

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50018229 (2-(4-Hydroxy-phenyl)-2-phenyl-propionic acid 2-die...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M2 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 [A65S,N67Q,E69T,I91L,F131V,K170E,L204A] (Homo sapiens (Human)) | BDBM210938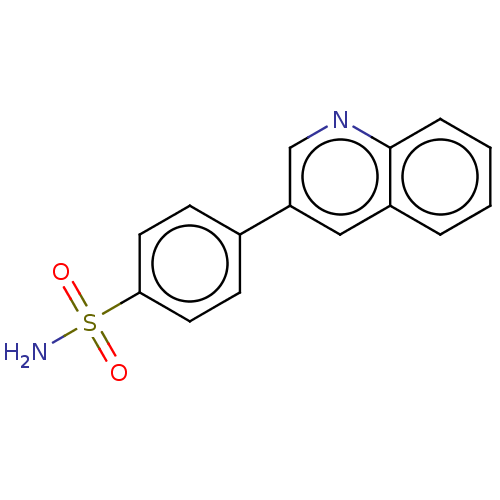 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 [A65S,N67Q,E69T,I91L,F131V,K170E,L204A] (Homo sapiens (Human)) | BDBM210935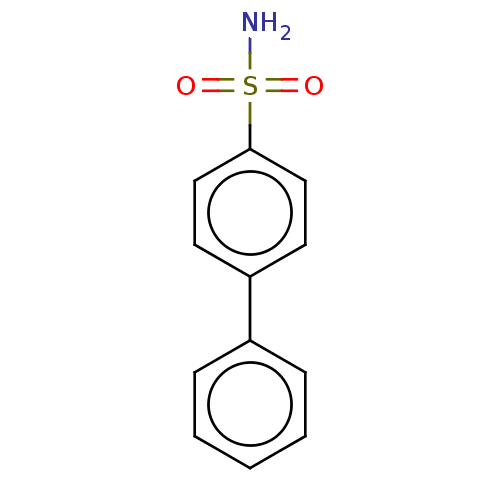 (4-(phenyl)-bezenesulfonamide (4a)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50491127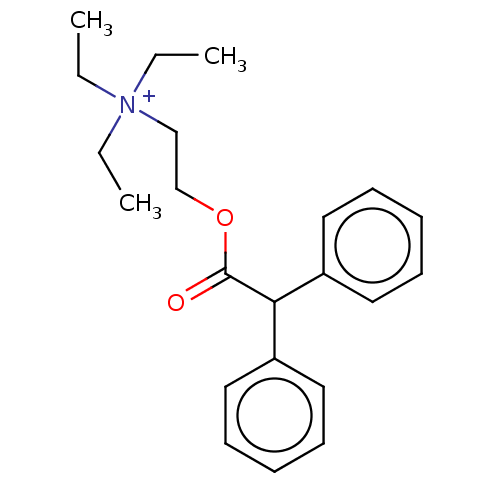 (CHEMBL2377383) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491126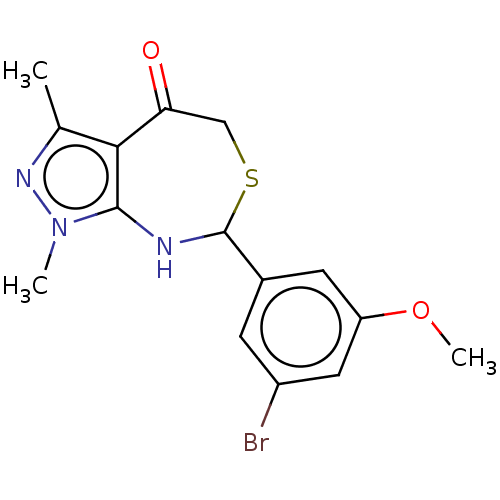 (CHEMBL2377266) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.594 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM210937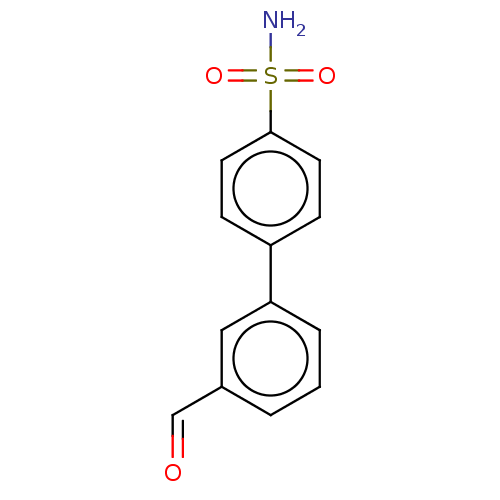 (4-(3-formylphenyl)-benzenesulfonamide (4e)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM210935 (4-(phenyl)-bezenesulfonamide (4a)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50018229 (2-(4-Hydroxy-phenyl)-2-phenyl-propionic acid 2-die...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.881 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.922 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM210936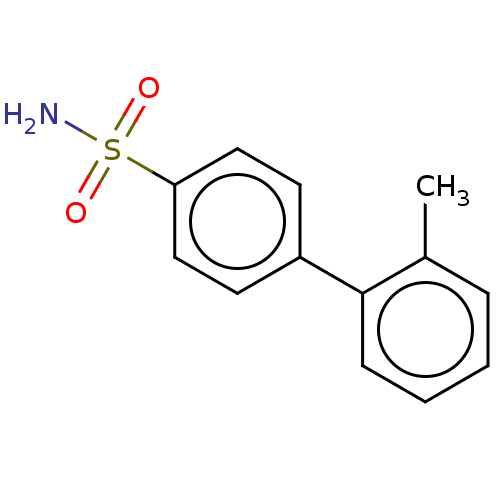 (4-(2-methylphenyl)-bezenesulfonamide (4b)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491119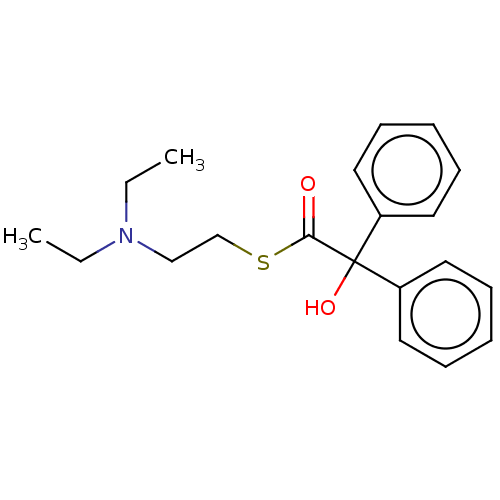 (CHEMBL2377387) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491124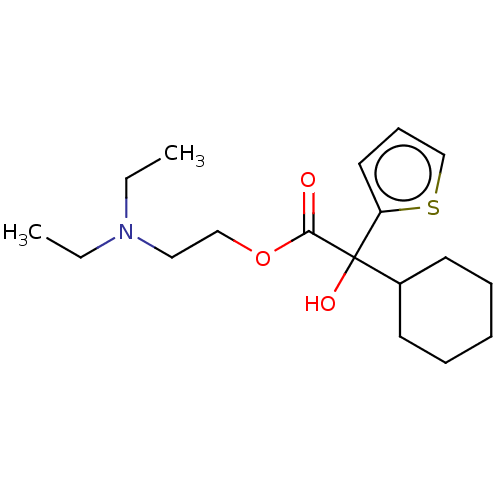 (CHEMBL2377267) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M4 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M2 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50491124 (CHEMBL2377267) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M4 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50491133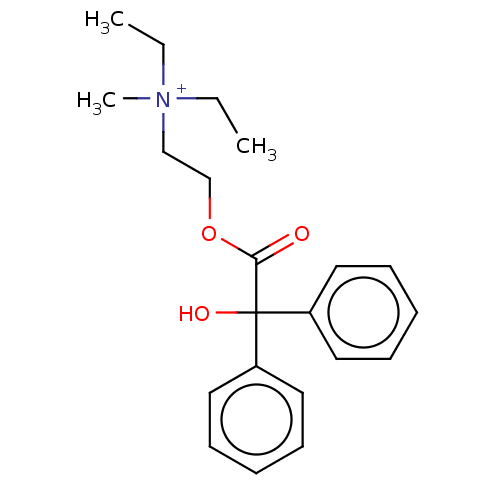 (Methylbenactyzium Bromide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M2 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491133 (Methylbenactyzium Bromide) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM210937 (4-(3-formylphenyl)-benzenesulfonamide (4e)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase-2 by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 2.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254203 (US10112937, Example 88 | US10150765, Example 88 | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat P2X7 expressed in human 1321N1 cells assessed as inhibition of BzATP-induced calcium flux preincubated for 30 ... | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50240680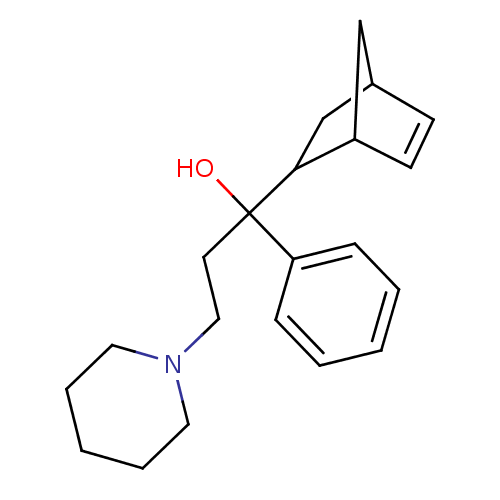 (1-(bicyclo[2.2.1]hept-5-en-2-yl)-1-phenyl-3-(piper...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 2.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10875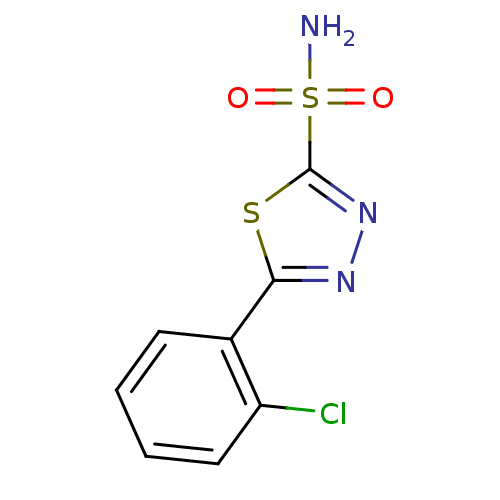 (5-(2-chlorophenyl)-1,3,4-thiadiazole-2-sulfonamide...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM210938 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10886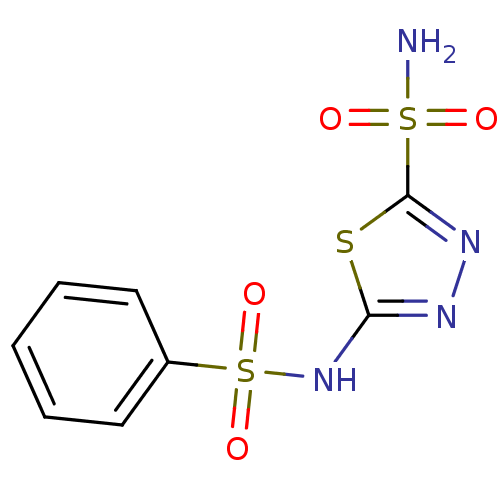 (2-N-benzene-1,3,4-thiadiazole-2,5-disulfonamide | ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase-2 by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50491119 (CHEMBL2377387) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM16510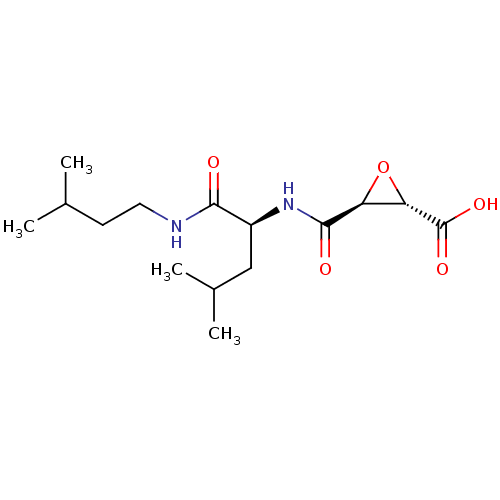 ((2S,3S)-3-[[(1S)-1-(isoamylcarbamoyl)-3-methyl-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory (CSIR-NCL) Curated by ChEMBL | Assay Description Inhibition of cathepsin B | Bioorg Med Chem 19: 7129-35 (2011) Article DOI: 10.1016/j.bmc.2011.09.058 BindingDB Entry DOI: 10.7270/Q2KW5GGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50491133 (Methylbenactyzium Bromide) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Sulfurihydrogenibium sp. (strain YO3AOP1)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10869 (5-imino-4-methyl-4,5-dihydro-1,3,4-thiadiazole-2-s...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM254266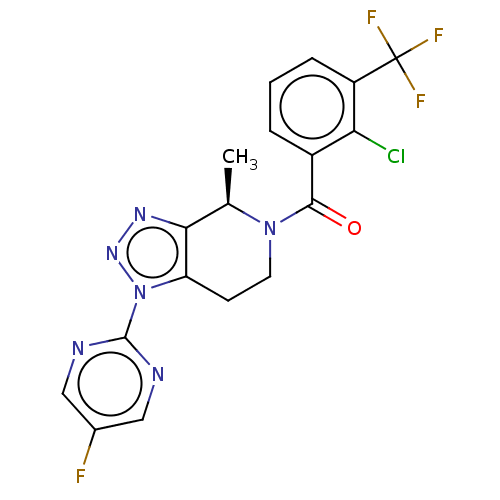 (US10112937, Example 158 | US10150765, Example 158 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Displacement of [3H]-A-804598 from recombinant human P2X7 expressed in human 1321N1 cells after 1 hr | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM254266 (US10112937, Example 158 | US10150765, Example 158 ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC , 3210 Merryfield Row, San Diego, California 92121, United States. Curated by ChEMBL | Assay Description Displacement of [3H]-A-804598 from recombinant rat P2X7 expressed in human 1321N1 cells after 1 hr | J Med Chem 60: 4559-4572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00408 BindingDB Entry DOI: 10.7270/Q2QN697T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Sulfurihydrogenibium sp. (strain YO3AOP1)) | BDBM13063 (4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50491119 (CHEMBL2377387) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M2 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Sulfurihydrogenibium sp. (strain YO3AOP1)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491127 (CHEMBL2377383) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50491121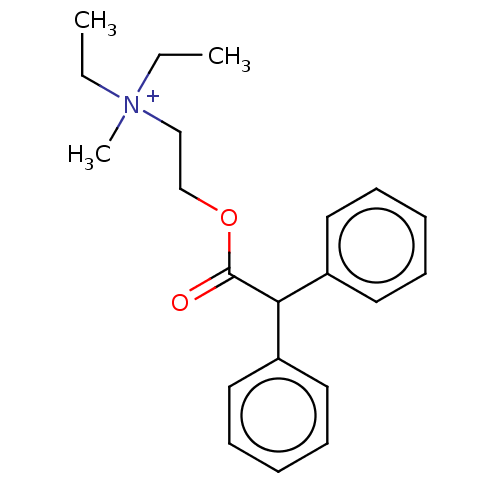 (CHEMBL2377385) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M1 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50491132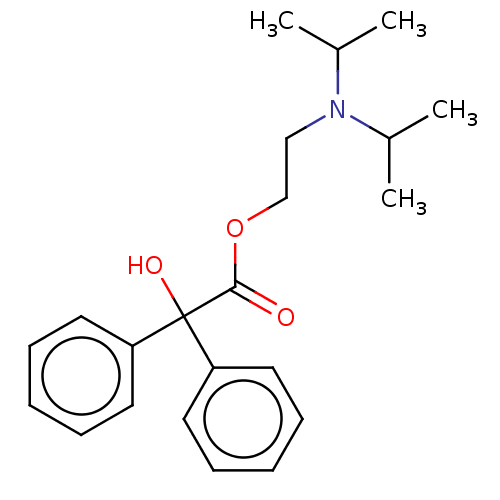 (CHEMBL2377261) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M2 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50240680 (1-(bicyclo[2.2.1]hept-5-en-2-yl)-1-phenyl-3-(piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 5.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50491128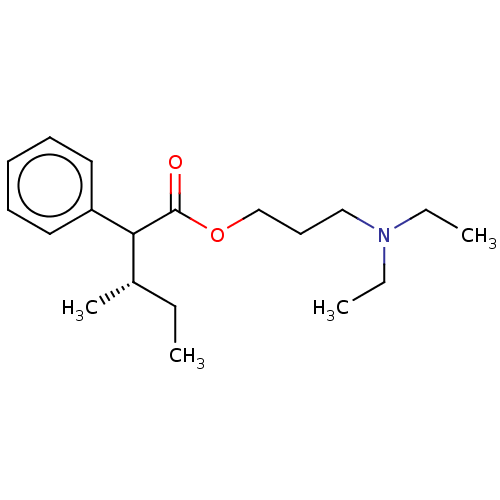 (CHEMBL2377392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM20461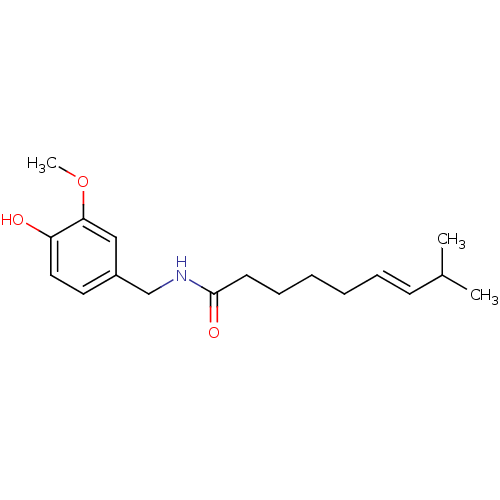 ((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Binding affinity to human recombinant TRPV1 | Bioorg Med Chem Lett 20: 7137-41 (2010) Article DOI: 10.1016/j.bmcl.2010.09.023 BindingDB Entry DOI: 10.7270/Q29W0FQ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase-1 by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Sulfurihydrogenibium sp. (strain YO3AOP1)) | BDBM10873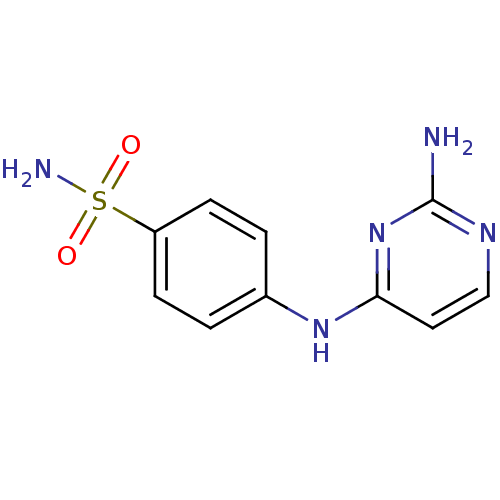 (4-[(2-aminopyrimidin-4-yl)amino]benzene-1-sulfonam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50491124 (CHEMBL2377267) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Walter Reed Army Institute of Research Curated by ChEMBL | Assay Description Displacement of [3H] N-methylscopolamine from human muscarinic M3 receptor expressed in CHOK1 cells after 30 mins by scintillation counting analysis | Bioorg Med Chem 21: 2651-62 (2013) Article DOI: 10.1016/j.bmc.2013.01.072 BindingDB Entry DOI: 10.7270/Q2X92F6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1195 total ) | Next | Last >> |