

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50346374 (3-(2-(4-(naphthalen-1-yl)piperazin-1-yl)ethyl)benz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.000178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli Federico II Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in Sprague-Dawley rat brain cortex homogenates after 30 mins by liquid scintillation counting | Eur J Med Chem 46: 2206-16 (2011) Article DOI: 10.1016/j.ejmech.2011.03.001 BindingDB Entry DOI: 10.7270/Q2M32W4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50359960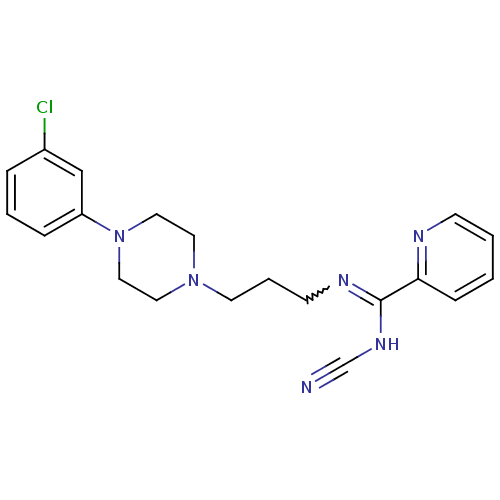 (CHEMBL1927094) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting | Eur J Med Chem 47: 520-9 (2012) Article DOI: 10.1016/j.ejmech.2011.11.023 BindingDB Entry DOI: 10.7270/Q29S1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50359961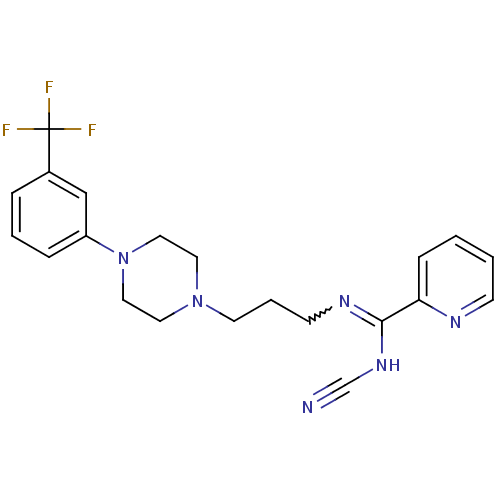 (CHEMBL1927095) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000778 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting | Eur J Med Chem 47: 520-9 (2012) Article DOI: 10.1016/j.ejmech.2011.11.023 BindingDB Entry DOI: 10.7270/Q29S1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50359954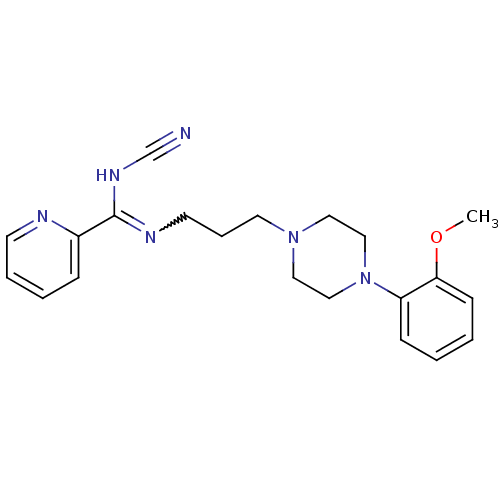 (CHEMBL1927088) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting | Eur J Med Chem 47: 520-9 (2012) Article DOI: 10.1016/j.ejmech.2011.11.023 BindingDB Entry DOI: 10.7270/Q29S1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50359962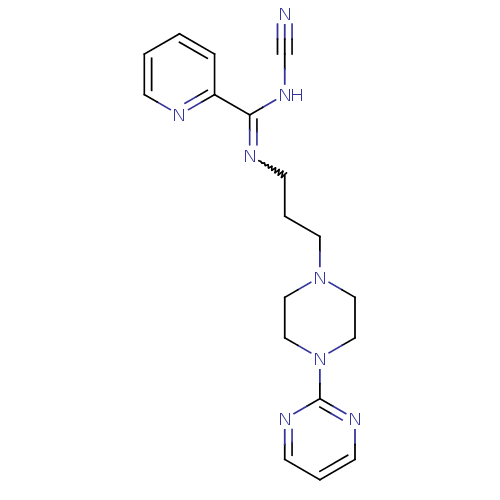 (CHEMBL1927096) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from Sprague-Dawley rat brain cortex serotonin 5-HT2A receptor after 15 mins by liquid scintillation counting | Eur J Med Chem 47: 520-9 (2012) Article DOI: 10.1016/j.ejmech.2011.11.023 BindingDB Entry DOI: 10.7270/Q29S1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50021919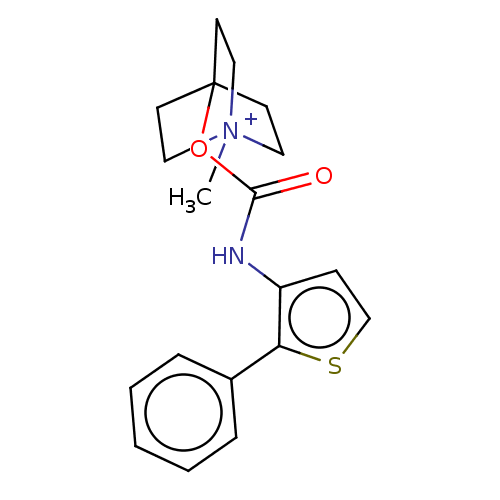 (CHEMBL3298595) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50021919 (CHEMBL3298595) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50021919 (CHEMBL3298595) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50420815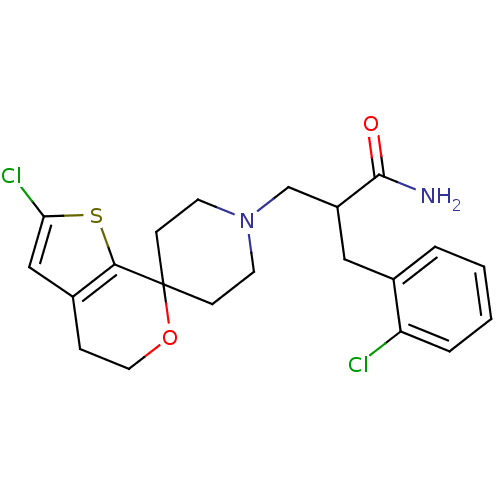 (CHEMBL2088054) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0373 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Agonist activity at human cloned NOP receptor expressed in CHO cell membranes after 30 mins by [35S]GTPgammaS binding assay | J Med Chem 55: 4955-67 (2012) Article DOI: 10.1021/jm201629q BindingDB Entry DOI: 10.7270/Q2TQ62SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50316964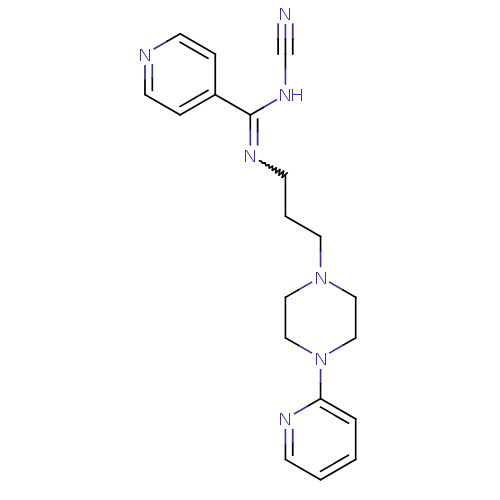 (CHEMBL1086961 | N'-cyano-N-(3-(4-(pyridin-2-yl)pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli Federico II Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-HT1A receptor in Sprague-Dawley rat brain cortex after 30 mins liquid scintillation spectrometer | Bioorg Med Chem Lett 20: 2978-82 (2010) Article DOI: 10.1016/j.bmcl.2010.02.106 BindingDB Entry DOI: 10.7270/Q2QR4X80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50021928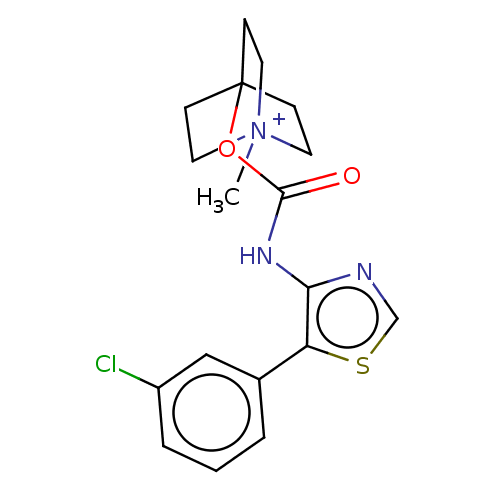 (CHEMBL3298599) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50021928 (CHEMBL3298599) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50021928 (CHEMBL3298599) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50021922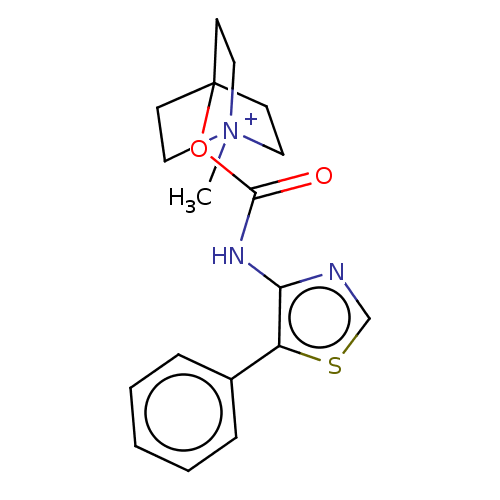 (CHEMBL3298596) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50021904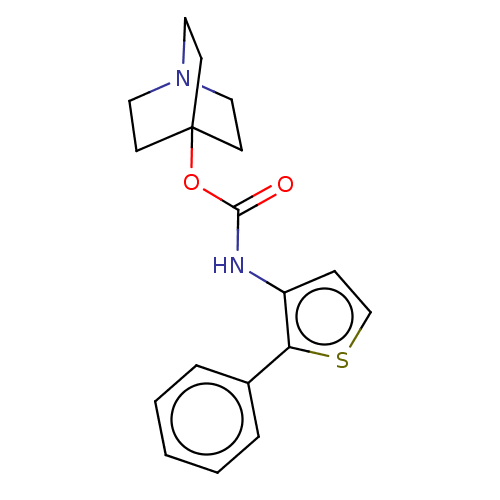 (CHEMBL3298588) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50021922 (CHEMBL3298596) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004193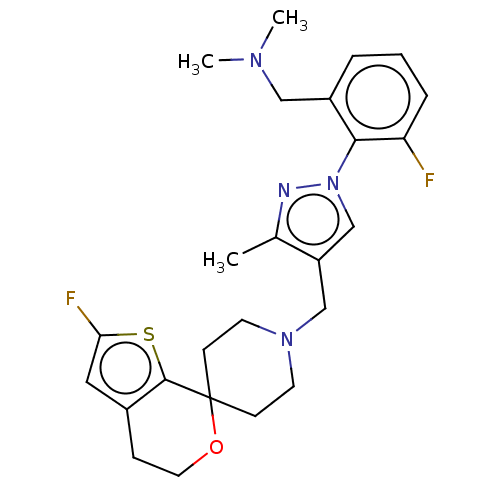 (CHEMBL3236476) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0648 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50420812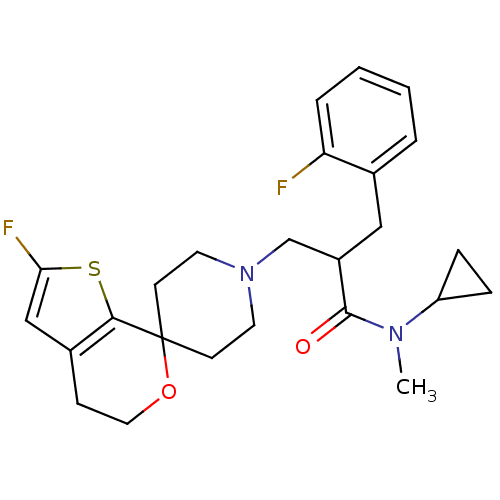 (CHEMBL2088061) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0672 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Agonist activity at human cloned NOP receptor expressed in CHO cell membranes after 30 mins by [35S]GTPgammaS binding assay | J Med Chem 55: 4955-67 (2012) Article DOI: 10.1021/jm201629q BindingDB Entry DOI: 10.7270/Q2TQ62SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004180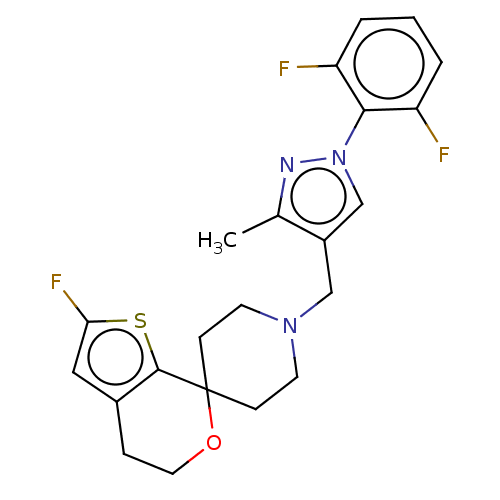 (CHEMBL3236474) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0711 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50021935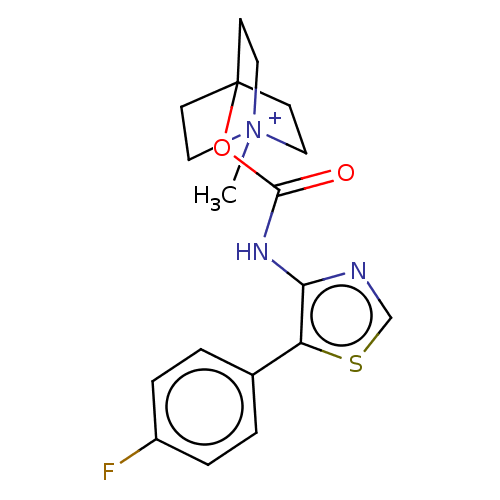 (CHEMBL3298600) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50359955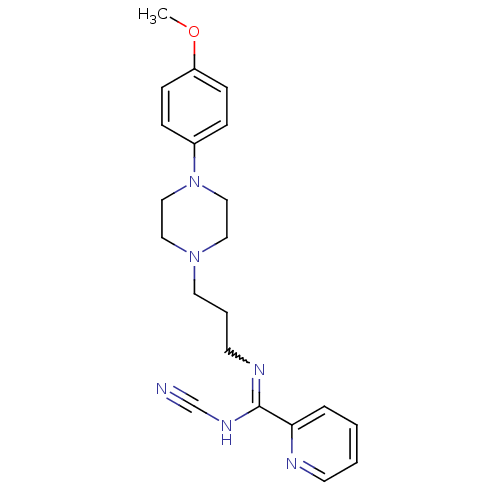 (CHEMBL1927089) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli"Federico II" Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from Sprague-Dawley rat brain cortex serotonin 5-HT1A receptor after 30 mins by liquid scintillation counting | Eur J Med Chem 47: 520-9 (2012) Article DOI: 10.1016/j.ejmech.2011.11.023 BindingDB Entry DOI: 10.7270/Q29S1RFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50420804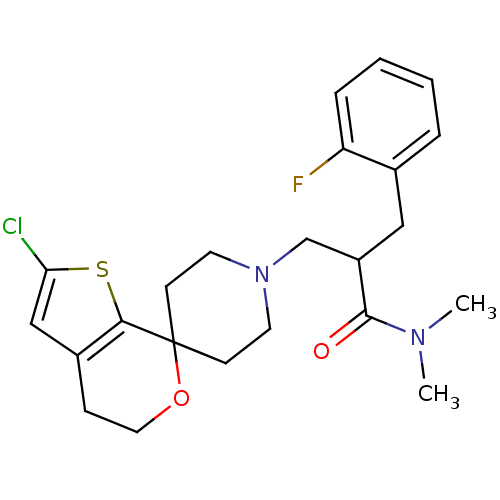 (CHEMBL2088055) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0892 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Agonist activity at human cloned NOP receptor expressed in CHO cell membranes after 30 mins by [35S]GTPgammaS binding assay | J Med Chem 55: 4955-67 (2012) Article DOI: 10.1021/jm201629q BindingDB Entry DOI: 10.7270/Q2TQ62SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50400866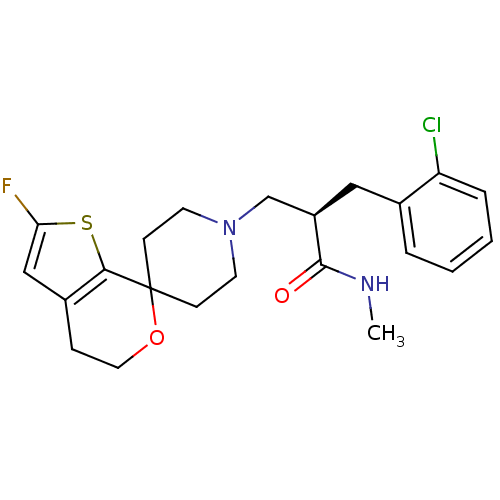 (CHEMBL2204348) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0902 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Agonist activity at human cloned NOP receptor expressed in CHO cell membranes after 30 mins by [35S]GTPgammaS binding assay | J Med Chem 55: 4955-67 (2012) Article DOI: 10.1021/jm201629q BindingDB Entry DOI: 10.7270/Q2TQ62SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004195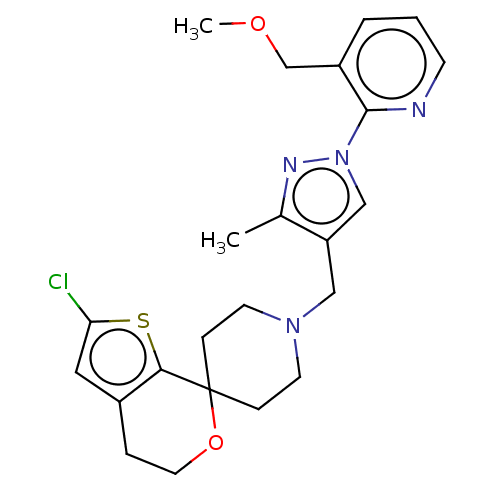 (CHEMBL3236478) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0908 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004175 (CHEMBL3236486) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0916 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004196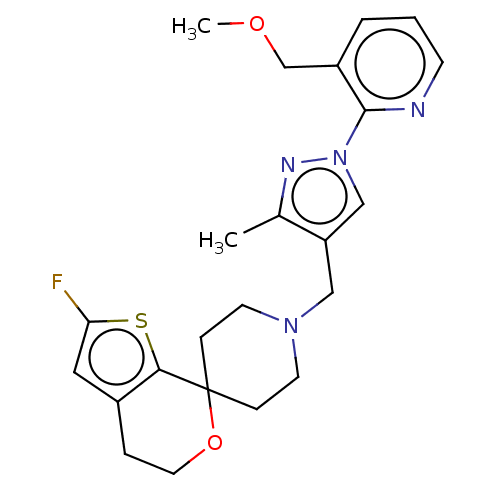 (CHEMBL3236479) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0939 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50021904 (CHEMBL3298588) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004181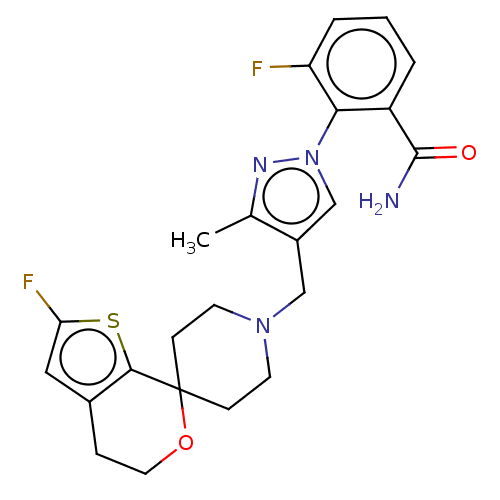 (CHEMBL3236475) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0954 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004174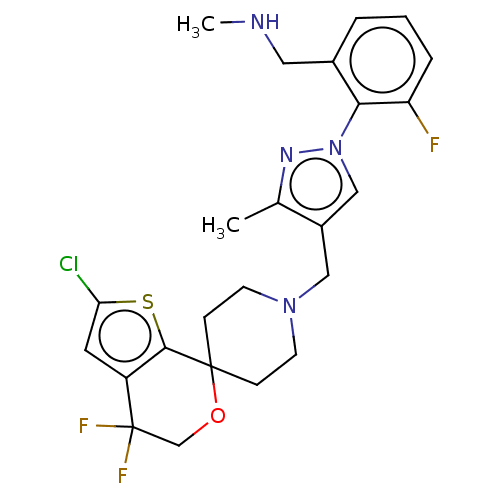 (CHEMBL3236485) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50021935 (CHEMBL3298600) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50516776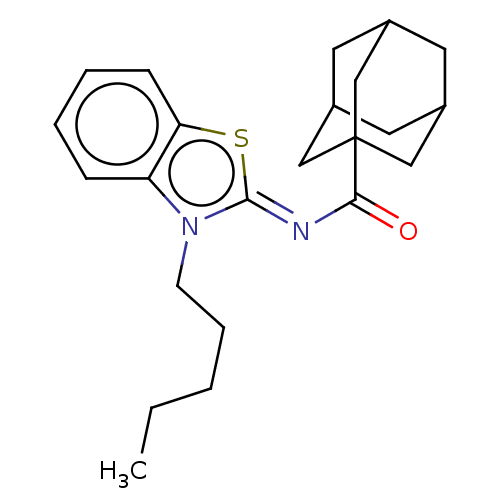 (CHEMBL4437741) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1R expressed in HEK293 cell membranes incubated for 90 mins by Cheng-Prusoff equation analysis | Eur J Med Chem 180: 154-170 (2019) Article DOI: 10.1016/j.ejmech.2019.07.002 BindingDB Entry DOI: 10.7270/Q2R214R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004176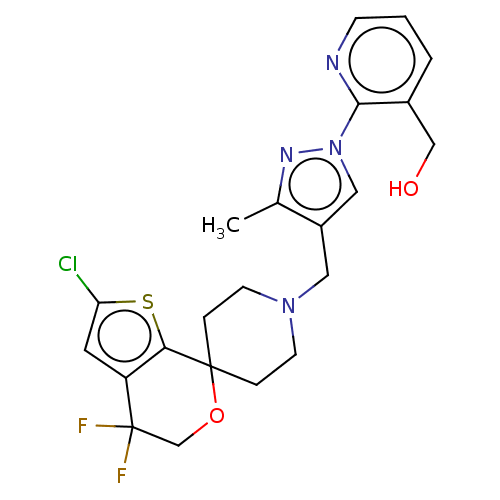 (CHEMBL3236487) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50400874 (CHEMBL2086410) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Agonist activity at human cloned NOP receptor expressed in CHO cell membranes after 30 mins by [35S]GTPgammaS binding assay | J Med Chem 55: 4955-67 (2012) Article DOI: 10.1021/jm201629q BindingDB Entry DOI: 10.7270/Q2TQ62SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50516789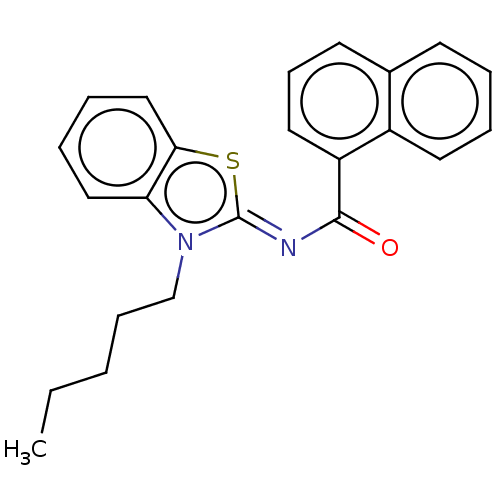 (CHEMBL4559193) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German University in Cairo Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from recombinant human CB1R expressed in HEK293 cell membranes incubated for 90 mins by Cheng-Prusoff equation analysis | Eur J Med Chem 180: 154-170 (2019) Article DOI: 10.1016/j.ejmech.2019.07.002 BindingDB Entry DOI: 10.7270/Q2R214R1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50045494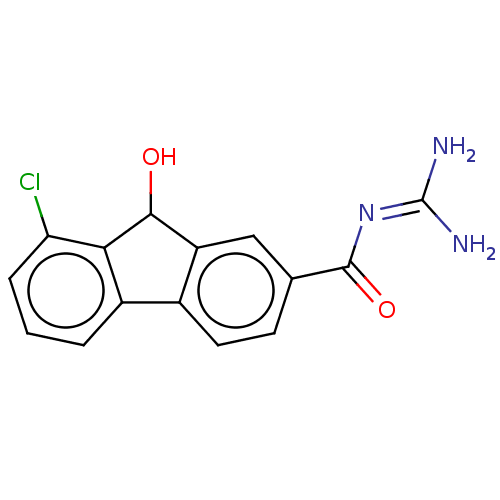 (CHEMBL3310115) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2B receptor expressed in HEK293-EBNA cells by liquid scintillation counting | Bioorg Med Chem 22: 4323-37 (2014) Article DOI: 10.1016/j.bmc.2014.05.027 BindingDB Entry DOI: 10.7270/Q2X63PK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50118178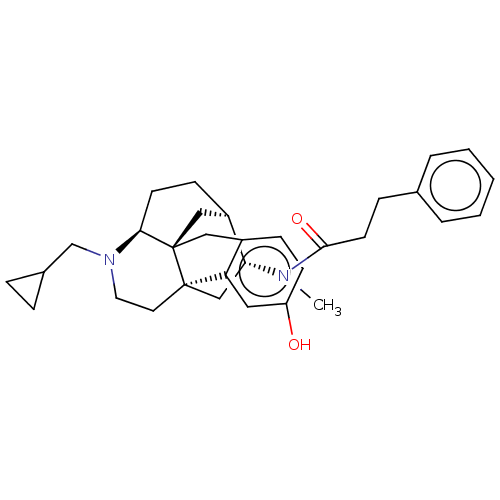 (CHEMBL3613172) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum | Bioorg Med Chem 23: 6271-9 (2015) Article DOI: 10.1016/j.bmc.2015.08.036 BindingDB Entry DOI: 10.7270/Q2V126M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004173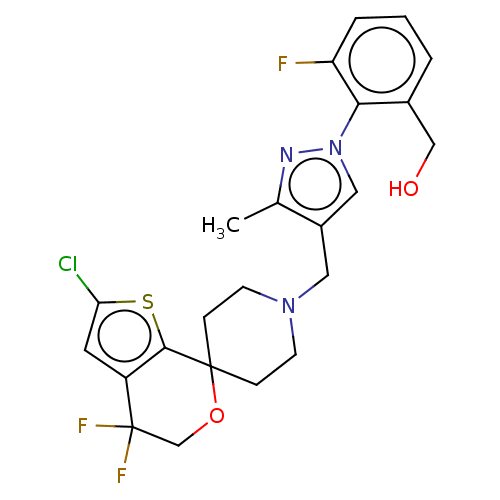 (CHEMBL3236484) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50420810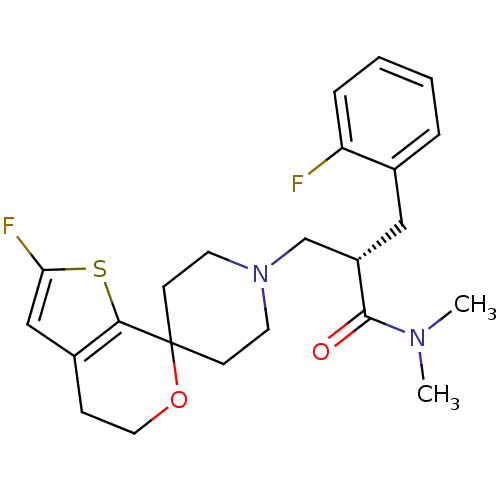 (CHEMBL2088060) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Agonist activity at human cloned NOP receptor expressed in CHO cell membranes after 30 mins by [35S]GTPgammaS binding assay | J Med Chem 55: 4955-67 (2012) Article DOI: 10.1021/jm201629q BindingDB Entry DOI: 10.7270/Q2TQ62SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50021922 (CHEMBL3298596) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M2 receptor in heart | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50021893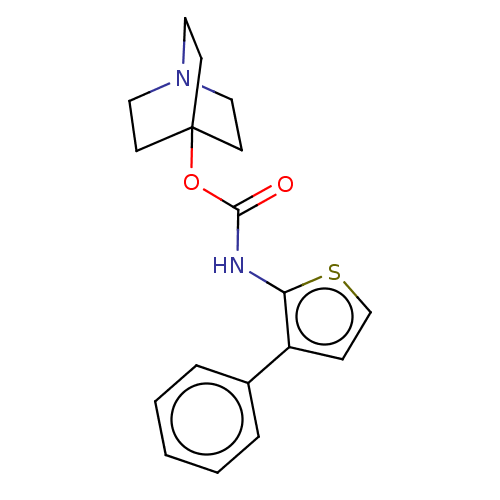 (CHEMBL3298333) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50021938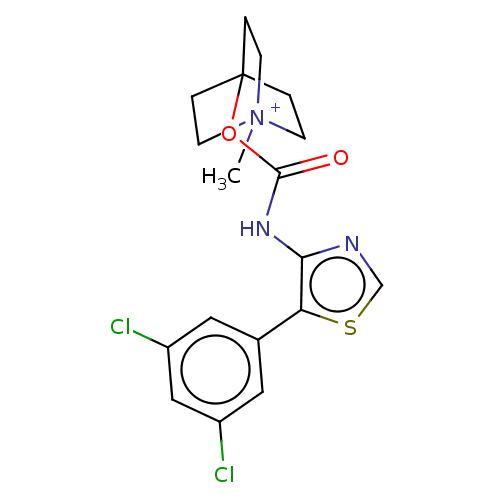 (CHEMBL3298763) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50021938 (CHEMBL3298763) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M1 receptor in cortex membrane | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004194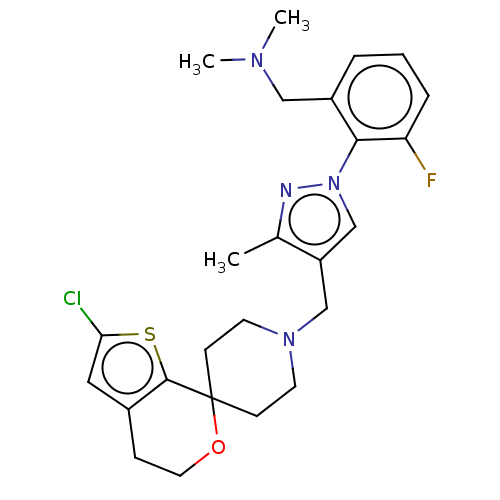 (CHEMBL3236477) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50420816 (CHEMBL2088053) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Agonist activity at human cloned NOP receptor expressed in CHO cell membranes after 30 mins by [35S]GTPgammaS binding assay | J Med Chem 55: 4955-67 (2012) Article DOI: 10.1021/jm201629q BindingDB Entry DOI: 10.7270/Q2TQ62SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50420814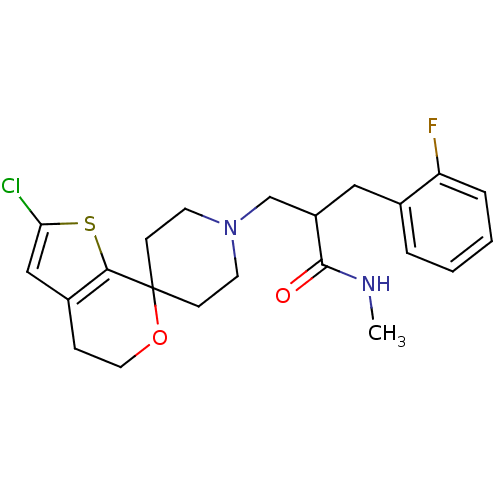 (CHEMBL2088056) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Agonist activity at human cloned NOP receptor expressed in CHO cell membranes after 30 mins by [35S]GTPgammaS binding assay | J Med Chem 55: 4955-67 (2012) Article DOI: 10.1021/jm201629q BindingDB Entry DOI: 10.7270/Q2TQ62SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50420803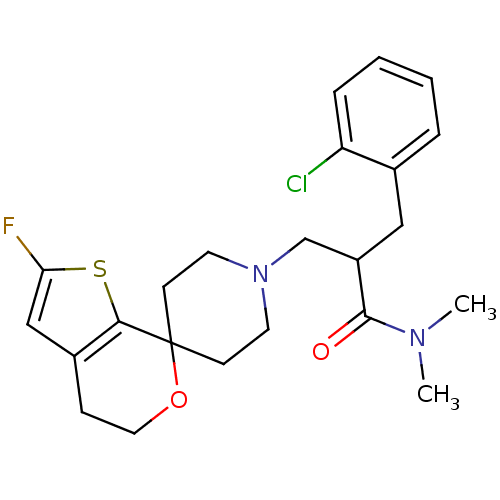 (CHEMBL2088057) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Agonist activity at human cloned NOP receptor expressed in CHO cell membranes after 30 mins by [35S]GTPgammaS binding assay | J Med Chem 55: 4955-67 (2012) Article DOI: 10.1021/jm201629q BindingDB Entry DOI: 10.7270/Q2TQ62SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50118191 (CHEMBL3613173) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum | Bioorg Med Chem 23: 6271-9 (2015) Article DOI: 10.1016/j.bmc.2015.08.036 BindingDB Entry DOI: 10.7270/Q2V126M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50400873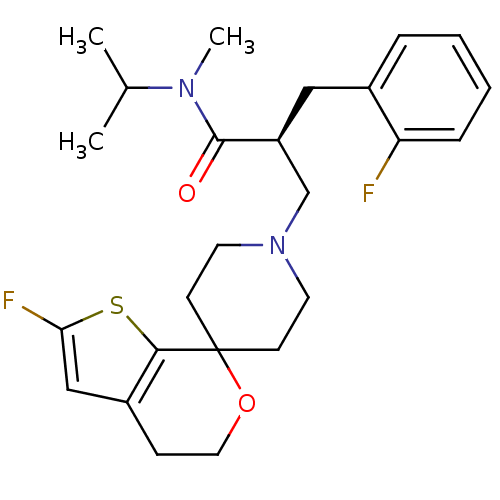 (CHEMBL2088029) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Agonist activity at human cloned NOP receptor expressed in CHO cell membranes after 30 mins by [35S]GTPgammaS binding assay | J Med Chem 55: 4955-67 (2012) Article DOI: 10.1021/jm201629q BindingDB Entry DOI: 10.7270/Q2TQ62SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004179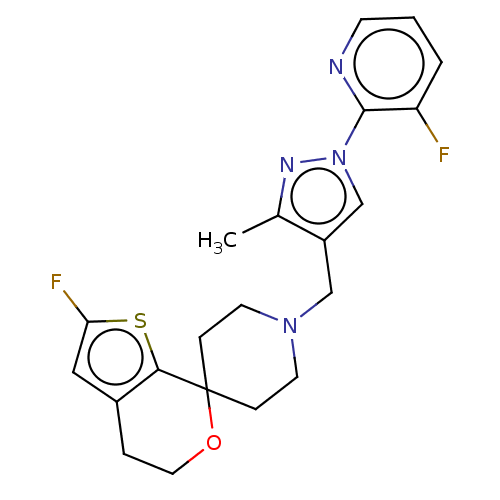 (CHEMBL3236473) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50344285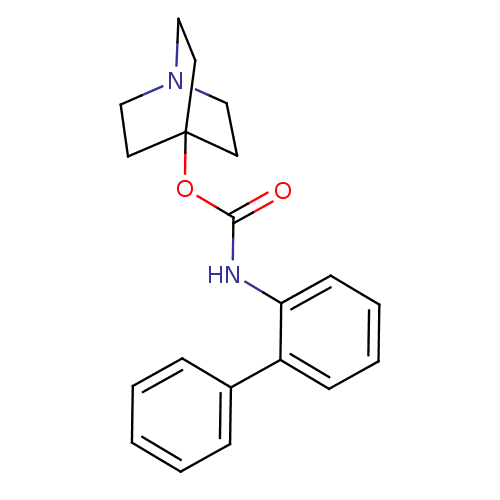 (CHEMBL1779047 | quinuclidin-4-yl biphenyl-2-ylcarb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from Sprague-Dawley rat muscarinic M3 receptor in submandibular gland | Bioorg Med Chem 22: 3478-87 (2014) Article DOI: 10.1016/j.bmc.2014.04.031 BindingDB Entry DOI: 10.7270/Q2XS5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3142 total ) | Next | Last >> |