

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340109 (CHEMBL1762827 | rac-4-[(13-Amino-10,11,12,14-tetra...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Mixed inhibition of Electrophorus electricus acetylcholinesterase using acetylcholine as substrate by Ellman's method | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50359391 (CHEMBL1929421) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Non-competitive inhibition of rat liver mitochondrial MAO-B using phenylethylamine as substrate by Line-Weaver Burk plot analysis | J Med Chem 54: 8251-70 (2011) Article DOI: 10.1021/jm200853t BindingDB Entry DOI: 10.7270/Q20P10G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50317074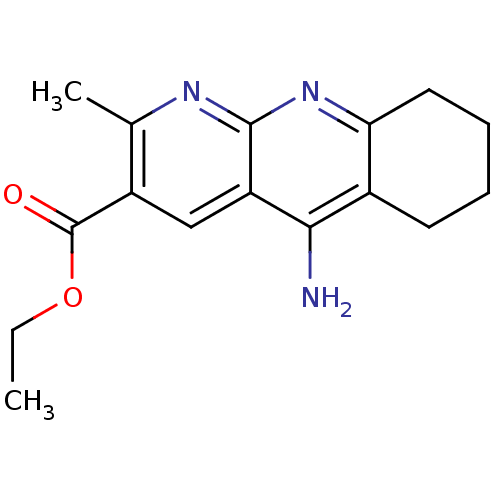 (CHEMBL1087194 | ethyl 5-amino-2-methyl-6,7,8,9-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE (unknown orign) by Lineweaver-Burke plot | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50448214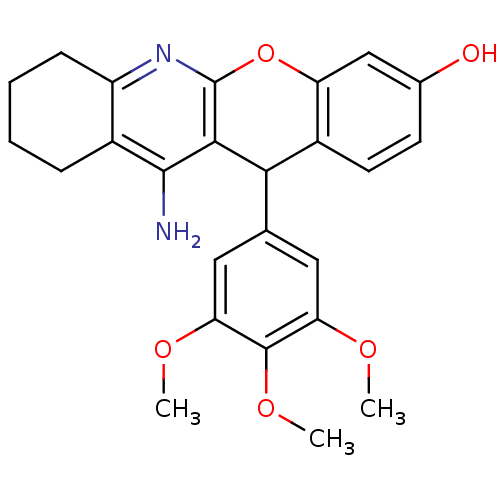 (CHEMBL3120707) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid (UCM) Curated by ChEMBL | Assay Description Noncompetitive inhibition of electric eel AchE using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured for ... | Eur J Med Chem 74: 491-501 (2014) Article DOI: 10.1016/j.ejmech.2013.12.021 BindingDB Entry DOI: 10.7270/Q2G73G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50381952 (CHEMBL2022933) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50401238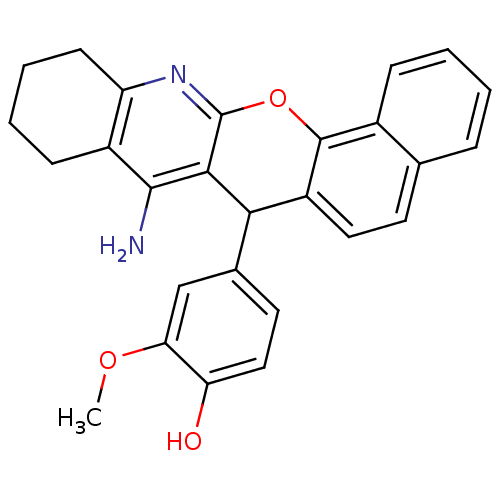 (CHEMBL2206895) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50359391 (CHEMBL1929421) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Mixed type inhibition of Electrophorus electricus AchE using acetylthiocholine as substrate by Line-Weaver Burk plot analysis | J Med Chem 54: 8251-70 (2011) Article DOI: 10.1021/jm200853t BindingDB Entry DOI: 10.7270/Q20P10G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384793 (CHEMBL2037384) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 428 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Química Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus AChE by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 5861-72 (2010) Article DOI: 10.1016/j.bmc.2010.06.095 BindingDB Entry DOI: 10.7270/Q23J3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50357943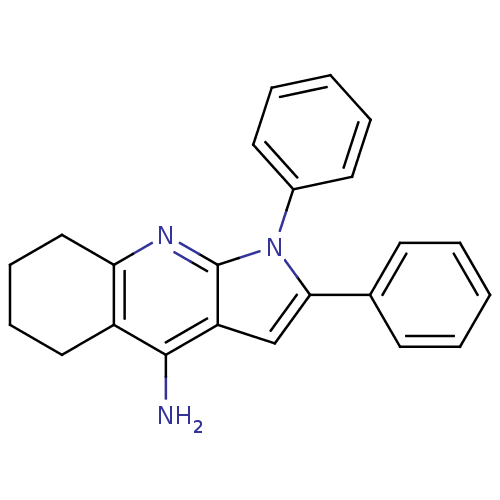 (CHEMBL1916768) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 621 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Mixed-type inhibition of Electrophorus electricus AChE assessed as hydrolysis of acetylthiocholine by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 6119-30 (2011) Article DOI: 10.1016/j.ejmech.2011.09.038 BindingDB Entry DOI: 10.7270/Q27M08BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50018671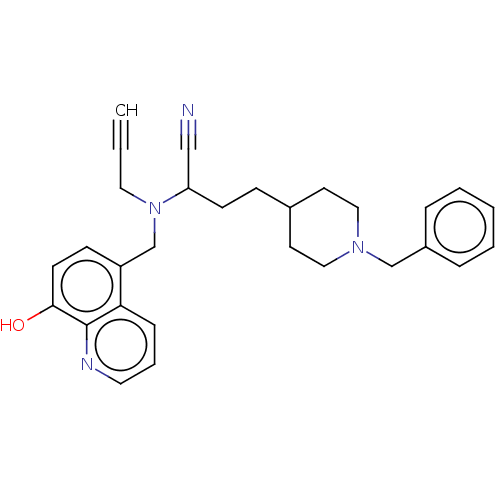 (CHEMBL3291019) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Reversible inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM50381961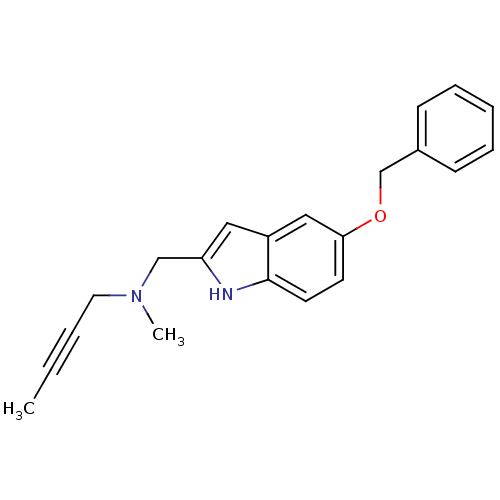 (CHEMBL2022928) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using acetylthiocholine as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM31904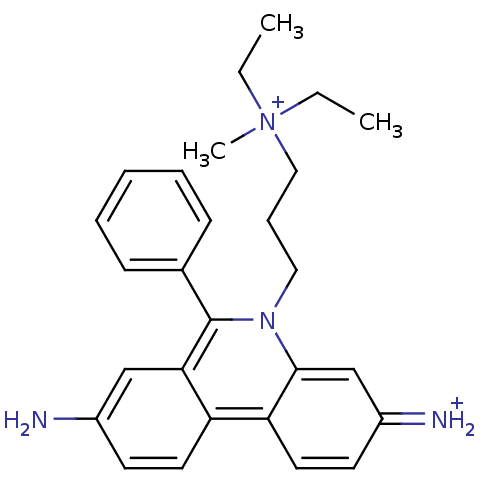 (CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Química Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Binding affinity to Electrophorus electricus AChE peripheral anionic site | Bioorg Med Chem 18: 5861-72 (2010) Article DOI: 10.1016/j.bmc.2010.06.095 BindingDB Entry DOI: 10.7270/Q23J3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM31904 (CHEMBL345124 | Propidium | Propidium Iodide, 2 | p...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Química Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Binding affinity to Electrophorus electricus AChE peripheral anionic site | Bioorg Med Chem 18: 5861-72 (2010) Article DOI: 10.1016/j.bmc.2010.06.095 BindingDB Entry DOI: 10.7270/Q23J3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50324068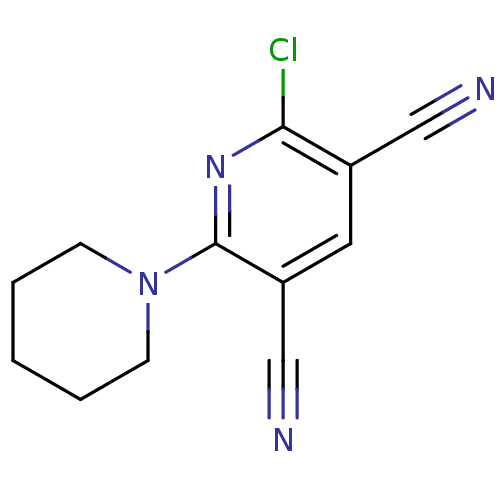 (2-Chloro-6-(piperidin-1-yl)pyridine-3,5-dicarbonit...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Química Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Non competitive inhibition of Electrophorus electricus AChE by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 5861-72 (2010) Article DOI: 10.1016/j.bmc.2010.06.095 BindingDB Entry DOI: 10.7270/Q23J3D5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50381960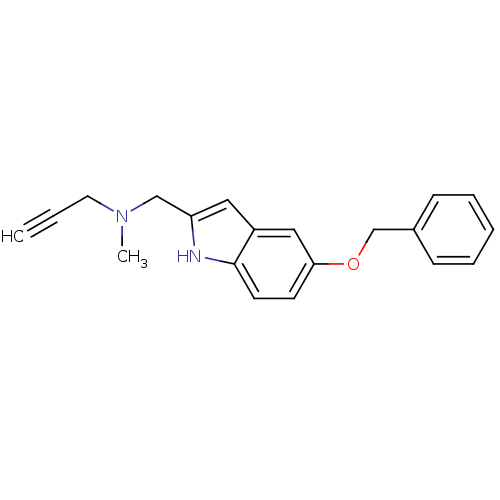 (CHEMBL2022927) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-B in rat liver homogenates using [14C]-phenylethylamine as substrate preincubated for 30 mins by liquid scintillation... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50381960 (CHEMBL2022927) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of mitochondrial MAO-A in rat liver homogenates using [14C]-(5-hydroxy-triptamine) as substrate preincubated for 30 mins by liquid scintil... | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50087800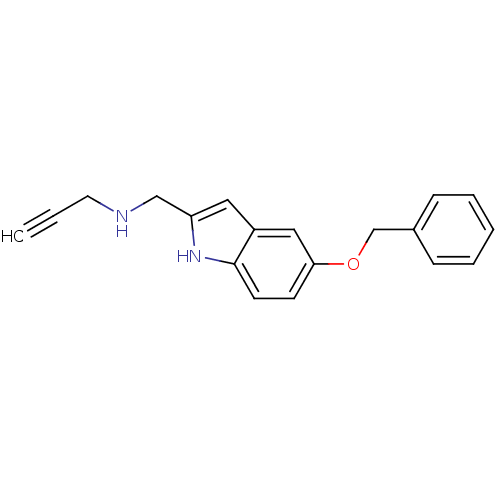 ((5-Benzyloxy-1H-indol-2-ylmethyl)-prop-2-ynyl-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Qu�mica M�dica, (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of human MAO-B expressed in baculovirus infected BTI insect cells preincubated for 15 mins | J Med Chem 57: 10455-63 (2014) Article DOI: 10.1021/jm501501a BindingDB Entry DOI: 10.7270/Q2WS8VX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Qu�mica M�dica, (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of human MAO-A expressed in baculovirus infected BTI insect cells preincubated for 15 mins | J Med Chem 57: 10455-63 (2014) Article DOI: 10.1021/jm501501a BindingDB Entry DOI: 10.7270/Q2WS8VX3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Consejo Superior de Investigaciones Cientificas; Universitat Autonoma de Barcelona; Universidad de Barcelona US Patent | Assay Description The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect... | US Patent US8999994 (2015) BindingDB Entry DOI: 10.7270/Q2NZ86CM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of horse serum BChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine iodide as substrate preincubated for 10 mins before substrate addition by Ellman's method | Eur J Med Chem 46: 6119-30 (2011) Article DOI: 10.1016/j.ejmech.2011.09.038 BindingDB Entry DOI: 10.7270/Q27M08BS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50359391 (CHEMBL1929421) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of rat liver mitochondrial MAO-A using [14C]-5-hydroxytryptamine after 30 mins by scintillation counting | J Med Chem 54: 8251-70 (2011) Article DOI: 10.1021/jm200853t BindingDB Entry DOI: 10.7270/Q20P10G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of Equus caballus BChE preincubated for 10 mins measured after 15 mins by Ellman's method | Eur J Med Chem 46: 4676-81 (2011) Article DOI: 10.1016/j.ejmech.2011.05.068 BindingDB Entry DOI: 10.7270/Q24T6KDT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50069802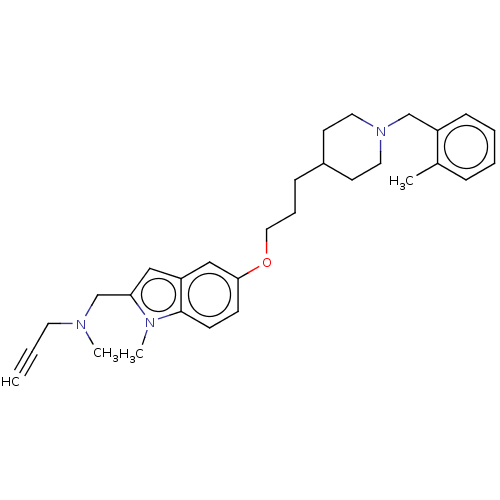 (CHEMBL3407581) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Qu�mica M�dica, (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of human MAO-A expressed in baculovirus infected BTI insect cells preincubated for 15 mins | J Med Chem 57: 10455-63 (2014) Article DOI: 10.1021/jm501501a BindingDB Entry DOI: 10.7270/Q2WS8VX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50359396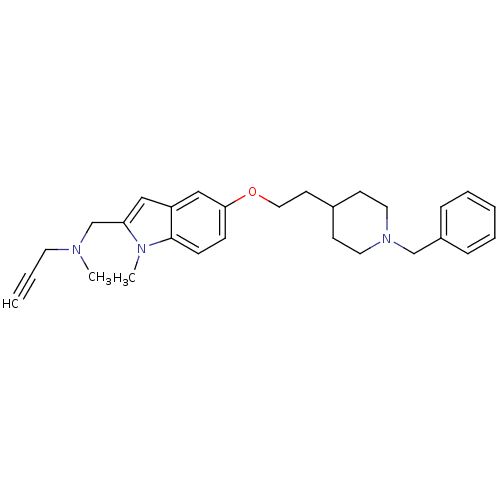 (CHEMBL1929420 | US8999994, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of rat liver mitochondrial MAO-A using [14C]-5-hydroxytryptamine after 30 mins by scintillation counting | J Med Chem 54: 8251-70 (2011) Article DOI: 10.1021/jm200853t BindingDB Entry DOI: 10.7270/Q20P10G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 15 mins by Ellman's method | Eur J Med Chem 52: 251-62 (2012) Article DOI: 10.1016/j.ejmech.2012.03.022 BindingDB Entry DOI: 10.7270/Q25H7H97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AchE using acetylthiocholine iodide as substrate preincubated for 10 mins measured after 15 mins of substrate ... | J Med Chem 54: 8251-70 (2011) Article DOI: 10.1021/jm200853t BindingDB Entry DOI: 10.7270/Q20P10G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Consejo Superior de Investigaciones Cientificas; Universitat Autonoma de Barcelona; Universidad de Barcelona US Patent | Assay Description The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect... | US Patent US8999994 (2015) BindingDB Entry DOI: 10.7270/Q2NZ86CM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of MAO-B in rat liver homogenate using [14C]-phenylethylamine as substrate preincubated for 30 mins followed by substrate addition measure... | Eur J Med Chem 80: 543-61 (2014) Article DOI: 10.1016/j.ejmech.2014.04.078 BindingDB Entry DOI: 10.7270/Q2BK1DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340109 (CHEMBL1762827 | rac-4-[(13-Amino-10,11,12,14-tetra...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50448218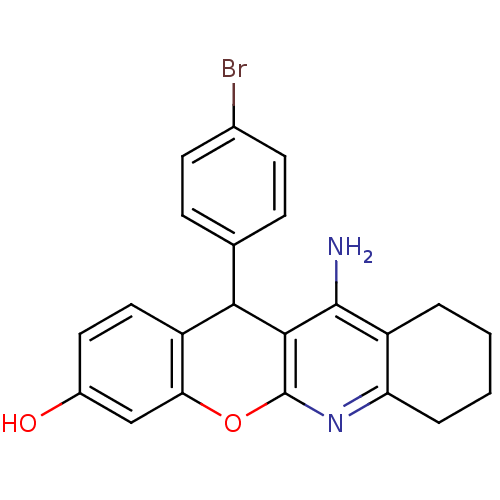 (CHEMBL3120710) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid (UCM) Curated by ChEMBL | Assay Description Inhibition of electric eel AchE using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured for 5 mins by Ellma... | Eur J Med Chem 74: 491-501 (2014) Article DOI: 10.1016/j.ejmech.2013.12.021 BindingDB Entry DOI: 10.7270/Q2G73G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM36552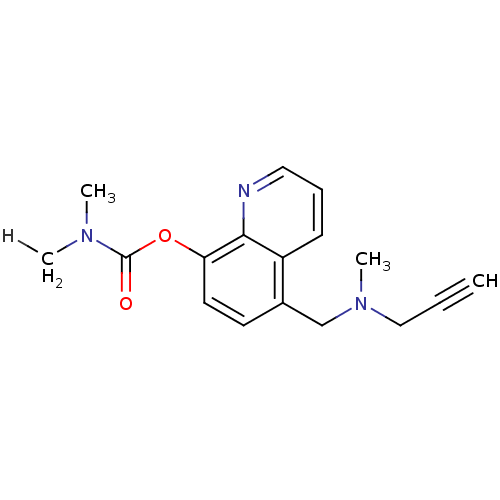 (Prochelators, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Qu�mica M�dica, (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of rat MAO-A | J Med Chem 57: 10455-63 (2014) Article DOI: 10.1021/jm501501a BindingDB Entry DOI: 10.7270/Q2WS8VX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50391207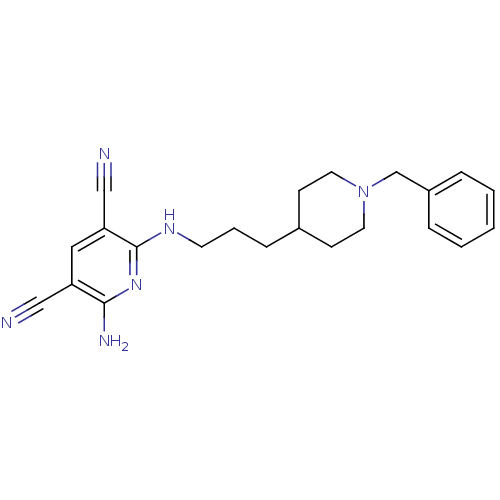 (CHEMBL2088785) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Org£nica General (CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate by Ellman method | Eur J Med Chem 57: 296-301 (2012) Article DOI: 10.1016/j.ejmech.2012.09.030 BindingDB Entry DOI: 10.7270/Q2MP54CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Org£nica General (CSIC) Curated by ChEMBL | Assay Description Inhibition of purified human erythrocyte AChE using acetylcholine as substrate by Ellman's method | Eur J Med Chem 67: 64-74 (2013) Article DOI: 10.1016/j.ejmech.2013.06.021 BindingDB Entry DOI: 10.7270/Q2KW5JZX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50359395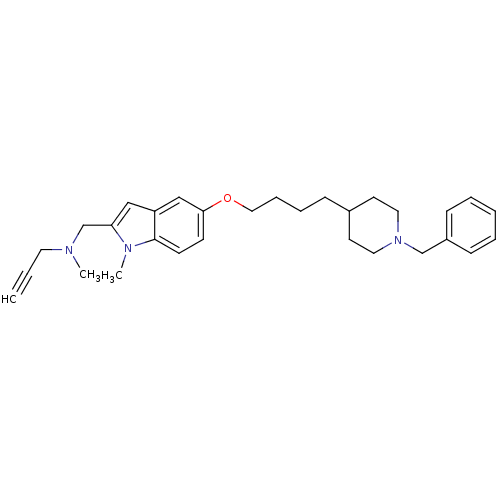 (CHEMBL1929422) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of rat liver mitochondrial MAO-A using [14C]-5-hydroxytryptamine after 30 mins by scintillation counting | J Med Chem 54: 8251-70 (2011) Article DOI: 10.1021/jm200853t BindingDB Entry DOI: 10.7270/Q20P10G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Org£nica General (CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate by Ellman method | Eur J Med Chem 57: 296-301 (2012) Article DOI: 10.1016/j.ejmech.2012.09.030 BindingDB Entry DOI: 10.7270/Q2MP54CK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50493231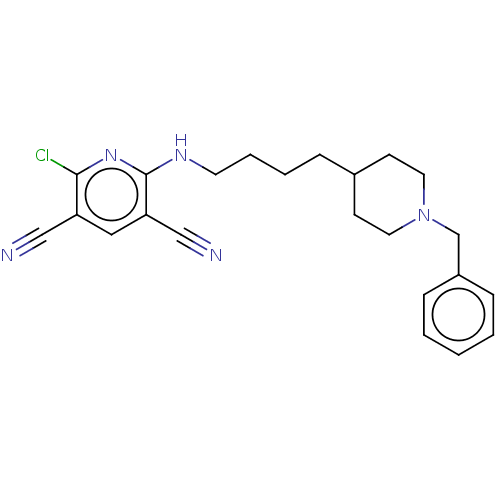 (CHEMBL2419688) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica Org£nica General (CSIC) Curated by ChEMBL | Assay Description Inhibition of purified human erythrocyte AChE using acetylcholine as substrate by Ellman's method | Eur J Med Chem 67: 64-74 (2013) Article DOI: 10.1016/j.ejmech.2013.06.021 BindingDB Entry DOI: 10.7270/Q2KW5JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Qu�mica M�dica (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ... | Eur J Med Chem 75: 82-95 (2014) Article DOI: 10.1016/j.ejmech.2013.12.028 BindingDB Entry DOI: 10.7270/Q22R3T4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50333148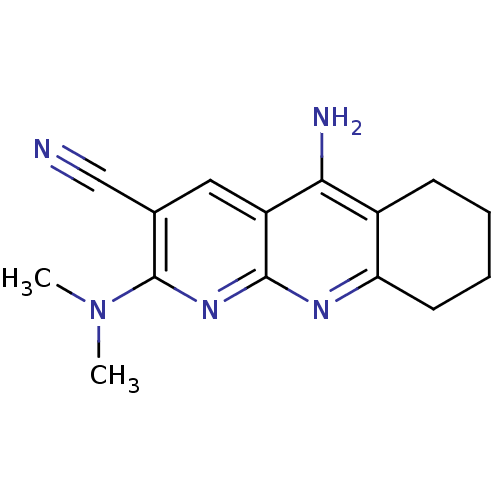 (5-Amino-2-(dimethylamino)-6,7,8,9-tetrahydrobenzo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Radicales Libres y Qu�mica Computacional (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE by Ellman's method | Bioorg Med Chem 19: 122-33 (2011) Article DOI: 10.1016/j.bmc.2010.11.040 BindingDB Entry DOI: 10.7270/Q22R3RX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Qu�mica M�dica, (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE expressed in HEK293 cells preincubated for 15 mins | J Med Chem 57: 10455-63 (2014) Article DOI: 10.1021/jm501501a BindingDB Entry DOI: 10.7270/Q2WS8VX3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340111 (CHEMBL1762829 | rac-14-(3'-Methoxyphenyl)-10,11,12...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorio de Qu�mica M�dica, (IQOG, CSIC) Curated by ChEMBL | Assay Description Inhibition of human MAO-B expressed in baculovirus infected BTI insect cells preincubated for 15 mins | J Med Chem 57: 10455-63 (2014) Article DOI: 10.1021/jm501501a BindingDB Entry DOI: 10.7270/Q2WS8VX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 657 total ) | Next | Last >> |