 Found 2506 hits with Last Name = 'pandey' and Initial = 'a'
Found 2506 hits with Last Name = 'pandey' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
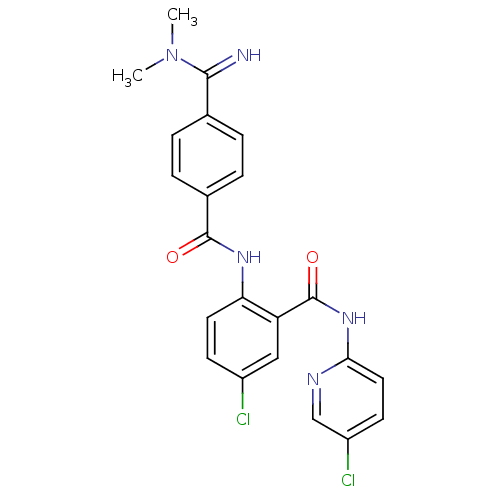
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249120
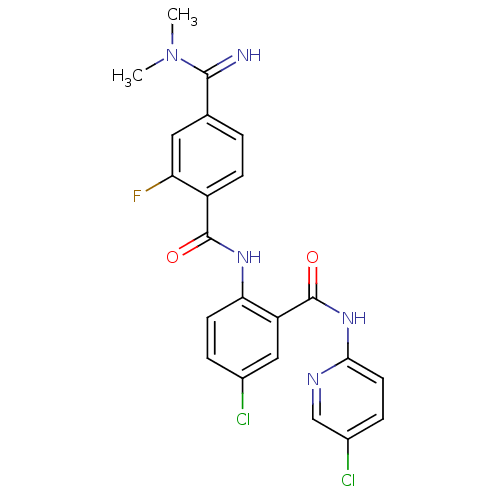
(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249423
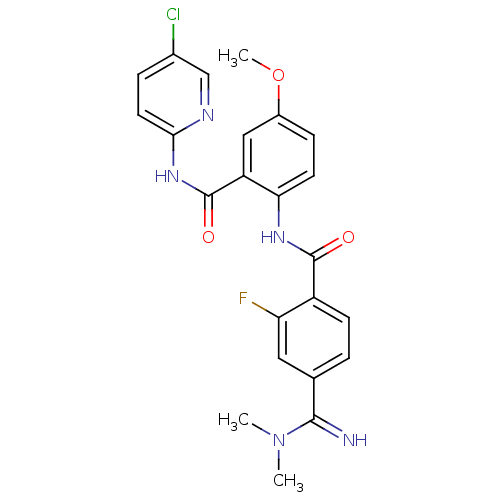
(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249298
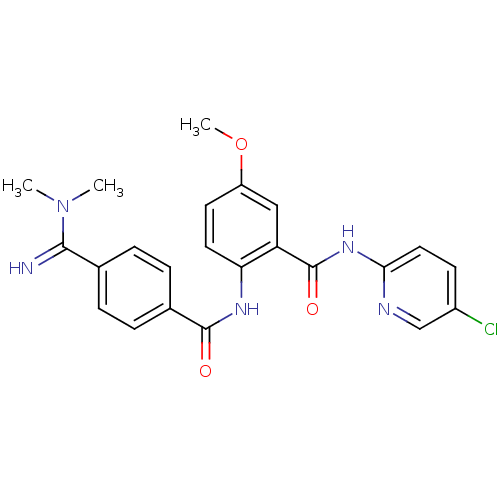
(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
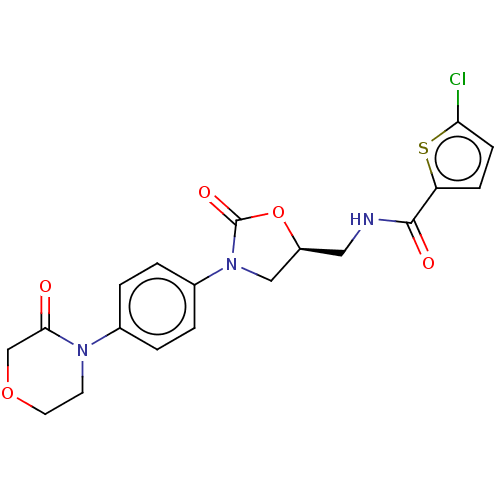
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249295
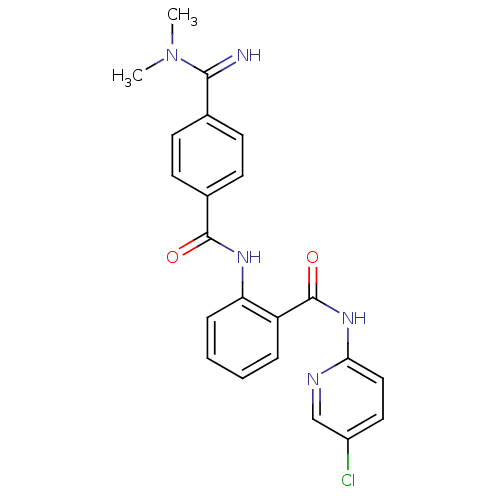
(CHEMBL471725 | N-(5-chloropyridin-2-yl)-2-(4-(N,N-...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccccc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H20ClN5O2/c1-28(2)20(24)14-7-9-15(10-8-14)21(29)26-18-6-4-3-5-17(18)22(30)27-19-12-11-16(23)13-25-19/h3-13,24H,1-2H3,(H,26,29)(H,25,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193861

(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193849

(CHEMBL219106 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2N2CCCCC2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H31ClN6O3/c1-34(2)26(30)18-7-10-21(24(15-18)35-13-5-4-6-14-35)27(36)32-23-11-9-20(38-3)16-22(23)28(37)33-25-12-8-19(29)17-31-25/h7-12,15-17,30H,4-6,13-14H2,1-3H3,(H,32,36)(H,31,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249120

(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249298

(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50249423

(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193842
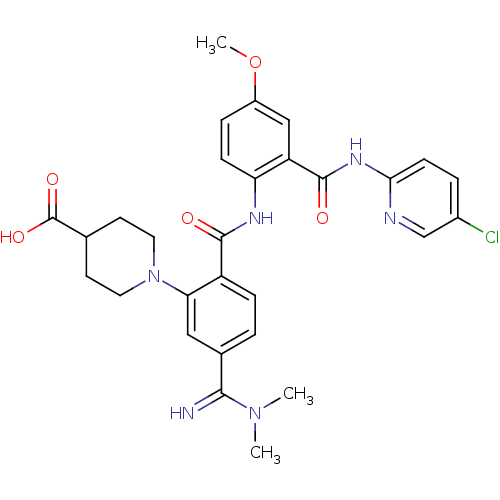
(1-(2-(2-(5-chloropyridin-2-ylcarbamoyl)-4-methoxyp...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2N2CCC(CC2)C(O)=O)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C29H31ClN6O5/c1-35(2)26(31)18-4-7-21(24(14-18)36-12-10-17(11-13-36)29(39)40)27(37)33-23-8-6-20(41-3)15-22(23)28(38)34-25-9-5-19(30)16-32-25/h4-9,14-17,31H,10-13H2,1-3H3,(H,33,37)(H,39,40)(H,32,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193850
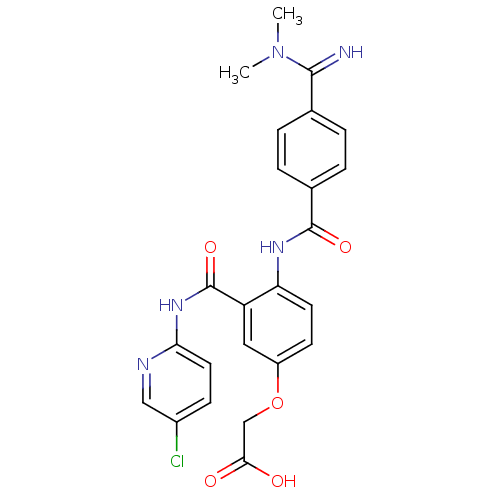
(2-(3-(5-chloropyridin-2-ylcarbamoyl)-4-(4-(N,N-dim...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(OCC(O)=O)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C24H22ClN5O5/c1-30(2)22(26)14-3-5-15(6-4-14)23(33)28-19-9-8-17(35-13-21(31)32)11-18(19)24(34)29-20-10-7-16(25)12-27-20/h3-12,26H,13H2,1-2H3,(H,28,33)(H,31,32)(H,27,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131871
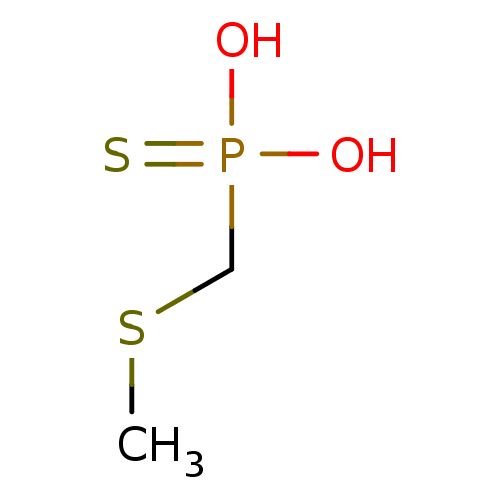
(CHEMBL122097 | Methylsulfanylmethyl-phosphonothioi...)Show InChI InChI=1S/C2H7O2PS2/c1-7-2-5(3,4)6/h2H2,1H3,(H2,3,4,6) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131868
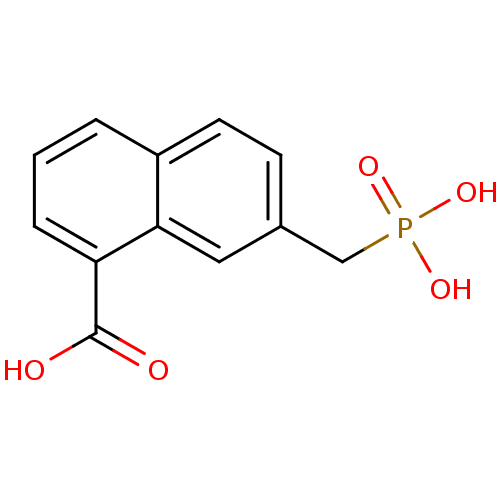
(7-Phosphonomethyl-naphthalene-1-carboxylic acid | ...)Show InChI InChI=1S/C12H11O5P/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-18(15,16)17/h1-6H,7H2,(H,13,14)(H2,15,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131866
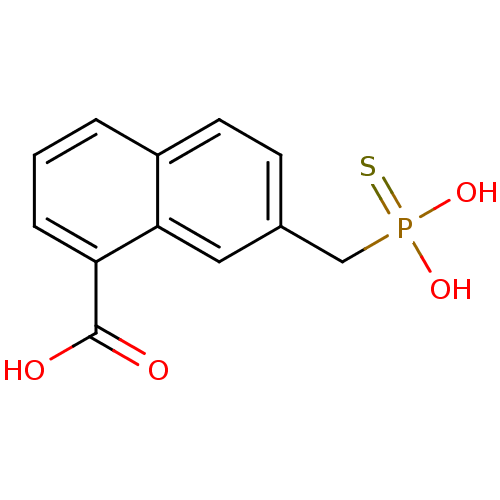
(7-Thiophosphonomethyl-naphthalene-1-carboxylic aci...)Show InChI InChI=1S/C12H11O4PS/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-17(15,16)18/h1-6H,7H2,(H,13,14)(H2,15,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131866

(7-Thiophosphonomethyl-naphthalene-1-carboxylic aci...)Show InChI InChI=1S/C12H11O4PS/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-17(15,16)18/h1-6H,7H2,(H,13,14)(H2,15,16,18) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131861
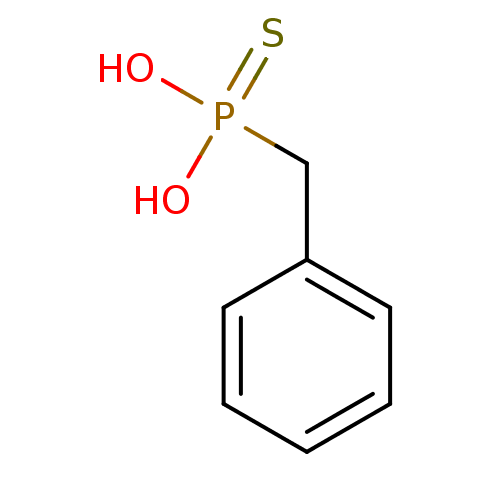
(Benzyl-phosphonothioic acid | CHEMBL123163)Show InChI InChI=1S/C7H9O2PS/c8-10(9,11)6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Rattus norvegicus) | BDBM50131868

(7-Phosphonomethyl-naphthalene-1-carboxylic acid | ...)Show InChI InChI=1S/C12H11O5P/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-18(15,16)17/h1-6H,7H2,(H,13,14)(H2,15,16,17) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against E. coli Alkaline Phosphatase |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131865
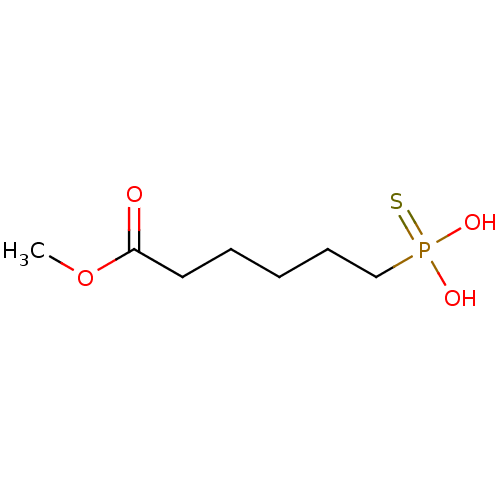
(6-Thiophosphono-hexanoic acid methyl ester | CHEMB...)Show InChI InChI=1S/C7H15O4PS/c1-11-7(8)5-3-2-4-6-12(9,10)13/h2-6H2,1H3,(H2,9,10,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131868

(7-Phosphonomethyl-naphthalene-1-carboxylic acid | ...)Show InChI InChI=1S/C12H11O5P/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-18(15,16)17/h1-6H,7H2,(H,13,14)(H2,15,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131860
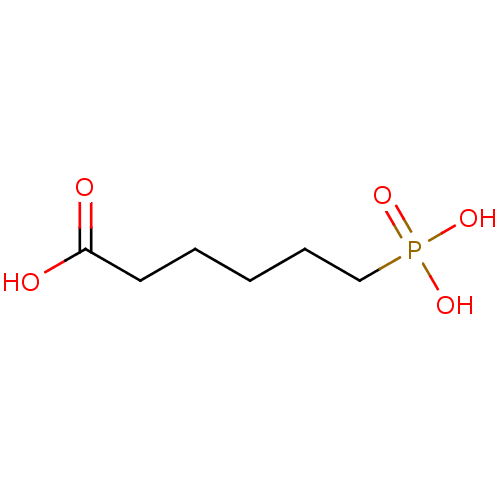
(6-Phosphono-hexanoic acid | CHEMBL122539)Show InChI InChI=1S/C6H13O5P/c7-6(8)4-2-1-3-5-12(9,10)11/h1-5H2,(H,7,8)(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131863
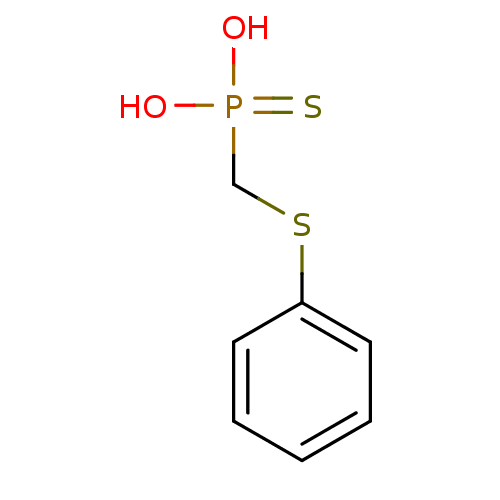
(CHEMBL331460 | Phenylsulfanylmethyl-phosphonothioi...)Show InChI InChI=1S/C7H9O2PS2/c8-10(9,11)6-12-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131863

(CHEMBL331460 | Phenylsulfanylmethyl-phosphonothioi...)Show InChI InChI=1S/C7H9O2PS2/c8-10(9,11)6-12-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131861

(Benzyl-phosphonothioic acid | CHEMBL123163)Show InChI InChI=1S/C7H9O2PS/c8-10(9,11)6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50080274
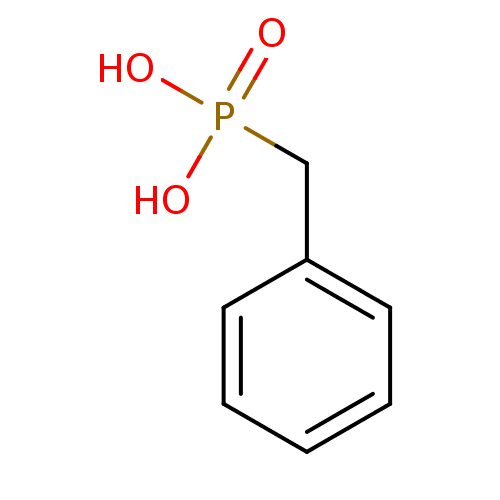
(Benzyl-phosphonic acid | CHEMBL299737 | Phenyl-met...)Show InChI InChI=1S/C7H9O3P/c8-11(9,10)6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131870
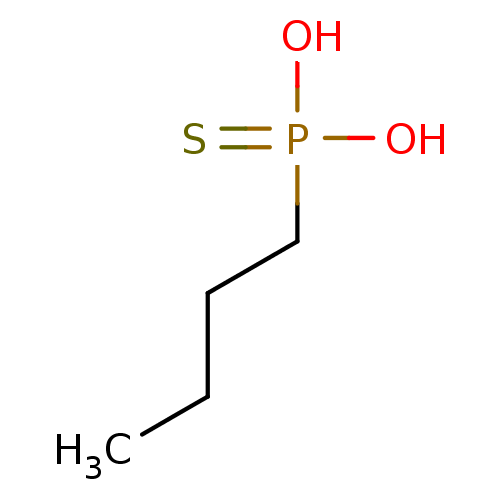
(Butyl-phosphonothioic acid | CHEMBL331046)Show InChI InChI=1S/C4H11O2PS/c1-2-3-4-7(5,6)8/h2-4H2,1H3,(H2,5,6,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, tissue-nonspecific isozyme
(Rattus norvegicus) | BDBM50131864
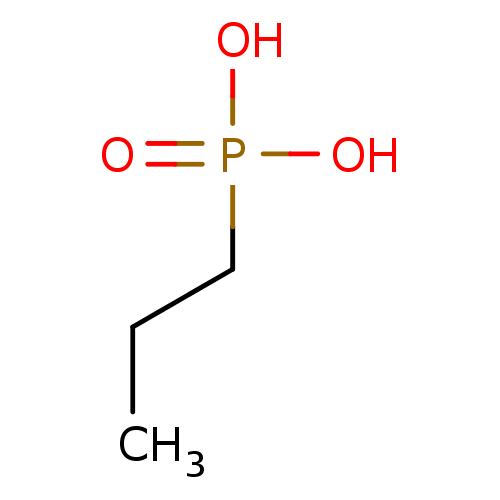
(CHEMBL125296 | Propyl-phosphonic acid)Show InChI InChI=1S/C3H9O3P/c1-2-3-7(4,5)6/h2-3H2,1H3,(H2,4,5,6) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against E. coli Alkaline Phosphatase |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131862

(CHEMBL122938 | methylphosphonic acid)Show InChI InChI=1S/CH5O3P/c1-5(2,3)4/h1H3,(H2,2,3,4) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.01E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131865

(6-Thiophosphono-hexanoic acid methyl ester | CHEMB...)Show InChI InChI=1S/C7H15O4PS/c1-11-7(8)5-3-2-4-6-12(9,10)13/h2-6H2,1H3,(H2,9,10,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131871

(CHEMBL122097 | Methylsulfanylmethyl-phosphonothioi...)Show InChI InChI=1S/C2H7O2PS2/c1-7-2-5(3,4)6/h2H2,1H3,(H2,3,4,6) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131867
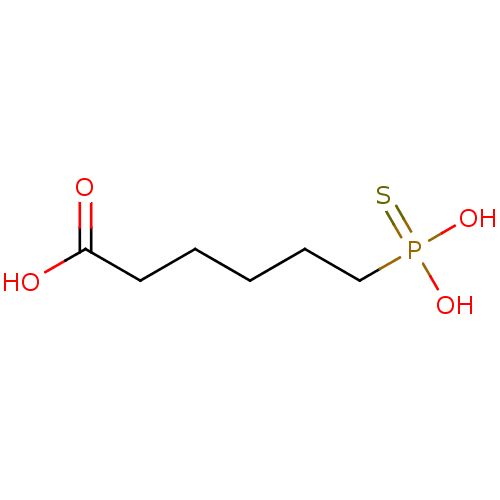
(6-Thiophosphono-hexanoic acid | CHEMBL123990)Show InChI InChI=1S/C6H13O4PS/c7-6(8)4-2-1-3-5-11(9,10)12/h1-5H2,(H,7,8)(H2,9,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131867

(6-Thiophosphono-hexanoic acid | CHEMBL123990)Show InChI InChI=1S/C6H13O4PS/c7-6(8)4-2-1-3-5-11(9,10)12/h1-5H2,(H,7,8)(H2,9,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131866

(7-Thiophosphonomethyl-naphthalene-1-carboxylic aci...)Show InChI InChI=1S/C12H11O4PS/c13-12(14)10-3-1-2-9-5-4-8(6-11(9)10)7-17(15,16)18/h1-6H,7H2,(H,13,14)(H2,15,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131864

(CHEMBL125296 | Propyl-phosphonic acid)Show InChI InChI=1S/C3H9O3P/c1-2-3-7(4,5)6/h2-3H2,1H3,(H2,4,5,6) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.55E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131860

(6-Phosphono-hexanoic acid | CHEMBL122539)Show InChI InChI=1S/C6H13O5P/c7-6(8)4-2-1-3-5-12(9,10)11/h1-5H2,(H,7,8)(H2,9,10,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131869
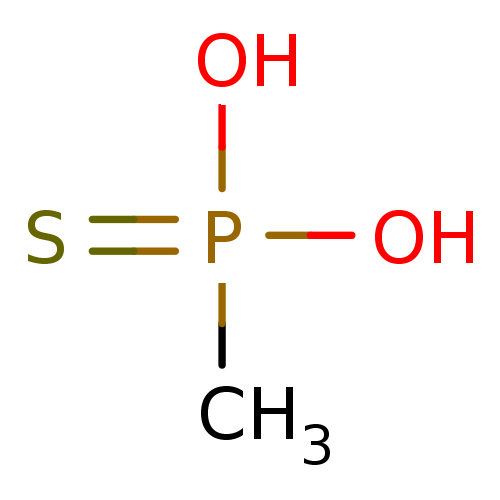
(CHEMBL122577 | Methyl-phosphonothioic acid)Show InChI InChI=1S/CH5O2PS/c1-4(2,3)5/h1H3,(H2,2,3,5) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131862

(CHEMBL122938 | methylphosphonic acid)Show InChI InChI=1S/CH5O3P/c1-5(2,3)4/h1H3,(H2,2,3,4) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131863

(CHEMBL331460 | Phenylsulfanylmethyl-phosphonothioi...)Show InChI InChI=1S/C7H9O2PS2/c8-10(9,11)6-12-7-4-2-1-3-5-7/h1-5H,6H2,(H2,8,9,11) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.58E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131869

(CHEMBL122577 | Methyl-phosphonothioic acid)Show InChI InChI=1S/CH5O2PS/c1-4(2,3)5/h1H3,(H2,2,3,5) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Alkaline phosphatase, germ cell type
(Homo sapiens (Human)) | BDBM50131869

(CHEMBL122577 | Methyl-phosphonothioic acid)Show InChI InChI=1S/CH5O2PS/c1-4(2,3)5/h1H3,(H2,2,3,5) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibition of Human Placental alkaline Phosphatase (PLAP) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase
(Enterobacteria phage lambda) | BDBM50131870

(Butyl-phosphonothioic acid | CHEMBL331046)Show InChI InChI=1S/C4H11O2PS/c1-2-3-4-7(5,6)8/h2-4H2,1H3,(H2,5,6,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131867

(6-Thiophosphono-hexanoic acid | CHEMBL123990)Show InChI InChI=1S/C6H13O4PS/c7-6(8)4-2-1-3-5-11(9,10)12/h1-5H2,(H,7,8)(H2,9,10,12) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131862

(CHEMBL122938 | methylphosphonic acid)Show InChI InChI=1S/CH5O3P/c1-5(2,3)4/h1H3,(H2,2,3,4) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50131860

(6-Phosphono-hexanoic acid | CHEMBL122539)Show InChI InChI=1S/C6H13O5P/c7-6(8)4-2-1-3-5-12(9,10)11/h1-5H2,(H,7,8)(H2,9,10,11) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) |
J Med Chem 46: 3703-8 (2003)
Article DOI: 10.1021/jm030106f
BindingDB Entry DOI: 10.7270/Q2MG7NXN |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition Protein kinase C (PKC) |
J Med Chem 45: 3772-93 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7JD9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50117342
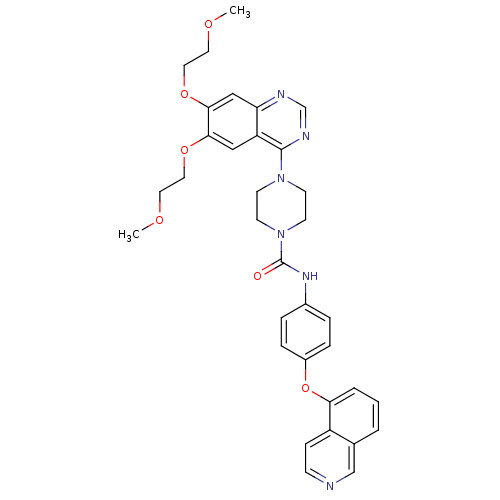
(4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...)Show SMILES COCCOc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4cccc5cnccc45)cc3)c2cc1OCCOC Show InChI InChI=1S/C34H36N6O6/c1-42-16-18-44-31-20-28-29(21-32(31)45-19-17-43-2)36-23-37-33(28)39-12-14-40(15-13-39)34(41)38-25-6-8-26(9-7-25)46-30-5-3-4-24-22-35-11-10-27(24)30/h3-11,20-23H,12-19H2,1-2H3,(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma |
J Med Chem 45: 3772-93 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7JD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249166

(2-(azetidin-1-yl)-N-(4-chloro-2-(5-chloropyridin-2...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(c1)N1CCC1 Show InChI InChI=1S/C25H24Cl2N6O2/c1-32(2)23(28)15-4-7-18(21(12-15)33-10-3-11-33)24(34)30-20-8-5-16(26)13-19(20)25(35)31-22-9-6-17(27)14-29-22/h4-9,12-14,28H,3,10-11H2,1-2H3,(H,30,34)(H,29,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50117334
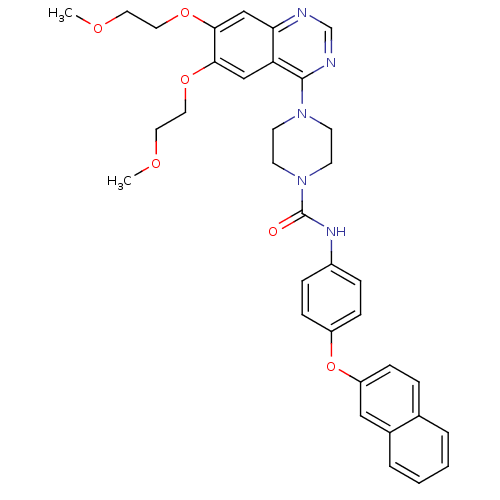
(4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...)Show SMILES COCCOc1cc2ncnc(N3CCN(CC3)C(=O)Nc3ccc(Oc4ccc5ccccc5c4)cc3)c2cc1OCCOC Show InChI InChI=1S/C35H37N5O6/c1-42-17-19-44-32-22-30-31(23-33(32)45-20-18-43-2)36-24-37-34(30)39-13-15-40(16-14-39)35(41)38-27-8-11-28(12-9-27)46-29-10-7-25-5-3-4-6-26(25)21-29/h3-12,21-24H,13-20H2,1-2H3,(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma |
J Med Chem 45: 3772-93 (2002)
BindingDB Entry DOI: 10.7270/Q2WW7JD9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data