

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328467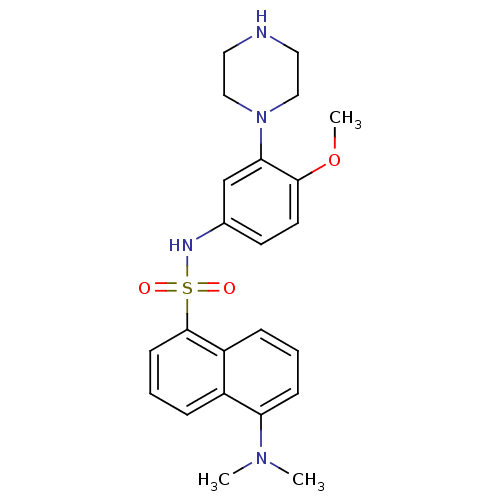 (5-(Dimethylamino)-N-(4-methoxy-3-piperazin-1-ylphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50014005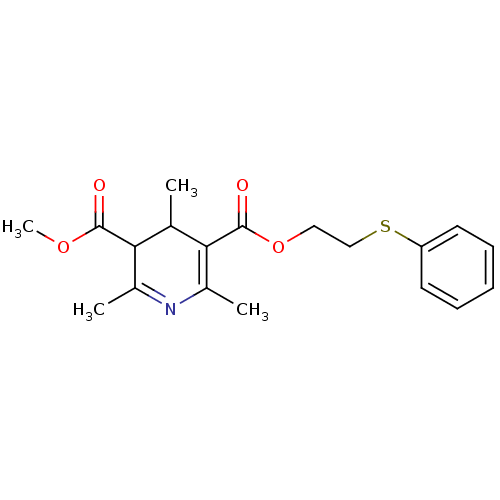 (2,4,6-Trimethyl-1,4-dihydro-pyridine-3,5-dicarboxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALTER, S.A. Curated by ChEMBL | Assay Description Tested for inhibition of [3H]PAF binding to rabbit platelet Platelet activating factor receptor | J Med Chem 33: 3205-10 (1991) BindingDB Entry DOI: 10.7270/Q2PV6KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328463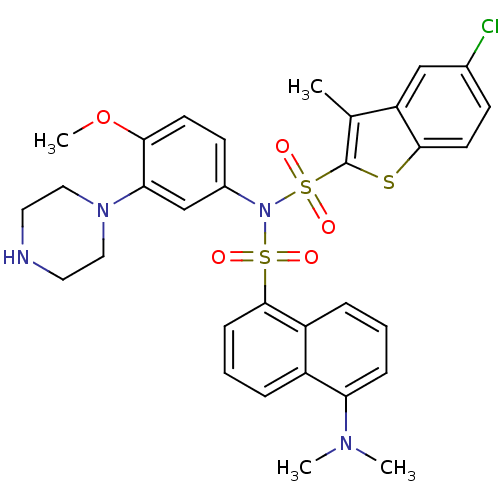 (5-Chloro-N-{[5-(dimethylamino)-1-naphthyl]sulfonyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50014007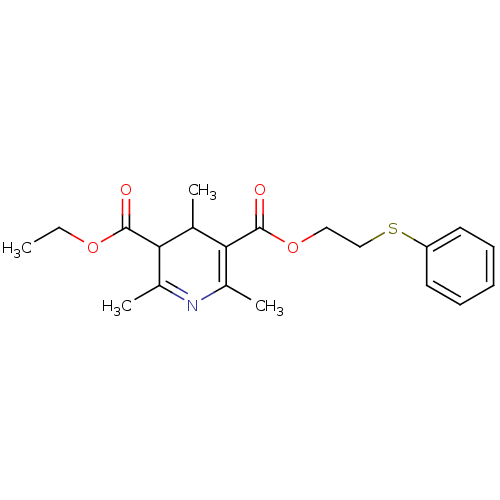 (2,4,6-Trimethyl-1,4-dihydro-pyridine-3,5-dicarboxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALTER, S.A. Curated by ChEMBL | Assay Description Tested for inhibition of [3H]PAF binding to rabbit platelet Platelet activating factor receptor | J Med Chem 33: 3205-10 (1991) BindingDB Entry DOI: 10.7270/Q2PV6KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328473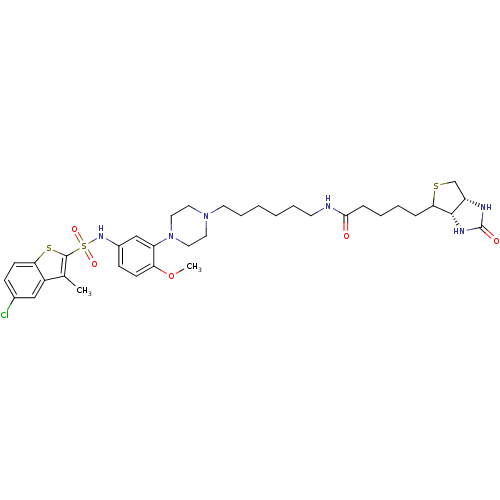 (CHEMBL1256172 | N-{6-[4-(5-{[(5-Chloro-3-methyl-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328468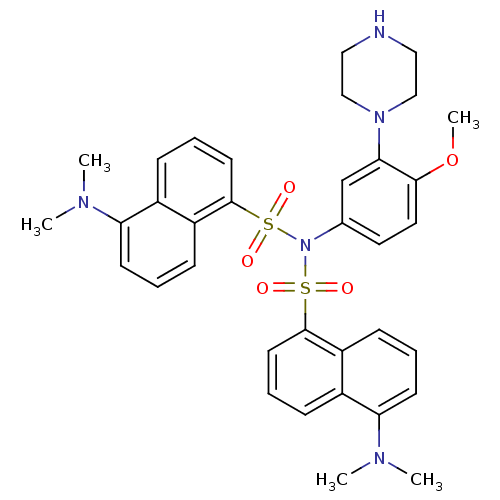 (5-(Dimethylamino)-N-(5-(dimethylamino)naphthalen-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328460 (5-Chloro-N-[3-(4-{[5-(dimethylamino)-1-naphthyl]su...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328469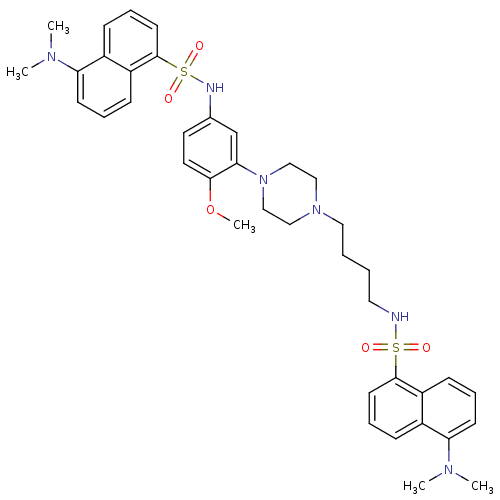 (5-(Dimethylamino)-N-(3-{4-[4-({[5-(dimethylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328471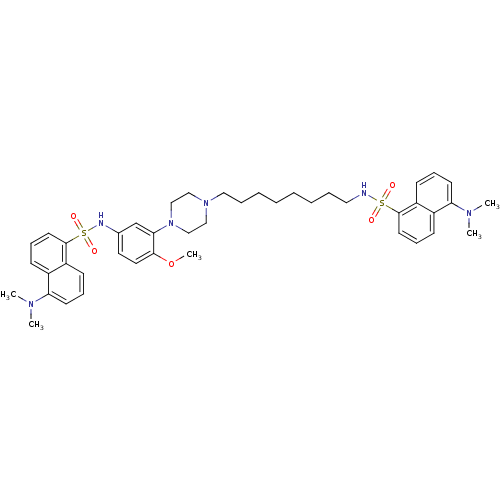 (5-(Dimethylamino)-N-(8-{4-[5-({[5-(dimethylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328465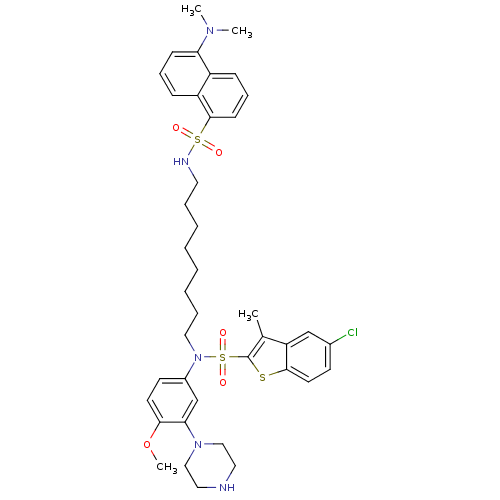 (5-Chloro-N-[8-({[5-(dimethylamino)-1-naphthyl]sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328461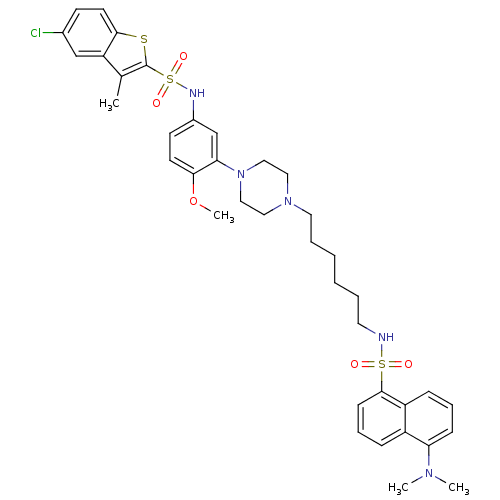 (5-Chloro-N-(3-{4-[6-({[5-(dimethylamino)-1-naphthy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 266 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328462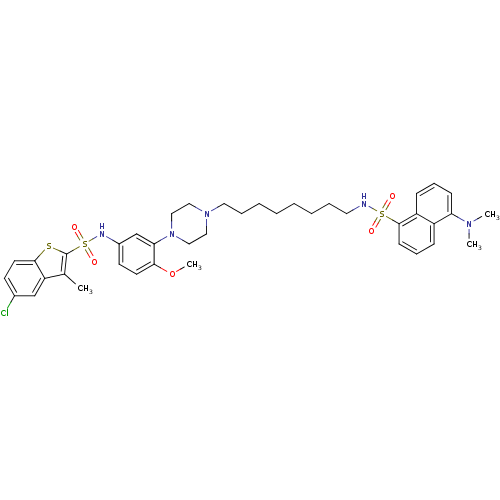 (5-Chloro-N-(3-{4-[8-({[5-(dimethylamino)-1-naphthy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50014009 (2,4,6-Trimethyl-1,4-dihydro-pyridine-3,5-dicarboxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 505 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALTER, S.A. Curated by ChEMBL | Assay Description Tested for inhibition of [3H]PAF binding to rabbit platelet Platelet activating factor receptor | J Med Chem 33: 3205-10 (1991) BindingDB Entry DOI: 10.7270/Q2PV6KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328472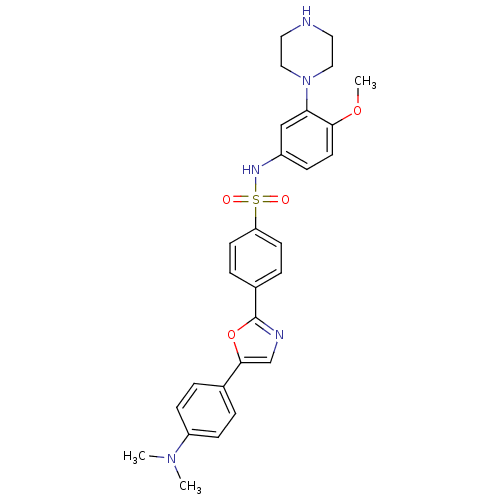 (4-{5-[4-(Dimethylamino)phenyl]-1,3-oxazol-2-yl}-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 523 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328470 (5-(Dimethylamino)-N-(3-{4-[6-({[5-(dimethylamino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 554 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50014013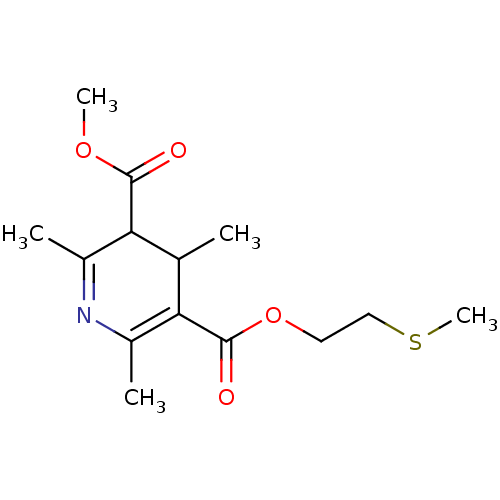 (2,4,6-Trimethyl-1,4-dihydro-pyridine-3,5-dicarboxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALTER, S.A. Curated by ChEMBL | Assay Description Tested for inhibition of [3H]PAF binding to rabbit platelet Platelet activating factor receptor | J Med Chem 33: 3205-10 (1991) BindingDB Entry DOI: 10.7270/Q2PV6KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328466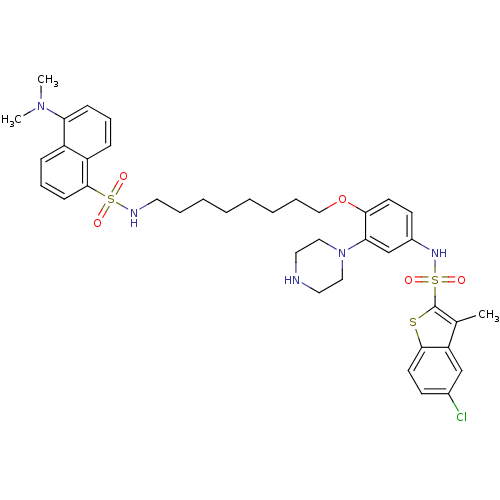 (5-Chloro-N-(4-{[8-({[5-(dimethylamino)-1-naphthyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50328464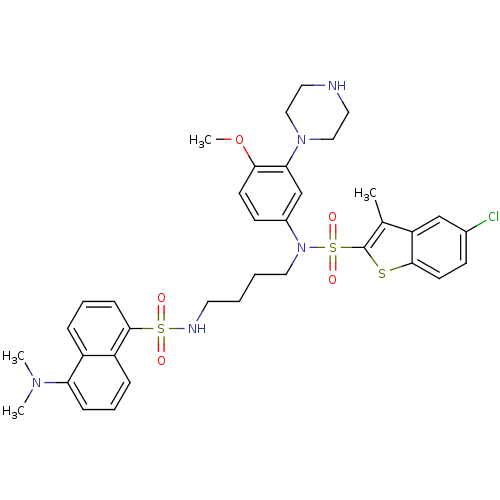 (5-Chloro-N-[4-({[5-(dimethylamino)-1-naphthyl]sulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 747 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense deMadrid Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor expressed in HEK293 cells | J Med Chem 53: 7095-106 (2010) Article DOI: 10.1021/jm1007177 BindingDB Entry DOI: 10.7270/Q2445MQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50014012 (2,4,6-Trimethyl-1,4-dihydro-pyridine-3,5-dicarboxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 913 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALTER, S.A. Curated by ChEMBL | Assay Description Tested for inhibition of [3H]PAF binding to rabbit platelet Platelet activating factor receptor | J Med Chem 33: 3205-10 (1991) BindingDB Entry DOI: 10.7270/Q2PV6KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50014004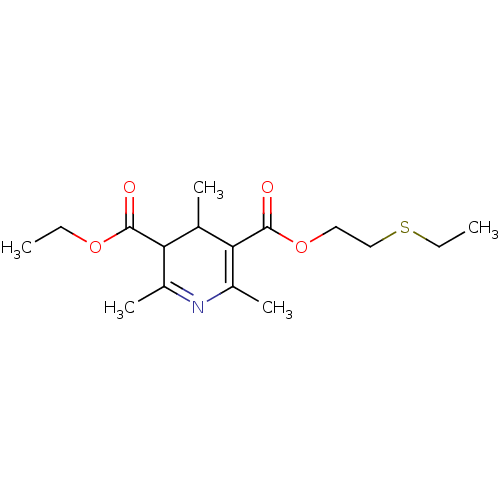 (2,4,6-Trimethyl-1,4-dihydro-pyridine-3,5-dicarboxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALTER, S.A. Curated by ChEMBL | Assay Description Tested for inhibition of [3H]PAF binding to rabbit platelet Platelet activating factor receptor | J Med Chem 33: 3205-10 (1991) BindingDB Entry DOI: 10.7270/Q2PV6KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50014011 (4-Ethyl-2,6-dimethyl-1,4-dihydro-pyridine-3,5-dica...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALTER, S.A. Curated by ChEMBL | Assay Description Tested for inhibition of [3H]PAF binding to rabbit platelet Platelet activating factor receptor | J Med Chem 33: 3205-10 (1991) BindingDB Entry DOI: 10.7270/Q2PV6KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50014008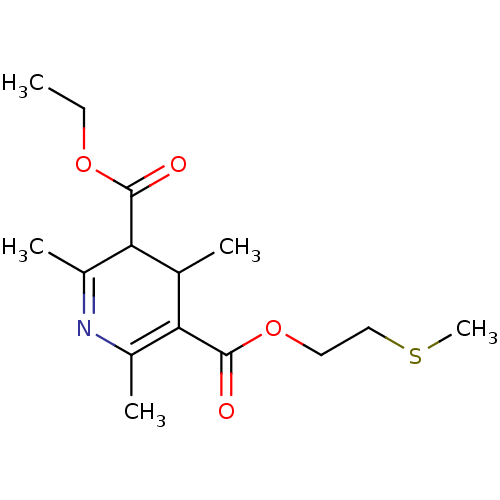 (2,4,6-Trimethyl-1,4-dihydro-pyridine-3,5-dicarboxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALTER, S.A. Curated by ChEMBL | Assay Description Tested for inhibition of [3H]PAF binding to rabbit platelet Platelet activating factor receptor | J Med Chem 33: 3205-10 (1991) BindingDB Entry DOI: 10.7270/Q2PV6KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50014010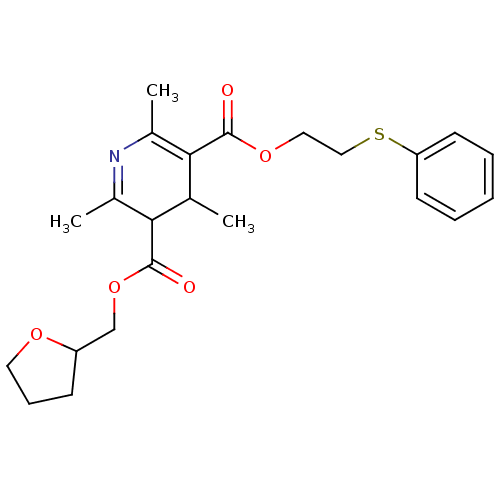 (2,4,6-Trimethyl-1,4-dihydro-pyridine-3,5-dicarboxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALTER, S.A. Curated by ChEMBL | Assay Description Tested for inhibition of [3H]PAF binding to rabbit platelet Platelet activating factor receptor | J Med Chem 33: 3205-10 (1991) BindingDB Entry DOI: 10.7270/Q2PV6KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase C (Bacillus cereus) | BDBM50332115 (3-(N-(benzyloxy)-2-(tert-butyloxycarbonylamino)dod...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advance Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Mixed-type inhibition of Bacillus cereus phosphatidylcholine preferred phospholipase C by Dixon plot analysis | Bioorg Med Chem 18: 8549-55 (2010) Article DOI: 10.1016/j.bmc.2010.10.031 BindingDB Entry DOI: 10.7270/Q2VH5P32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase C (Bacillus cereus) | BDBM50332109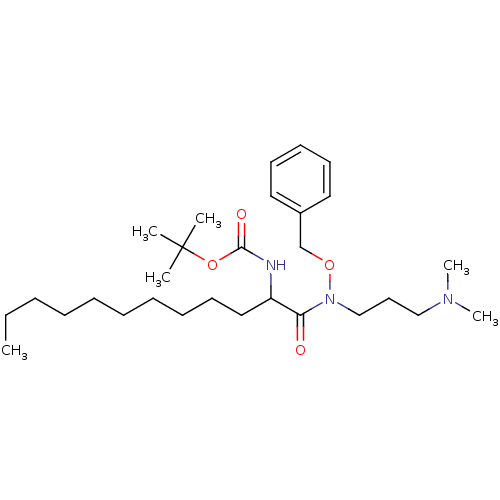 (CHEMBL1287861 | Tert-butyl 1-(benzyloxy[3-(dimethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advance Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Non-competitive inhibition of Bacillus cereus phosphatidylcholine preferred phospholipase C by Dixon plot analysis | Bioorg Med Chem 18: 8549-55 (2010) Article DOI: 10.1016/j.bmc.2010.10.031 BindingDB Entry DOI: 10.7270/Q2VH5P32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50014006 (2,4,6-Trimethyl-1,4-dihydro-pyridine-3,5-dicarboxy...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALTER, S.A. Curated by ChEMBL | Assay Description Tested for inhibition of [3H]PAF binding to rabbit platelet Platelet activating factor receptor | J Med Chem 33: 3205-10 (1991) BindingDB Entry DOI: 10.7270/Q2PV6KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase C (Bacillus cereus) | BDBM50332105 (1-(hydroxy(2-(trimethylammonio)ethyl)amino)-1-oxod...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advance Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Uncompetitive inhibition of Bacillus cereus phosphatidylcholine preferred phospholipase C by Dixon plot analysis | Bioorg Med Chem 18: 8549-55 (2010) Article DOI: 10.1016/j.bmc.2010.10.031 BindingDB Entry DOI: 10.7270/Q2VH5P32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase C (Bacillus cereus) | BDBM50332119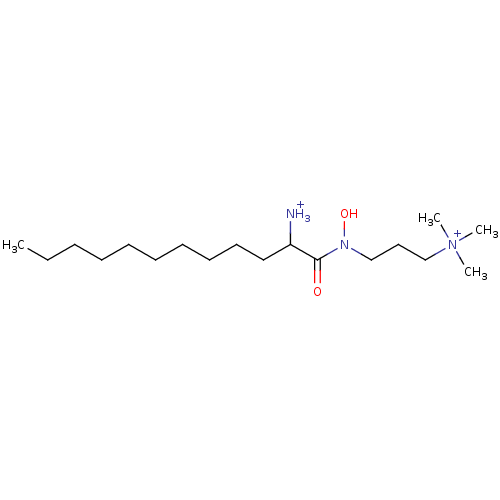 (1-(hydroxy[3-(trimethylammonio)propyl]amino)-1-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advance Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Mixed-type inhibition of Bacillus cereus phosphatidylcholine preferred phospholipase C by Dixon plot analysis | Bioorg Med Chem 18: 8549-55 (2010) Article DOI: 10.1016/j.bmc.2010.10.031 BindingDB Entry DOI: 10.7270/Q2VH5P32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Cavia porcellus) | BDBM50318494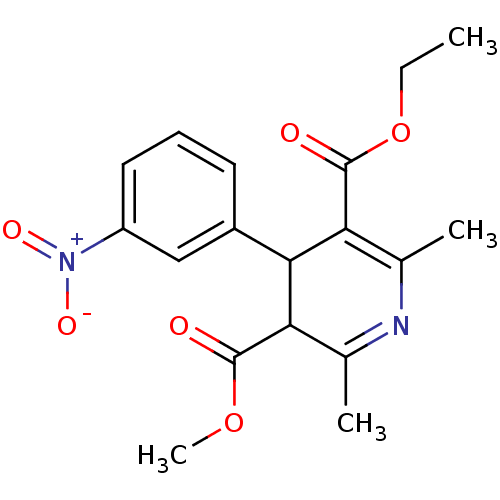 (3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ALTER, S.A. Curated by ChEMBL | Assay Description Tested for inhibition of [3H]PAF binding to rabbit platelet Platelet activating factor receptor | J Med Chem 33: 3205-10 (1991) BindingDB Entry DOI: 10.7270/Q2PV6KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002644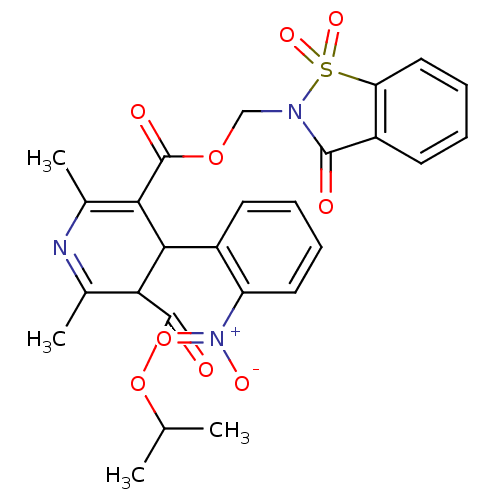 (2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50336640 ((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002628 (4-(2,3-Dichloro-phenyl)-2,6-dimethyl-1,4-dihydro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002603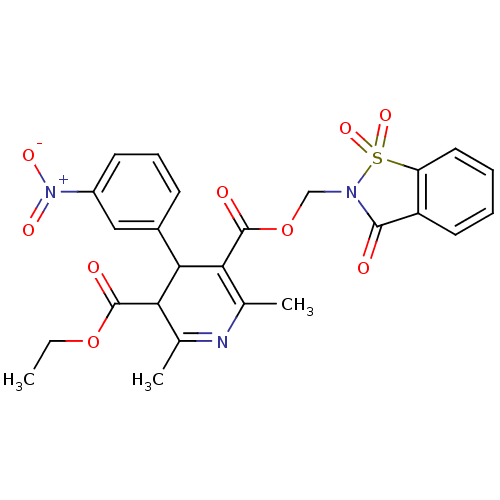 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002613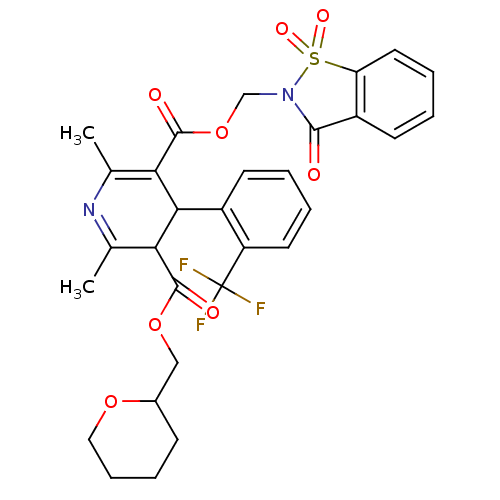 (2,6-Dimethyl-4-(2-trifluoromethyl-phenyl)-1,4-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002626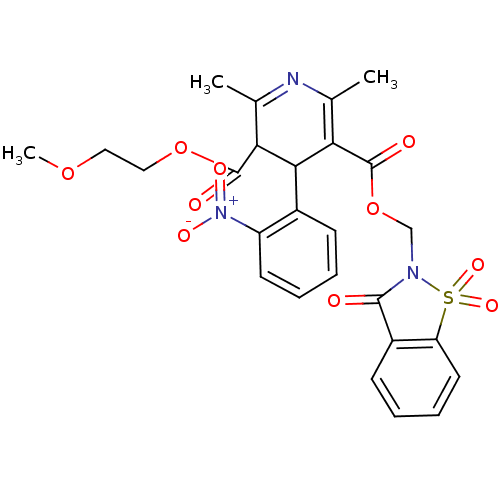 (2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002607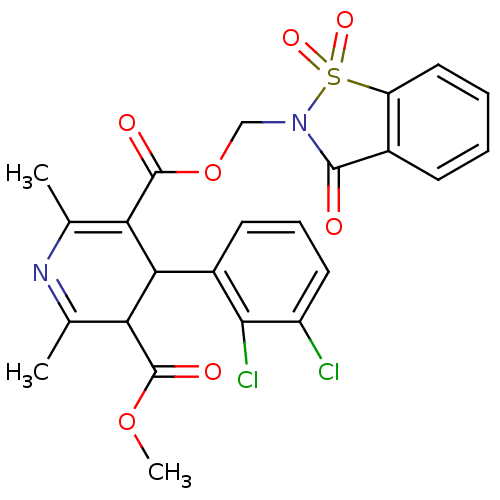 (4-(2,3-Dichloro-phenyl)-2,6-dimethyl-1,4-dihydro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002600 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002643 (4-(2-Chloro-phenyl)-2,6-dimethyl-1,4-dihydro-pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002635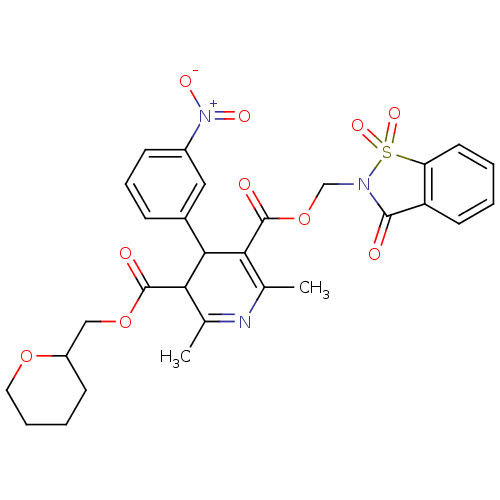 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002621 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002580 (2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002631 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002589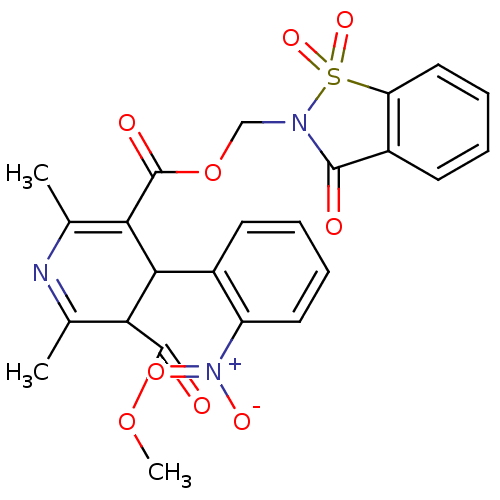 (2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002604 (2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Oryctolagus cuniculus) | BDBM50002637 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Alter, S.A. Curated by ChEMBL | Assay Description Blockade of calcium-evoked contractions in depolarized aortic strips | J Med Chem 35: 2407-14 (1992) BindingDB Entry DOI: 10.7270/Q2BV7H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline kinase alpha (Homo sapiens (Human)) | BDBM50166208 (Bisquinolinium derivative | CHEMBL191595) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Ex vivo inhibitory concentration against human choline kinase | J Med Chem 48: 3354-63 (2005) Article DOI: 10.1021/jm049061o BindingDB Entry DOI: 10.7270/Q2WD403P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline kinase alpha (Homo sapiens (Human)) | BDBM50154646 (4N-methyl-4N-phenyl-1-{3-[3-(4-methylanilino-1-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Grenada Curated by ChEMBL | Assay Description Inhibitory activity against human choline kinase enzyme | J Med Chem 47: 5433-40 (2004) Article DOI: 10.1021/jm0496537 BindingDB Entry DOI: 10.7270/Q22V2FM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline kinase alpha (Homo sapiens (Human)) | BDBM50166185 (Bisquinolinium derivative | CHEMBL372667) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Ex vivo inhibitory concentration against human choline kinase | J Med Chem 48: 3354-63 (2005) Article DOI: 10.1021/jm049061o BindingDB Entry DOI: 10.7270/Q2WD403P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline kinase alpha (Homo sapiens (Human)) | BDBM50166194 (Bisquinolinium derivative | CHEMBL363853) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Ex vivo inhibitory concentration against human choline kinase | J Med Chem 48: 3354-63 (2005) Article DOI: 10.1021/jm049061o BindingDB Entry DOI: 10.7270/Q2WD403P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50250903 ((5Z,9Z)-5,9-heptacosadienoic acid | CHEMBL463437) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Puerto Rico Curated by ChEMBL | Assay Description Inhibition of human DNA topoisomerase 1 | J Nat Prod 65: 1715-8 (2002) BindingDB Entry DOI: 10.7270/Q2WW7HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 161 total ) | Next | Last >> |