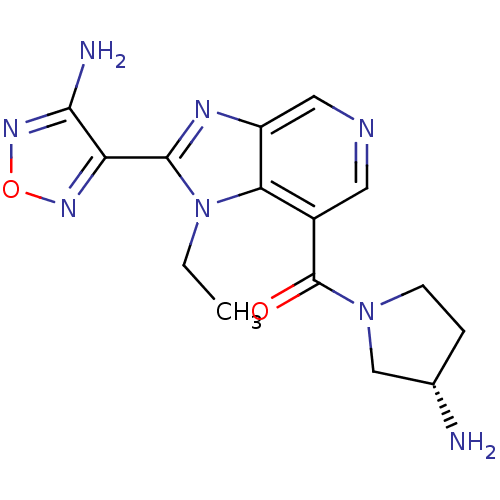
(CHEMBL1956071 | GSK screening, 29)Show SMILES CCn1c(nc2cncc(C(=O)N3CC[C@H](N)C3)c12)-c1nonc1N Show InChI InChI=1S/C15H18N8O2/c1-2-23-12-9(15(24)22-4-3-8(16)7-22)5-18-6-10(12)19-14(23)11-13(17)21-25-20-11/h5-6,8H,2-4,7,16H2,1H3,(H2,17,21)/t8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MSK1 |
J Pharmacol Exp Ther 320: 89-98 (2006)
Article DOI: 10.1124/jpet.106.110635
BindingDB Entry DOI: 10.7270/Q2DV1KBV |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24990
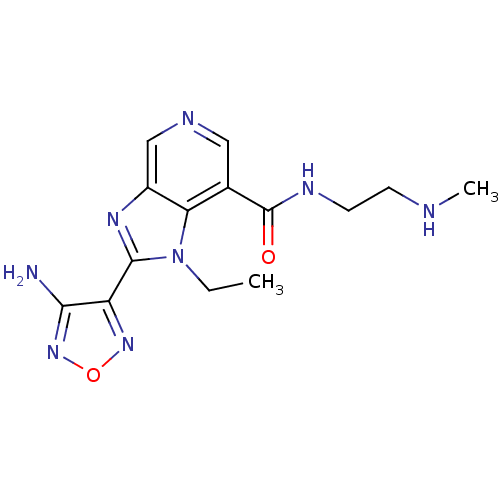
(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-N-[2-(met...)Show InChI InChI=1S/C14H18N8O2/c1-3-22-11-8(14(23)18-5-4-16-2)6-17-7-9(11)19-13(22)10-12(15)21-24-20-10/h6-7,16H,3-5H2,1-2H3,(H2,15,21)(H,18,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... |
J Med Chem 51: 5663-79 (2008)
Article DOI: 10.1021/jm8004527
BindingDB Entry DOI: 10.7270/Q29G5K3H |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
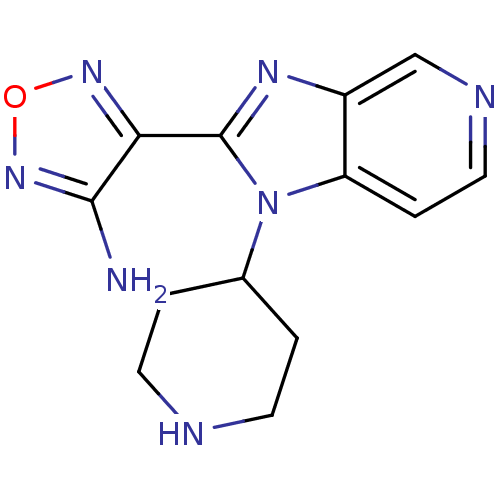
(Homo sapiens (Human)) | BDBM50168601
(4-(1-Piperidin-4-yl-1H-imidazo[4,5-c]pyridin-2-yl)...)Show InChI InChI=1S/C13H15N7O/c14-12-11(18-21-19-12)13-17-9-7-16-6-3-10(9)20(13)8-1-4-15-5-2-8/h3,6-8,15H,1-2,4-5H2,(H2,14,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration exhibited towards MSK-1 |
Bioorg Med Chem Lett 15: 3402-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.021
BindingDB Entry DOI: 10.7270/Q2Z037P9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data